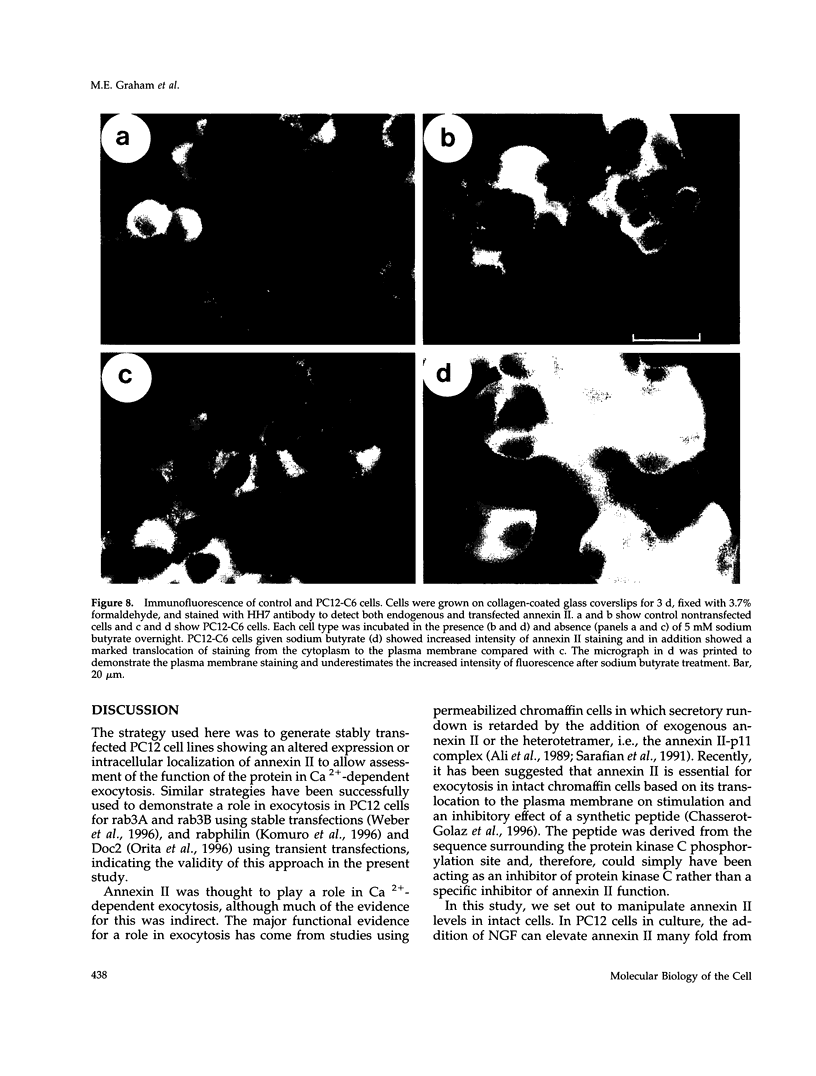
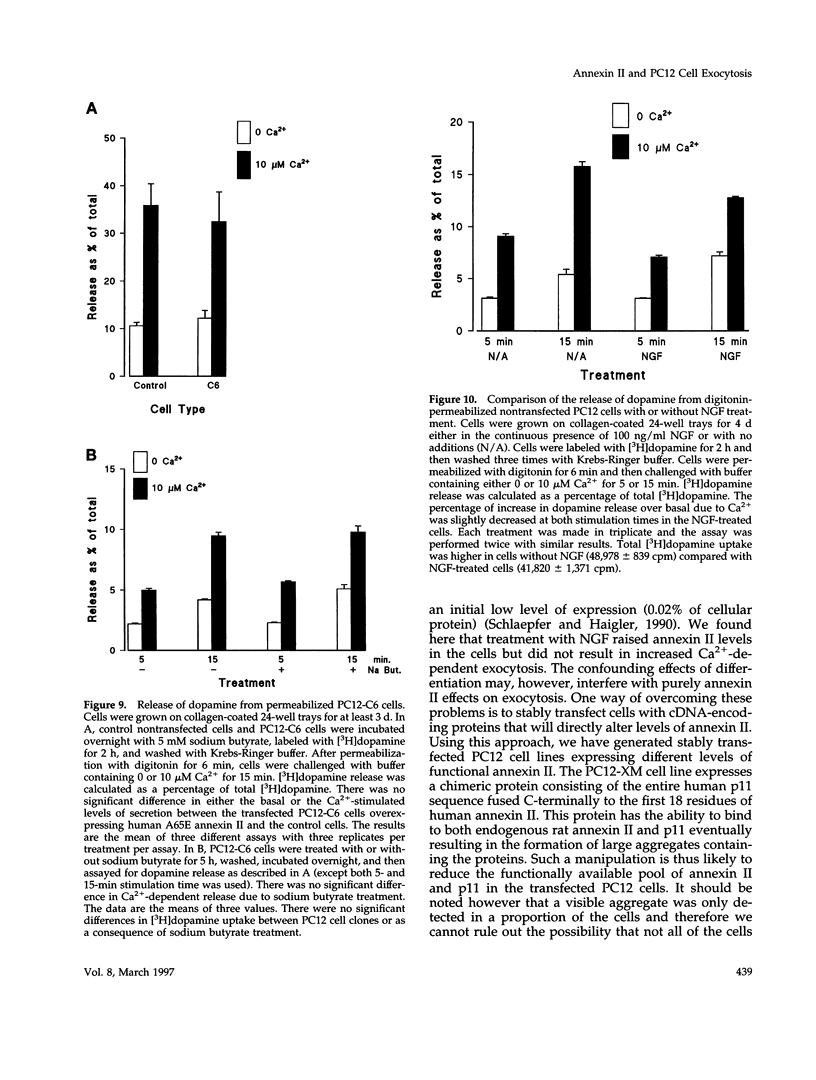
Abstract
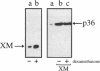
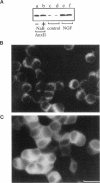
The Ca2+/phospholipid/cytoskeletal-binding protein annexin II has been proposed to play an important role in Ca(2+)-dependent exocytosis; however, the evidence for this role is inconclusive. More direct evidence obtained by manipulating annexin II levels in cells is still required. We have attempted to do this by generating stably transfected PC12 cell lines expressing proteins which elevate or lower functional annexin II levels and using these cell lines to investigate Ca(2+)-dependent exocytosis. Three cell lines were generated: one expressing an annexin II mutant which aggregates annexin II in at least a proportion of the cells, thereby removing functional protein from the cell; a mixed clonal cell line constitutively overexpressing human annexin II; and a clonal cell line capable of over-expressing annexin II in the presence of sodium butyrate. After digitonin permeabilization, Ca(2+)-dependent dopamine release from these cell lines was compared with that from control nontransfected cells, and, in addition, release was compared in induced to uninduced cells. There were no significant differences in Ca(2+)-dependent exocytosis between any of the transfected cell lines before or after induction and the control cells. In addition, nontransfected PC12 cells treated with nerve growth factor, which elevates annexin II levels severalfold, failed to increase Ca(2+)-dependent exocytosis after digitonin permeabilization, compared with control cells. We conclude that annexin II is not an important regulator of Ca(2+)-dependent exocytosis in PC12 cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Ellis C. A., Harris A., Sellers L. A., Toker A. Kinase and neurotransmitters. Nature. 1990 Apr 12;344(6267):594–594. doi: 10.1038/344594a0. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Burgoyne R. D. The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signal. 1990;2(3):265–276. doi: 10.1016/0898-6568(90)90054-e. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Clague M. J. Annexins in the endocytic pathway. Trends Biochem Sci. 1994 Jun;19(6):231–232. doi: 10.1016/0968-0004(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Evidence for a role of calpactin in calcium-dependent exocytosis. Biochem Soc Trans. 1990 Dec;18(6):1101–1104. doi: 10.1042/bst0181101. [DOI] [PubMed] [Google Scholar]
- Chamberlain L. H., Roth D., Morgan A., Burgoyne R. D. Distinct effects of alpha-SNAP, 14-3-3 proteins, and calmodulin on priming and triggering of regulated exocytosis. J Cell Biol. 1995 Sep;130(5):1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasserot-Golaz S., Vitale N., Sagot I., Delouche B., Dirrig S., Pradel L. A., Henry J. P., Aunis D., Bader M. F. Annexin II in exocytosis: catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J Cell Biol. 1996 Jun;133(6):1217–1236. doi: 10.1083/jcb.133.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E., Dowling L. G., Sando J. J., Villar-Palasi C., Whipple J. H., Zaks W. J. Characterization of the chromobindins. Soluble proteins that bind to the chromaffin granule membrane in the presence of Ca2+. J Biol Chem. 1983 Dec 10;258(23):14664–14674. [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr Calpactins: calcium-regulated membrane-skeletal proteins. Bioessays. 1987 Oct;7(4):173–175. doi: 10.1002/bies.950070408. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Emans N. Annexins in membrane traffic. Trends Cell Biol. 1993 Jul;3(7):224–227. doi: 10.1016/0962-8924(93)90116-i. [DOI] [PubMed] [Google Scholar]
- Handel S. E., Rennison M. E., Wilde C. J., Burgoyne R. D. Annexin II (calpactin I) in the mouse mammary gland: immunolocalization by light- and electron microscopy. Cell Tissue Res. 1991 Jun;264(3):549–554. doi: 10.1007/BF00319044. [DOI] [PubMed] [Google Scholar]
- Harder T., Gerke V. The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J Cell Biol. 1993 Dec;123(5):1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993 Dec 9;366(6455):572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hirt R. P., Poulain-Godefroy O., Billotte J., Kraehenbuhl J. P., Fasel N. Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene. 1992 Feb 15;111(2):199–206. doi: 10.1016/0378-1119(92)90687-k. [DOI] [PubMed] [Google Scholar]
- Johnsson N., Johnsson K., Weber K. A discontinuous epitope on p36, the major substrate of src tyrosine-protein-kinase, brings the phosphorylation site into the neighbourhood of a consensus sequence for Ca2+/lipid-binding proteins. FEBS Lett. 1988 Aug 15;236(1):201–204. doi: 10.1016/0014-5793(88)80314-4. [DOI] [PubMed] [Google Scholar]
- Komuro R., Sasaki T., Orita S., Maeda M., Takai Y. Involvement of rabphilin-3A in Ca2+-dependent exocytosis from PC12 cells. Biochem Biophys Res Commun. 1996 Feb 15;219(2):435–440. doi: 10.1006/bbrc.1996.0251. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Berón W., Sarrouf M. N., Colombo M. I., Creutz C., Stahl P. D. Calcium-dependent fusion among endosomes. J Biol Chem. 1994 Dec 9;269(49):30927–30934. [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. A role for soluble NSF attachment proteins (SNAPs) in regulated exocytosis in adrenal chromaffin cells. EMBO J. 1995 Jan 16;14(2):232–239. doi: 10.1002/j.1460-2075.1995.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Morgan A., Wilkinson M., Burgoyne R. D. Identification of Exo2 as the catalytic subunit of protein kinase A reveals a role for cyclic AMP in Ca(2+)-dependent exocytosis in chromaffin cells. EMBO J. 1993 Oct;12(10):3747–3752. doi: 10.1002/j.1460-2075.1993.tb06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T., Sobue K., Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol. 1990 Jan;110(1):13–25. doi: 10.1083/jcb.110.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita S., Sasaki T., Komuro R., Sakaguchi G., Maeda M., Igarashi H., Takai Y. Doc2 enhances Ca2+-dependent exocytosis from PC12 cells. J Biol Chem. 1996 Mar 29;271(13):7257–7260. doi: 10.1074/jbc.271.13.7257. [DOI] [PubMed] [Google Scholar]
- Osborn M., Johnsson N., Wehland J., Weber K. The submembranous location of p11 and its interaction with the p36 substrate of pp60 src kinase in situ. Exp Cell Res. 1988 Mar;175(1):81–96. doi: 10.1016/0014-4827(88)90257-1. [DOI] [PubMed] [Google Scholar]
- Roth D., Burgoyne R. D. Stimulation of catecholamine secretion from adrenal chromaffin cells by 14-3-3 proteins is due to reorganisation of the cortical actin network. FEBS Lett. 1995 Oct 23;374(1):77–81. doi: 10.1016/0014-5793(95)01080-x. [DOI] [PubMed] [Google Scholar]
- Roth D., Morgan A., Burgoyne R. D. Identification of a key domain in annexin and 14-3-3 proteins that stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. FEBS Lett. 1993 Apr 12;320(3):207–210. doi: 10.1016/0014-5793(93)80587-k. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semich R., Gerke V., Robenek H., Weber K. The p36 substrate of pp60src kinase is located at the cytoplasmic surface of the plasma membrane of fibroblasts; an immunoelectron microscopic analysis. Eur J Cell Biol. 1989 Dec;50(2):313–323. [PubMed] [Google Scholar]
- Thiel C., Weber K., Gerke V. Characterization of a Ca(2+)-binding site in human annexin II by site-directed mutagenesis. J Biol Chem. 1991 Aug 5;266(22):14732–14739. [PubMed] [Google Scholar]
- Waisman D. M. Annexin II tetramer: structure and function. Mol Cell Biochem. 1995 Aug-Sep;149-150:301–322. doi: 10.1007/BF01076592. [DOI] [PubMed] [Google Scholar]
- Walent J. H., Porter B. W., Martin T. F. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992 Sep 4;70(5):765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Weber E., Jilling T., Kirk K. L. Distinct functional properties of Rab3A and Rab3B in PC12 neuroendocrine cells. J Biol Chem. 1996 Mar 22;271(12):6963–6971. doi: 10.1074/jbc.271.12.6963. [DOI] [PubMed] [Google Scholar]
- Wu Y. N., Vu N. D., Wagner P. D. Anti-(14-3-3 protein) antibody inhibits stimulation of noradrenaline (norepinephrine) secretion by chromaffin-cell cytosolic proteins. Biochem J. 1992 Aug 1;285(Pt 3):697–700. doi: 10.1042/bj2850697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. N., Wagner P. D. Calpactin-depleted cytosolic proteins restore Ca(2+)-dependent secretion to digitonin-permeabilized bovine chromaffin cells. FEBS Lett. 1991 Apr 22;282(1):197–199. doi: 10.1016/0014-5793(91)80476-j. [DOI] [PubMed] [Google Scholar]
- Zokas L., Glenney J. R., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987 Nov;105(5):2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]