Abstract
Objective
To determine the effects of interleukin (IL)-2 treatment on inflammatory and thrombotic biomarkers in chronically HIV-infected adults receiving antiretroviral therapy (ART).
Methods
Cryopreserved plasma was evaluated retrospectively for CRP and D-dimer at baseline, end of an IL-2 cycle, and long-term follow-up from 2 randomized, controlled trials: 1) 57 IL-2-naive adults receiving either 3–6 cycles of IL-2 plus ART (nucleoside analogues) or ART alone for 12 months; 2) 40 IL-2-experienced adults on HAART who either interrupted or continued therapy for 6 months after a baseline IL-2 cycle. High-sensitivity CRP (hsCRP) was measured by immunonephelometry (detection limit 0.175 mg/L) and D-dimer by latex agglutination (detection limit 0.20 mg/L). Median within-group differences and pre- and post-IL-2 changes between groups were assessed via non-parametric Wilcoxon Signed-Rank and Mann-Whitney U tests. Spearman Rank test was used to assess correlations between changes in hsCRP, D-dimer, and HIV-RNA viral load.
Results
Significant increases in hsCRP (Study 1: 138.6 mg/L; Study 2: 58.9 mg/L) and D-dimer (Study 1: 3.1 mg/L; Study 2: 0.4 mg/L, all p < 0.0001) occurred by the end of the initial IL-2 cycle, returning to baseline by end of study. No correlations were seen between changes in hsCRP or D-dimer and HIV-RNA, CD4 T cell count or proliferation (Ki67 expression). No thrombotic or cardiovascular serious adverse events occurred during these study periods.
Conclusions
IL-2 dosing caused transient increases in plasma hsCRP and D-dimer levels, unassociated with HIV-RNA viral load, suggesting the possibility of increased risk for thrombotic events.
Keywords: Interleukin-2, C-reactive protein, D-dimer, HIV infections, Randomized Controlled Trial
Introduction
Several Phase I/II studies have demonstrated that intermittent cycling of IL-2 leads to long-term CD4 T cell increases in chronic HIV-infection [1, 2]. Recently, data from large-scale multicenter phase III trials (ESPRIT and SILCAAT) [3, 4] showed that these CD4 T cell increases are not associated with clinical benefit and may be associated with more Grade 4 clinical events [5, 6].
IL-2 causes the release of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-1β, and IL-6 [7–10], which are known to increase acute phase reactants such as C-reactive protein (CRP) and activate pro-thrombotic pathways [11, 12]. Multiple studies have established associations between levels of CRP and the fibrinogen breakdown product D-dimer with both cardiovascular and thrombotic disease [10, 13-15]. These biomarkers have also been shown to be elevated in HIV-infected patients [16, 17]. Recent findings from the Strategies for the Management of Antiretroviral Therapy (SMART) trial demonstrated that baseline levels of CRP and D-dimer were predictors of all-cause mortality in a chronically HIV-infected sample of patients who were undergoing highly active antiretroviral treatment (HAART) interruption [18]. Understanding the acute and chronic effects of IL-2 on these markers may thus provide insights into potential IL-2-related complications.
In this study, plasma CRP and D-dimer levels were analyzed longitudinally in 2 randomized clinical trials that evaluated IL-2 as an adjuvant therapy in HIV-infection. The first study [19, 20] evaluated the effect of 3–6 IL-2 cycles added to single or dual nucleoside analogue therapy administered over 1 year compared to antiretroviral therapy alone. The second study [21, 22] evaluated the effect of a baseline IL-2 cycle in patients who either subsequently interrupted or continued HAART for 6 months. Biomarkers and immune parameters were analyzed pre- and post-IL-2 administration, as well as long-term at the end of each trial, in order to characterize the duration of any IL-2 effect, its relationship to HIV viremia and changes in CD4 T cell proliferation.
Methods
Clinical Trials
The methodology and primary outcomes of both these trials have been published previously [19–22]. In the first study [19] 57 chronically HIV-infected IL-2-naive adults on nucleoside reverse transcriptase inhibitor (NRTI) mono- or combination therapy were randomized 1:1 to receive either 3–6 intermittent cycles of IL-2 plus ART or ART alone and were followed for one year. Cycles of human recombinant IL-2 (Novartis, Emeryville, CA) were administered no more frequently than every 8 weeks for 5 consecutive days as continuous infusions of 6–18 million international units per day.
The second study [21, 22] consisted of 40 chronically HIV-infected IL-2-experienced adults who were on HAART therapy prior to receiving a baseline cycle of IL-2 (subcutaneous injections of 7.5 million international units twice daily). Subjects were then randomized 2:1 either to interrupt or continue HAART for 6 months, during which time additional IL-2 cycles were administered with 10 days of peri-cycle HAART, if CD4 T cell counts fell below 90% of baseline.
Participants in both trials were evaluated monthly throughout their study periods. Intracellular Ki67 expression in CD4 T cells was determined via flow cytometry at baseline in both studies, at 12 months follow-up in Study 1, and at 1 month post-HAART interruption in Study 2, as described in [20].
CRP and D-dimer measurements
Concentrations of CRP and D-dimer were determined from cryopreserved plasma collected at baseline, upon completion of the first IL-2 cycle (Day 6 in the first study and Day 5 in the second), and at the end of the study period (Month 12 in Study 1 and Month 6 in Study 2). For subjects in Study 2 who interrupted HAART, levels were also measured 1 month after the baseline IL-2 cycle.
Plasma CRP levels were determined using high-sensitivity (hsCRP) immunonephelometry (BN-II, Siemens) with a detection limit of 0.175 mg/L. D-dimer concentration was determined via latex agglutination (Liatest, Diagnostica Stago) with a detection limit of 0.20 mg/L.
Statistical Methods
Median within-group differences from baseline to either the end of the first IL-2 cycle or the entire study period were assessed via the non-parametric Wilcoxon Signed-Rank test. Between-group comparisons between randomized treatment groups within each trial were assessed via the non-parametric Mann-Whitney U test. Spearman Rank correlation tests and linear regression analysis were used to evaluate the bivariate associations between magnitude of change in hsCRP, D-dimer, CD4 T cells, and HIV-RNA plasma levels at these same time points.
Results
Participant Characteristics
Demographic characteristics and median values for baseline immune parameters and biomarkers for both treatment groups in each trial are summarized in Table 1. No thrombotic or cardiovascular serious adverse events occurred in either trial during these study periods.
Table 1.
Baseline characteristics, immune parameters, inflammatory, and thrombotic biomarkers. Median values are shown.
| STUDY 1 IL-2 recipients | Controls | STUDY 2 | |
|---|---|---|---|
| N | 28 | 29 | 40 |
| Sex (% male) | 97 | 100 | 98 |
| Age (years) | 38 | 36.5 | 46 |
| IL-2-experienced | No | No | Yes (≥3 cycles) |
| Baseline IL-2 cycle | Yes | No | Yes |
| Additional IL-2 cycles* | 3–6 | 0 | 0–1 |
| CD4 T cells/μl | 416 | 371 | 947 |
| HIV-RNA VL (copies/mL) | 3580 | 5926 | < 50 |
| hsCRP (mg/L) | 0.91 | 0.90 | 1.15 |
| D-dimer (mg/L) | 0.25 | 0.21 | 0.19 |
Range of additional IL-2 cycles given over study period; IL = interleukin; VL = viral load; hsCRP = high sensitivity; C-reactive protein
Effects of IL-2 on hsCRP and D-dimer
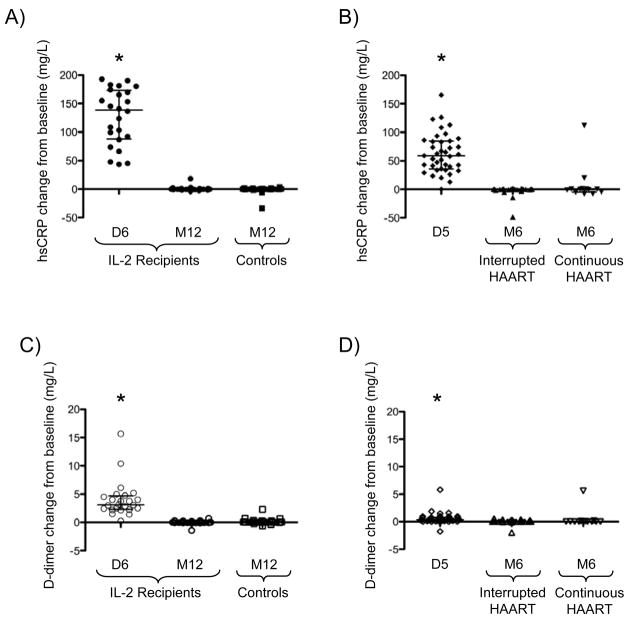
By the end of the initial IL-2 cycle, IL-2 recipients from both trials demonstrated significant increases (all p < 0.0001) in median plasma levels of hsCRP (Study 1 = 138.6 mg/L, interquartile range (IQR): 87.9–173 mg/L; Study 2 = 58.9 mg/L, IQR: 35.9–84.5 mg/L) and D-dimer (Study 1 = 3.1 mg/L, IQR: 2.3–4.7 mg/L; Study 2 = 0.4 mg/L, IQR: 0.2–0.8 mg/L). However, as shown in Figure 1, these increases were transient, as hsCRP (Panels A and B) and D-dimer (Panels C and D) levels returned to baseline in IL-2 recipients from each trial by the end of their respective study periods (Figure 1). In the second trial, both hsCRP and D-dimer had returned to baseline as early as 1 month post-IL-2 administration (data not shown).
Figure 1. IL-2-associated changes in biomarker levels.
Significant increases were evident immediately following interleukin (IL)-2 cycling in plasma high-sensitivity C-reactive protein (hsCRP) and D-dimer levels in Study 1 (A and C) and Study 2 (B and D), which normalized by long-term follow-up, regardless of the presence of HAART and, hence, HIV viremia. HAART = highly active antiretroviral treatment; D = day, M = month; *p<0.0001
Association between Changes in hsCRP, D-dimer, and Immune Parameters
Peri-IL-2 cycle increases in hsCRP and D-dimer positively correlated among IL-2 recipients in Study 1 (R = 0.43, p = 0.036) and Study 2 (R = 0.57, p = 0.0002); however, levels of hsCRP and D-dimer were not associated at baseline in either trial. These end of IL-2 cycle increases in hsCRP and D-dimer were not correlated to baseline HIV-RNA viral load or CD4 T cell count in either study. Long-term changes (baseline to either Month 12 in Study 1 or Month 6 in Study 2) in hsCRP or D-dimer with either HIV-RNA viral load or CD4 T cell count were also not associated (data not shown). In addition, no correlations were observed between changesin CD4 T cell proliferation (Ki67 expression), hsCRP, or D-dimer levels over the same time periods in either trial (data not shown).
Discussion
This study demonstrates that IL-2 cycles cause marked transient increases in plasma levels of the acute phase reactant CRP and the thrombotic marker D-dimer, both in the setting of inadequate HIV-RNA suppression (i.e., mono or dual nucleoside analogue therapy) and with viral suppression to <50 copies/ml on HAART.
Significant associations between inflammatory biomarkers and endothelial activation markers have been demonstrated in HIV-infected patients [17], suggesting a potential mechanism by which such markers may portend cardiovascular or thrombotic disease. In addition, data from the SMART study suggested increases in biomarker levels observed following HAART interruption were associated with increased rates of non-HIV-related adverse events [18]. However, the clinical significance of the hsCRP and D-dimer elevations observed in these two trials is unclear, as these levels returned to baseline by 1 month post-IL-2 and remained at baseline when measured up to 12 months after an initial IL-2 cycle. This finding is consistent with studies in malignant melanoma patients, which demonstrated that IL-2-induced increases in several pro-coagulant markers, including thrombin-antithrombin and plasmin-antiplasmin complexes, returned to baseline within 24 hours post-IL-2 administration [11]. Moreover, no serious clinical events related to thromboses, emboli, or cardiovascular events were reported during the primary study periods in either of these trials. In contrast to findings from the SMART study [18], increased adverse clinical events were not observed among subjects in Study 2 who interrupted HAART, possibly due to the small sample size, shorter duration of follow-up (6 months), and better immune status (median CD4 T cell count = 947 cells/μl) compared to subjects in the SMART study.
Of note, however, a significantly greater number of serious cardiovascular events were observed in the IL-2 treatment arm compared to the placebo arm (40 versus 14 events, respectively) of the multi-center ESPRIT trial, the most common being peripheral emboli and thromboses (13 versus 2 events, respectively) [6]. Given the randomized design of the ESPRIT trial, this finding supports a causal relationship for these adverse events to IL-2. However, the timing of their clinical presentation raises questions as to the underlying pathologic mechanism.
These data offer the first randomized and controlled comparative analysis of inflammatory and thrombotic biomarkers in HIV-infected IL-2 recipients versus non-recipients, both in the presence and absence of HAART. The striking impact of IL-2 treatment on these biomarkers should warrant evaluating thrombotic and cardiovascular risk factors prior to IL-2 treatment, regardless of HIV status. With outcome data from the SILCAAT and ESPRIT trials indicating that IL-2 provides no clinical benefit in chronic HIV infection treated with HAART [5, 6], the present findings suggest a possible mechanism to explain some of the adverse clinical events associated with IL-2 treatment.
Acknowledgments
The authors would like to thank all study participants and the staff of Outpatient Clinic 8 at NIAID for their help in completing both trials. B. Porter contributed to data organization, analysis, and interpretation, and wrote the manuscript. J. Kovacs, R. Davey, and H. Lane contributed to the development and implementation of both trials, and review of the manuscript. J. Shen and C. Rehm contributed to data collection, organization, and analysis, and review of the manuscript. J. Lozier, G. Csako, K. Ngheim, and R. Costello contributed to sample analysis, data organization, and review of the manuscript. I. Sereti contributed to the development and implementation of the study, data organization, analysis, and interpretation, and editing of the manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Clinical and Molecular Retrovirology Section (Bethesda, MD). Human recombinant IL-2 was provided by Novartis (Emeryville, CA).
Sources of Support
This work was supported by the Intramural Research Programs of the Clinical and Molecular Retrovirology Section of the National Institute of Allergy and Infectious Diseases and the Clinical Center of the National Institutes of Health (Bethesda, MD). Human recombinant IL-2 was provided by Novartis (Emeryville, CA).
Footnotes
Conflicts of Interest: The US Government has been granted a patent for the use of intermittent subcutaneous IL-2 as therapy in HIV infection, listing H. C. Lane and J.A. Kovacs as co-inventors.
Prior Publication Disclosure
This work was presented in an abstract format at the 16th Conference on Retroviruses and Opportunistic Infections (CROI), held in Montreal, Canada from 2/8/09 to 2/11/09.
Contributor Information
Brian O. Porter, National Institutes of Health, National Institute of Allergy & Infectious Diseases
Jean Shen, NIH, Critical Care Medicine Department.
Joseph A. Kovacs, NIH, Clinical Center [CC]
Richard T. Davey, NIH, NIAID
Catherine Rehm, NIH, NIAID.
Jay Lozier, NIH, CC, Department of Laboratory Medicine [DLM].
Gyorgy Csako, NIH, CC, DLM.
Khanh Nghiem, NIH, CC, DLM.
Rene Costello, NIH, CC, DLM.
H. Clifford Lane, NIH, NIAID.
Irini Sereti, NIH, NIAID.
References
- 1.Arduino RC, Nannini EC, Rodriguez-Barradas M, Schrader S, Losso M, Ruxrungtham K, et al. CD4 cell response to 3 doses of subcutaneous interleukin 2: meta-analysis of 3 Vanguard studies. Clin Infect Dis. 2004;39:115–122. doi: 10.1086/421775. [DOI] [PubMed] [Google Scholar]
- 2.Farel CE, Chaitt DG, Hahn BK, Tavel JA, Kovacs JA, Polis MA, et al. Induction and maintenance therapy with intermittent interleukin-2 in HIV-1 infection. Blood. 2004;103:3282–3286. doi: 10.1182/blood-2003-09-3283. [DOI] [PubMed] [Google Scholar]
- 3.Emery S, Abrams DI, Cooper DA, Darbyshire JH, Lane HC, Lundgren JD, Neaton JD. The evaluation of subcutaneous proleukin (interleukin-2) in a randomized international trial: rationale, design, and methods of ESPRIT. Control Clin Trials. 2002;23:198–220. doi: 10.1016/s0197-2456(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 4.Pett SL, Wand H, Law MG, Arduino R, Lopez JC, Knysz B, et al. Evaluation of Subcutaneous Proleukin (interleukin-2) in a Randomized International Trial (ESPRIT): geographical and gender differences in the baseline characteristics of participants. HIV Clin Trials. 2006;7:70–85. doi: 10.1310/4733-acqf-f3p4-2qac. [DOI] [PubMed] [Google Scholar]
- 5.Levy Y. Effect of interleukin-2 (IL-2) on clinical outcomes in patients with CD4+ cell count 50–299/mm3: Primary results of the SILCAAT Study. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 6.Losso M, Abrams D. Effect of interleukin-2 (IL-2) on clinical outcomes in patients with CD4+ cell count >300/mm3: Preliminary results of the ESPRIT study. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 7.Fortis C, Soldini L, Ghezzi S, Colombo S, Tambussi G, Vicenzi E, et al. Tumor necrosis factor alpha, interleukin 2, and soluble interleukin 2 receptor levels in human immunodeficiency virus type 1-infected individuals receiving intermittent cycles of interleukin 2. AIDS Res Hum Retroviruses. 2002;18:491–499. doi: 10.1089/088922202317406637. [DOI] [PubMed] [Google Scholar]
- 8.Mier JW, Vachino G, van der Meer JW, Numerof RP, Adams S, Cannon JG, et al. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988;8:426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- 9.Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res. 1993;53:2597–2602. [PubMed] [Google Scholar]
- 10.Sereti I, Herpin B, Metcalf JA, Stevens R, Baseler MW, Hallahan CW, et al. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. Aids. 2001;15:1765–1775. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 11.Baars JW, de Boer JP, Wagstaff J, Roem D, Eerenberg-Belmer AJ, Nauta J, et al. Interleukin-2 induces activation of coagulation and fibrinolysis: resemblance to the changes seen during experimental endotoxaemia. Br J Haematol. 1992;82:295–301. doi: 10.1111/j.1365-2141.1992.tb06421.x. [DOI] [PubMed] [Google Scholar]
- 12.Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL-2. Clin Exp Immunol. 1994;95:366–372. doi: 10.1111/j.1365-2249.1994.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roumen-Klappe EM, den Heijer M, van Uum SH, van der Ven-Jongekrijg J, van der Graaf F, Wollersheim H. Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 2002;35:701–706. doi: 10.1067/mva.2002.121746. [DOI] [PubMed] [Google Scholar]
- 14.Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. Jama. 2003;290:932–940. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 15.Lowe GD. Circulating inflammatory markers and risks of cardiovascular and non-cardiovascular disease. J Thromb Haemost. 2005;3:1618–1627. doi: 10.1111/j.1538-7836.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185:456–462. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, Armentrout R, O’Riordan MA, Storer N, Rizk N, Harrill D, et al. Endothelial Activation Markers Are Linked to HIV Status and Are Independent of Antiretroviral Therapy and Lipoatrophy. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs JA, Vogel S, Albert JM, Falloon J, Davey RT, Jr, Walker RE, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 20.Sereti I, Anthony KB, Martinez-Wilson H, Lempicki R, Adelsberger J, Metcalf JA, et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004;104:775–780. doi: 10.1182/blood-2003-12-4355. [DOI] [PubMed] [Google Scholar]
- 21.Porter BO, Anthony KB, Shen J, Hahn B, Keh CE, Maldarelli F, et al. Inferiority of IL-2 alone versus IL-2 with HAART in maintaining CD4 T cell counts during HAART interruption: a randomized controlled trial. Aids. 2009;23:203–212. doi: 10.1097/QAD.0b013e32831cc114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keh CE, Shen JM, Hahn B, Hallahan CW, Rehm CA, Thaker V, et al. Interruption of antiretroviral therapy blunts but does not abrogate CD4 T-cell responses to interleukin-2 administration in HIV infected patients. Aids. 2006;20:361–369. doi: 10.1097/01.aids.0000206502.24407.9f. [DOI] [PubMed] [Google Scholar]