Abstract
This review focuses on presympathetic neurons in the medulla oblongata including the adrenergic cells groups C1-C3 in the rostral ventrolateral medulla and the serotonergic, GABAergic and glycinergic neurons in the ventromedial medulla. The phenotypes of these neurons including colocalized neuropeptides (e.g. neuropeptide Y, enkephalin, thyrotropin-releasing hormone, substance P) as well as their relative anatomical location are considered in relation to predicting their function in control of sympathetic outflow, in particular the sympathetic outflows controlling blood pressure and thermoregulation. Several explanations are considered for how the neuroeffectors coexisting in these neurons might be functioning, although their exact purpose remains unknown. Although there is abundant data on potential neurotransmitters and neuropeptides contained in the presympathetic neurons, we are still unable to predict function and physiology based solely on the phenotype of these neurons.
Keywords: phenotype, neuropeptide, serotonin, catecholamine, thermoregulation, central cardiovascular control, sympathetic nervous system
1. Introduction
The neurons that innervate the spinal sympathetic preganglionic neurons (SPGNs) are termed presympathetic neurons. The SPGNs are the final common pathway for many reflexes important to homeostasis (e.g., maintaining blood pressure or body temperature at appropriate levels). Thus the presympathetic neurons are in the position to orchestrate these reflexes. Presympathetic neurons are located in the upper cervical spinal cord, medulla, pons and hypothalamus. This review will focus on the neurotransmitters and peptides associated with the presympathetic neurons located in the medulla oblongata and what the various phenotypes of these neurons might reveal about sympathetic control, with particular focus on cardiovascular control and thermoregulation.
2. Bulbospinal Presympathetic Neurons
The sympathetic nervous system is responsible for “resting state” homeostatic functions as well as responding to stressful conditions such as exercise, disease or “fight or flight” situations. These functions are orchestrated through a complex network operating through SPGNs in the spinal cord that project to sympathetic ganglia. The neurons directly innervating the SPGNs are the presympathetic neurons. The presympathetic neurons are in the position to control a range of functions from blood pressure to bladder control. This review will focus on the presympathetic neurons in the medulla oblongata that control primarily cardiovascular and thermoregulatory functions.
Loewy and coworkers (1995a; 1997) found many groups of presympathetic neurons in the upper cervical spinal cord, medulla, pons and forebrain using the pseudorabies virus (PRV). The PRV is taken up by cells in a post-ganglionic target (e.g., adrenal gland or heart), replicates within the cell and then buds out and infects the next cell(s) in closest physical proximity, that is those that are synaptically connected (Card et al., 1990). This cycle of replication and infection continues through chains of synaptically connected neurons, thus revealing the sympathetic preganglionic neurons, sympathetic interneurons within the spinal cord (not normally referred to as “presympathetic” although they are directly antecedent to SPGNs) and the presympathetic neurons in the brainstem (as well as pons and forebrain). This method is useful in determining candidate presympathetic neurons and has identified the main groups of these presympathetic neurons in the medulla: the rostral ventrolateral medulla (RVLM) that contains the C1 cells, the rostral ventromedial medulla (RVMM) with the various raphe nuclei and the dorsal area around the medial longitudinal fasciculus/lateral tegmental field containing the C3 neurons. These groups of neurons will be discussed by primary phenotype and function.
3. RVLM Presympathetic Neurons
3.1. Catecholaminergic presympathetic RVLM neurons, the C1 cells
Fuxe and coworkers introduced the first technique that allowed identification of various monoaminergic neurons in brain and showed that some of these neurons had spinal projections (Carlsson et al., 1964; Dahlstrom and Fuxe, 1965). The C1 adrenergic neurons were first described by Hokfelt and coworkers (1974) who also suggested that C1 cells might be involved in vasomotor control (Bolme et al., 1974). The defining characteristic of these neurons is that they contain the catecholamine synthetic enzymes necessary for the production of epinephrine, i.e. tyrosine hydroxylase (TH), dopamine beta-hydroxylase (DBH) and phenylethanolamine-N-methyltransferase (PNMT). The first demonstration that C1 neurons contain all the catecholamine synthetic enzymes was only relatively recently demonstrated by Phillips et al. (2001).
Reis and coworkers added substantially to the hypothesis that the C1 cells were presympathetic by identifying the spinally projecting PNMT-immunoreactive (ir) C1 neurons in the ventrolateral medulla and showing that PNMT terminals contact the SPGNs in rat (Tucker et al., 1987; Milner et al., 1988). Anderson et al. (1989) and Bernstein-Goral & Bohn (1989) replicated these findings of PNMT terminals in contact with SPGNs in the rat. Minson et al. (1990) added more evidence for C1 bulbospinal neurons and found that some of the PNMT innervation of the spinal cord comes from the more dorsal C2 and C3 adrenergic neurons (discussed in more detail below).
3.2. Other neuroactive substances present in C1 neurons
The RVLM C1 bulbospinal neurons contain several other neurochemicals including enkephalin (Stornetta et al., 2001), NPY (Blessing et al., 1987; Tseng et al., 1993; Stornetta et al., 1999), cocaine- and amphetamine-regulated transcript (CART) (Dun et al., 2002; Burman et al., 2004), pre-pro-tachykinin (substance P) (Li et al., 2005) and calbindin (Goodchild et al., 2000). The colocalization of potential transmitter substances in the C1 cells has been previously reviewed by Pilowsky & Goodchild (2002). The most recently described potential transmitter found to be colocalized in the C1 neurons is the sympatho-excitatory neurochemical pituitary adenylate cyclase-activating polypeptide (PACAP) (Farnham et al., 2008).
To date, the phenotypic identification of cotransmitters in C1 neurons has yielded some limited predictive value on the projection pattern and conduction velocity of C1 cells with specific colocalized neuropeptides. One instance is that NPY tends to be in the C1 neurons projecting to the hypothalamus rather than to the spinal cord (only 10% of spinally projecting C1 cells contain NPY where 96% of C1 cells projecting to hypothalamus are NPY positive (Stornetta et al., 1999)). Although C1 neurons have a variety of conduction velocities, from a faster conduction suggestive of lightly myelinated axons to a slower conduction velocity associated with unmyelinated axons, there is some correlation of the colocalized peptide in C1 neurons with their conduction velocity. The C1-NPY neurons have a slow conduction velocity (Stornetta et al., 1999) while enkephalin containing C1 neurons tend to have a faster conduction velocity than non-enkephalinergic C1 cells (Stornetta et al., 2001).
We know something of the effects of some of these colocalized neuropeptides on SPGNs. For instance, using the technique of direct iontophoresis or pressure ejection onto identified neurons, substance P increased the firing rate of SPGNs (Gilbey et al., 1983; Backman and Henry, 1984; Dun and Mo, 1988; Backman et al., 1990). PACAP excites SPGNs (Lai et al., 1997) by directly potentiating NMDA-receptor-mediated responses (Wu and Dun, 1997). Although enkephalin itself has not been directly tested, another opiate, morphine, inhibits the activity of SPGNs (Guyenet and Stornetta, 1982) consistent with the generally inhibitory effect of enkephalin elsewhere in the CNS.
CART is excitatory to SPGNs (if one assumes that intrathecal application and measurement of blood pressure and heart rate are indicative of a direct effect on SPGNs), since CART applied intrathecally caused sympathoactivation at nanomolar concentrations, while at lower concentrations, CART dramatically increased the cardiovascular effects of intrathecal glutamate (Scruggs et al., 2005; Dun et al., 2007). The effects of intrathecal injections of NPY are variable depending on concentration (nanomolar concentrations produce pressor responses (Hassessian et al., 1990; Wager-Page et al., 1993) while sub-nanomolar concentrations produce depressor responses (Westfall et al., 1988; Chen and Westfall, 1993). The effect of NPY on SPGNs has not been directly tested. Elsewhere in the CNS, NPY has an inhibitory effect that is presynaptically mediated (Vezzani et al., 1999).
Although we know what the effects of the isolated substances on SPGNs might be, the combined actions of the colocalized neuropeptides in the C1 cells have not yet been fully determined. One possible explanation for the colocalized “inhibitory” peptides (e.g. enkephalin), is that when the system is undergoing maximal stimulation (given that peptides are released with higher stimulation frequencies (Lundberg and Hokfelt, 1983; Pernow et al., 1989; Iverfeldt et al., 1989; Drake et al., 1994; Vilim et al., 2000)), an inhibitory peptide could serve as an autoregulatory inhibitory feedback mechanism to bring the system back towards normal firing. Another hypothesis on the role of the colocalized neuropeptides put forward by Fuxe and coworkers (Zoli et al., 1998) is that the peptides are involved in “volume” transmission while the ionotropic transmitters are part of “wiring” transmission, i.e., normal synaptic transmission. This theory posits that because the locations of high affinity peptide receptors are often mismatched with the location of the peptide-containing terminals that the peptides would signal by diffusion from the terminals to the more distant receptor sites. Another variant on this theory was recently described by Leng and Ludwig (2008) where peptides have a low probability of release but can be released in large amounts under certain conditions and thus “shout” rather than “whisper” like normal ionotropic transmission. Perhaps another explanation is that peptides could set a general membrane bias or tone where the synaptic transmission is accomplished by the ionotropic transmitter. However, these theories are still not tested in the C1 neuronal transmission to SPGNs and the exact role of the coexisting peptides in the C1 cells remains undecided at present.
3.3. Can the phenotype of particular combinations of colocalized substances in C1 neurons predict function?
So far, no phenotypic identification of the C1 cells including colocalized neuromodulators has yielded answers to other questions such as are particular subsets of C1 neurons specifically involved in the control of particular end organs. For example, which C1 cells are responsive to glucose levels (Ritter et al., 1998) and control sympathetic output to epinephrine-secreting adrenal chromaffin cells (Morrison and Cao, 2000) vs. which are the barosensitive C1 cells that regulate sympathetic outflow to blood vessels (Lipski et al., 1995; Schreihofer and Guyenet, 1997) and norepinephrine secreting adrenal chromaffin cells (Morrison and Cao, 2000)? The rostral/caudal location of a C1 neuron may be as good a predictor of its function as the specific colocalized peptide. A couple of examples: 1) Ritter (1998) found that the slightly more caudal C1 cells located closer to the A1 cells were likely to be glucosensitive, and 2) the more caudal C1 cells are likely to project to the hypothalamus (Verberne et al., 1999). McAllen has proposed the idea of the RVLM vasomotor area (termed the subretrofacial nucleus in the cat) being organized geographically with different subsets of vasomotor neurons driving, for example, skin vs. muscle sympathetic activity (McAllen, 1986; Dampney and McAllen, 1988; McAllen and Dampney, 1989; 1990; McAllen et al., 1995; 1997). These different geographic subsets of neurons have not been found in the rat, perhaps due to the relatively smaller area and denser packing of the neurons in the RVLM vasomotor area of the rat brain. One study (Polson et al., 1992) did attempt to phenotype the cells of the subretrofacial nucleus in the cat based on geographic region; however this study was anatomical and did not definitely characterize the physiological properties of any of the neurons.
3.4. Non-C1 RVLM presympathetic neurons
Although many of the bulbospinal neurons within the RVLM are C1 neurons, from 30-50% of the bulbospinal neurons in this area are non-C1 neurons (Ruggiero et al., 1994; Stornetta et al., 2002b). Guyenet and colleagues first brought attention to these non-C1 neurons in work on slices with bulbospinal cells labeled by retrograde tracers (Sun et al., 1988a; 1988b). The labeled cells were recorded in vitro and found to have intrinsic pacemaker properties that some believe to be part of the origin of sympathetic tone. None of these cells were immunoreactive for either PNMT or TH. These results may have been confounded by the recording technique where it is possible that the catecholamine enzymes were diluted by the contents of the intracellular recording pipette. However, the non-C1 presympathetic neurons were later confirmed by both intracellular and juxtacellular recording in vivo in adult animals (Lipski et al., 1995; Schreihofer and Guyenet, 1997), although they constitute a minority (∼25-30%) of the presympathetic neurons in the RVLM. This relative percentage of C1 vs. non-C1 presympathetic neurons is in agreement with Ruggiero and coworkers who found that 72% of neurons in the RVLM retrogradely labeled from spinal cord injections were PNMT-ir (Ruggiero et al., 1994), (assuming that all RVLM spinally projecting neurons are presympathetic). The presympathetic C1 neurons generally have slower conduction velocities indicative of unmyelinated axons in agreement with the report from the Reis group (Milner et al., 1988) that most PNMT-ir axons in the IML region are unmyelinated. However, in another Reis collaboration, Morrison et al. (1988) reported some lightly myelinated C1 axons and found a range of conduction velocities for C1 neurons from slow to fast, corroborated by Schreihofer and Guyenet (1997). While the non-C1 presympathetic neurons generally have faster conduction velocities (Schreihofer and Guyenet, 1997), whether or not a cell is catecholaminergic is not a prefect predictor of its conduction velocity.
3.5. Neuropeptides in non-C1 RVLM presympathetic neurons
Most of the peptides mentioned above that are present in C1 presympathetic neurons have also been found in non-C1 presympathetic neurons in the same general area of the RVLM. These include enkephalin (Stornetta et al., 2001), substance P (Li et al., 2005), NPY (Stornetta et al., 1990; Stornetta et al., 1999), CART (Burman et al., 2004) and PACAP (Farnham et al., 2008). While all these substances are found throughout the brain, substance P, NPY and PACAP in the medulla show a more restricted distribution, similar to that of the catecholamines, as far as the appearance in the area of the NTS, the lateral tegmental field and the RVLM. NPY also appears in the spinal trigeminal nucleus and substance P is also present in serotonergic neurons in the midline and RVMM. Some PACAP neurons are also found in the area of the midline raphe. Enkephalin has a much more widespread distribution throughout the medulla.
Burman et al. (2004) suggested that all presympathetic barosensitive neurons contain CART, whether or not they are immunoreactive (ir) for TH. This is based on c-Fos responses to nitroprusside injections (theoretically causing activation of presympathetic neurons by baroreceptor unloading but also potentially activating the neurons downstream of the barosensitive neurons) as well as direct recording and juxtacellular labeling of barosensitive neurons antidromically activated from spinal cord. While almost all c-Fos-ir neurons contained CART, CART was found in some neurons that did not express c-Fos so one must withhold some enthusiasm on the conclusion that CART expression marks a neuron as a barosensitive presympathetic neuron. It is possible that CART might mark presympathetic neurons with other sympathetic functions not related to barosensitive cardiovascular outflows. This has not yet been directly tested.
3.6. RVLM glutamatergic presympathetic neurons
Glutamate is most likely the agent of fast excitatory drive to sympathetic outflow controlling blood pressure. The origin of a glutamatergic presympathetic drive comes from the same area of the RVLM where the C1 cells are located (Dampney et al., 1982; Morrison et al, 1988; Reis et al, 1989; Morrison and Reis, 1991; Cechetto and Chen, 1992). Basil and Gordon (1993) noted that NMDA agonists were responsible for exciting SPGNs. Deuchars et al. (1995) demonstrated excitatory postsynaptic potentials (EPSPs) in SPGNs elicited by electrical stimulation of the RVLM and also showed evidence that this pathway was at least partly monosynaptic and glutamatergic, although the conclusions are limited by the fact that fibers of passage could also have been stimulated.
Morrison (2003) provided a brief review of this topic and noted that release of both glutamate agonists as well as electrical stimulation of the RVLM caused excitation of SPGNs and this excitation could be blocked with glutamate antagonists. Aicher et al (2000) also found NMDA receptors on neurons (likely SPGNs) postsynaptic to adrenergic terminals in the intermediolateral cell column (IML) in thoracic spinal cord. The finding that vesicular glutamate transporter type 2 (VGLUT2) was present in both C1 and non-C1 bulbospinal barosensitive neurons (Stornetta et al., 2002a; 2002b) suggested that the C1 neurons could be partly responsible for the glutamatergic presympathetic drive. Further support for this concept derives from the report of Nakamura et al. (2004b), who found VGLUT2 in asymmetric synapses onto choline acetyltransferase-ir neurons (SPGNs) in intermediolateral cell column of thoracic spinal cord. The VGLUT2 was present in DBH-ir (adrenergic or noradrenergic) terminals.
3.7. C1 and non-C1 neurons and cardiovascular control via catecholamines vs. glutamate
Whether or not the C1 cells participate in the control of blood pressure is a controversial topic which still has not been definitively proven. Lipski and coworkers (1995) found both adrenergic (TH-ir) and nonadrenergic bulbospinal barosensitive neurons. Guyenet and collaborators (1997) verified and extended this finding of both C1 and non-C1 presympathetic blood pressure sensitive neurons in the C1 area by recording barosensitive neurons antidromically activated from the spinal cord that were PNMT-ir. They also found non-PNMT-ir neurons that were presympathetic and barosensitive. Lesions of over 80% of the bulbospinal C1 cells result in only modest decreases in blood pressure (Madden et al., 1999; Madden and Sved, 2003), although some sympathetic reflexes are greatly attenuated (Schreihofer and Guyenet, 2000). Sved has argued that the C1 cells might not release epinephrine onto SPGNs (1989) (based on lack of HPLC detection of epinephrine in the spinal cord) and epinephrine as the “excitatory” transmitter of the presympathetic cells is controversial. Although catecholamines can produce an excitatory effect on SPGNs via alpha-1 receptors (Yoshimura et al., 1987a; 1987b; 1989; Inokuchi et al., 1992; Malhotra et al., 1993; Huangfu et al., 1994), catecholamines can also inhibit SPGNs via alpha-2 receptors (Franz et al., 1982; Guyenet and Stornetta, 1982; Inokuchi et al., 1992; Stornetta et al., 1995). Thus the catecholamines released by the C1 neurons, although not considered as “fast transmitters” and perhaps not responsible for the fast activation elicited by electrical stimulation from RVLM to SPGNs (Morrison & Reis, 1991), nonetheless affect the state of membrane polarization (the “gain”) and have a major influence on the degree of excitability of cardiovascular-related SPGNs and thus a major influence on blood pressure.
4. Dorsal Medullary Presympathetic Neurons: the C2 (?) and C3 cells
While much attention has been paid to the C1 adrenergic neurons of the RVLM as major regulators of sympathetic control of cardiovascular function, there are other catecholaminergic cell groups that project to the IML and may be presympathetic neurons. These presumed catecholaminergic presympathetic neurons include the C2 and C3 nuclei. Both C2 and C3 cell groups have been reported to project to the spinal cord (Sawchenko and Bohn, 1989; Minson et al., 1990; Farnham et al., 2008). However, whether the C2 neurons are indeed projecting to SPGNs is not certain. While Loewy and coworkers (Strack et al., 1989a; Loewy et al., 1994; Jansen et al., 1995b) report consistent findings on C3 neurons as presympathetic neuronal candidates, these same experiments show negligible C2 labeling from PRV injections into sympathetic targets. These findings call into question whether the C2 neurons are indeed presympathetic. However there is a report that C2 neurons could be presynaptic to parasympathetic pancreatic vagal motor neurons (Loewy et al., 1994). Certainly the C2 neurons could have an autonomic function due to their putative visceral inputs (Appleyard et al., 2007) (although these authors could not discriminate C2 from A2), inputs from the area postrema (Cunningham et al., 1994), activation by hemorrhage (Chan and Sawchenko, 1995; Buller et al., 1996; Dayas et al., 2001) as well as their projections to autonomic areas in the midbrain (Phillipson and Bohn, 1994) and hypothalamus (Cunningham et al., 1990). The C2 neurons could be in a position to coordinate sympathetic and parasympathetic systems. However, reports on the exact physiological role of the C2 neurons are still lacking. The C2 neurons are quite distinct from the C3 neurons. They differ in their morphology and in their projection patterns. Restricting reports to those that have confirmed co-localization of substances in spinally-projecting neurons, most of the dorsal medullary adrenergic spinally projecting neurons found to contain neuropeptides were the C3 neurons. NPY was found either rarely or not at all in the C2 neurons but abundantly in the C3 spinally projecting neurons (Jansen et al., 1995b; Stornetta et al., 1999). Enkephalin was absent in both C2 and C3 neurons (Stornetta et al., 2001). In agreement with the lack of enkephalin in C2 or C3 neurons, Loewy and coworkers found no (in C2) or extremely limited (in C3) immunoreactivity for the enkephalin-like peptide, MERGL, in neurons labeled with PRV trans-synaptically transported from sympathetic targets (Strack et al., 1989b; Jansen et al., 1995b). Other than the lack of colocalized enkephalin, the C3 neurons seem very similar to the C1 neurons in terms of phenotype and projection characteristics and may be similar in terms of physiology. However, due to the sparsely scattered distribution of these neurons within the reticulum of the brainstem, experiments to determine their function as presympathetic neurons are difficult.
5. RVMM Presympathetic Neurons
5.1. Serotonergic presympathetic neurons
Carlsson et al. (1963) noted that levels of serotonin in rabbit spinal cord decreased dramatically in spinal segments caudal to a spinal transection, although this study could not identify the exact location of the descending source of serotonin. Bjorklund and Skagerberg (1979), using the fluorescent method for detection of indoleamines, determined that many of these spinally projecting serotonin neurons were located in the medulla oblongata by double labeling with a fluorescent retrograde marker. The existence of bulbospinal serotonergic neurons in the raphe pallidus (B1), parapyramidal area, obscurus (B2) and parts of magnus (B3) has been confirmed by many others (Bowker et al., 1981a; 1981b; Minson et al., 1984; Millhorn et al., 1987; 1989; Bowker and Abbott, 1990; Kwiat and Basbaum, 1990; Sasek et al., 1990; Jones et al., 1991; Johnson et al., 1993). However, the serotonin bulbospinal system is a good example of why the presence of a spinal projection does not necessarily identify a neuron as presympathetic. There is a plentiful serotonin innervation of the dorsal horn (Kwiat and Basbaum, 1992; Allen and Cechetto, 1994; Hökfelt et al., 2000; Geranton et al., 2008) involved in pain modulation (Mason, 2001). There is also a serotonergic innervation of motor neurons in the ventral horn (Zhan et al., 1989; Allen and Cechetto, 1994; Yates et al., 1999). Interestingly, the largest responses of serotonergic neurons in freely moving animals occur in relation to motor activity (Jacobs et al., 2002).
Bacon et al. (1990) used anterograde tracing and electron microscopy to show direct monosynaptic projections from RVMM to SPGNs. That some serotonin neurons in the RVMM (which includes the B1 and B3 serotonin groups) are indeed presympathetic was demonstrated by Loewy and coworkers with the PRV (Jansen et al., 1995b; Smith et al., 1998) and confirmed by others using similar methods (Stornetta et al., 2004; Nakamura et al., 2004a). Using the technique of anterograde tracing of serotonergic terminals on SPGNs from labeling in raphe also confirms the presence of serotonergic presympathetic neurons (Nakamura et al., 2004a). There is some evidence from double retrograde viral tracing studies that the same serotonin neurons that are presympathetic are also innervating motor neurons (Kerman et al., 2003; 2008) and may be involved in coordinating somatic and sympathetic efferents, for instance in the anticipation of exercise.
There is considerable evidence that at least some of the serotonergic presympathetic neurons have a role in thermoregulation (Morrison, 2004; Hodges et al., 2008) and some of these presympathetic thermoregulatory serotonin neurons also have the capacity for glutamate release (they contain VGLUT3 as discussed below) (Nakamura et al., 2004a). Much of this evidence derives from studies where PRV was injected in the major effectors of heat generation or loss in rats, i.e., brown adipose tissue or tail artery (Smith et al., 1998; Cano et al., 2003; Yoshida et al., 2003; Nakamura et al., 2004a). Serotonergic neurons in the RVMM were consistently labeled at short time courses suggesting that these neurons were synaptically connected to the SPGNs involved in regulating these temperature controlling outputs.
There are also suggestions that the serotonergic presympathetic neurons in the RVMM may have an excitatory effect on cardiovascular sympathetic outflow (Ma and Dun, 1986; Lewis et al., 1993; Pickering et al., 1994; Ramage, 2001). Serotonin iontophoresed onto SPGNs in thoracic cord of cats (Coote et al., 1981; McCall, 1983) excites these neurons. However, serotonin seems to excite all SPGNs regardless of function (e.g., those innervating adrenal gland vs. those innervating other sympathetic ganglia (Backman et al., 1990)), so the serotonergic presympathetic neurons are not necessarily limited to those with vasomotor function. Serotonin could have a depolarizing effect on the post synaptic membrane via its action on 5HT2(2A) receptors (Ramage, 2001). Serotonin could also be a “neruomodulator” in changing the level of membrane polarization and thus setting the gain of the SPGN excitability much like the catecholamines (McCall, 1988).
Another explanation for sympathoactivation from stimulation in the raphe could be via glutamate. Madden and Morrison (2006; 2008) recently reported that spinal cord microinjections of serotonin can potentiate NMDA stimulated brown adipose tissue (BAT) sympathetic nerve activity. Whether glutamate could be coreleased from the serotonin terminals is controversial. The vesicular glutamate transporter, VGLUT3 is present both in bulbospinal serotonin neurons and in terminals making presumed excitatory contacts onto SPGNs (Nakamura et al., 2004a; Stornetta et al., 2005). However, VGLUT3 is also expressed by GABAergic neurons (Nakamura et al., 2004a; Stornetta et al., 2005) and, as noted above, serotonin is present in some GABA neurons. VGLUT3 is also found in bulbospinal neurons without serotonin or GABA (Nakamura et al., 2004a; Stornetta et al., 2005). Dissecting out the role of these different serotonergic neurons in sympathetic control is still a hot topic.
5.2. Presence of other neuroactive substances in RVMM serotonergic neurons
Bulbospinal serotonergic neurons and their terminals apposing SPGNs contain a variety of neuropeptides (for review see Hokfelt et al., 2000) including thyrotropin-releasing hormone (TRH) and substance P (Johansson et al., 1981; Helke et al., 1982; Appel et al., 1987; Nicholas et al., 1992; Johnson et al., 1993; Pilowsky et al., 1995; Hokfelt et al., 2000), somatostatin and enkephalin (Holets and Elde, 1983; Krukoff et al., 1985; Maxwell et al., 1996). Loewy has reported all of these neuropeptides in medullary presympathetic neurons using the PRV method (Jansen et al., 1995b). Both TRH and substance P increase the firing rate of SPGNs when applied by iontophoresis or direct application in slices (Gilbey et al., 1983; Backman and Henry, 1984; Dun and Mo, 1988) while somatostatin and enkephalin have inhibitory effects when tested on neurons in, for example, locus coeruleus (Inoue et al., 1988; Travagli et al., 1995) (although no one has specifically tested these peptides directly on SPGNs). Some of these cotransmitters may act synergistically with serotonin. Franck et al. (1989) reported that substance P could increase the evoked release of serotonin in the spinal cord. This would result in a feed forward effect; when conditions prevail for release of substance P (high firing as mentioned previously), even more serotonin would be released. The inhibitory neuropeptides could act as a brake on this system. Serotonin itself might change downstream excitability by acting on presynaptic 5HT1B “autoreceptors” to decrease release of serotonin (note that this 5HT receptor subtype has been found in spinal cord but not necessarily localized to SPGNs (Matsumoto et al., 1992)).
There is obviously much complexity inherent in the serotonin system for controlling sympathetic outflow, including whether serotonin is acting pre- or post-synaptically at SPGNs, which of the many serotonin receptors (Hannon and Hoyer, 2008) are activated and how the colocalized neuroeffectors might be interacting to synergize or negate the effects of serotonin. Research on serotonin and its cotransmitters at the level of the SPGNs is needed to determine these effects with greater precision.
5.3. RVMM inhibitory presympathetic neurons
Coote and Macleod (1974) had suggested that some bulbospinal neurons in the RVMM exerted an inhibitory influence on SPGNs. Electrical stimulation within the RVLM/RVMM produces inhibitory postsynaptic potentials (IPSPs) in SPGNs (Deuchars et al., 1997), although electrical stimulation also excites fibers of passage as noted earlier, thus limiting the interpretation of the exact location of the IPSP-inducing neurons. Up to 50% of the contacts on SPGNs are inhibitory (Llewellyn-Smith et al., 1995; 1998), although some of these contacts come from spinal interneurons (Dun et al., 1992; Deuchars et al., 2005). Bulbospinal GABAergic neurons located in the RVLM/RVMM have been described by many different laboratories (Millhorn et al., 1987; Jones et al., 1991; Miura et al., 1994; Matsumoto et al., 1994). In his work with the PRV, Loewy described a large group of neurons in the RVMM that are presympathetic (Strack et al., 1989a). Some of these neurons are GABAergic and/or glycinergic. This was first illustrated by identifying GAD-67 mRNA in neurons retrogradely labeled from spinal cord fluorescent tracer injections (Stornetta and Guyenet, 1999). While a bulbospinal projection is necessary to identify a presympathetic neuron, it is not sufficient, and thus the subsequent use of PRV to identify presympathetic neurons was combined with GAD-67 and glycine transporter-2 (GLYT2) mRNAs in the virally infected neurons in the RVMM to identify these bulbospinal presumed inhibitory neurons as presympathetic (Stornetta et al., 2004). Some of the GABAergic bulbospinal neurons also could be glycinergic (i.e. express GLYT2: Stornetta et al., 2004), serotoninergic (i.e. express tryptophan hydroxylase-ir: Millhorn et al., 1987; Stornetta and Guyenet, 1999; Stornetta et al., 2004; 2005) as well as glutamatergic (i.e. express VGLUT3: Stornetta et al., 2005). It is possible that the sympathetic depressor area in the gigantocellular region of the RVLM described by Aicher (Aicher et al., 1994; 1995; 1997) might have its effects via these bulbospinal GABAergic/glycinergic neurons, although the proof for this is still lacking. There are a great number of presympathetic neurons in the RVMM whose function is still unknown and, according to the PRV results from Loewy and colleagues, this area remains one of the largest unexplored potential inputs to the SPGNs.
6. Summary
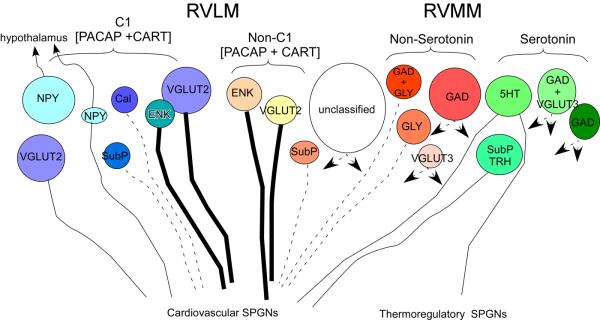
Many of the presympathetic neurons in the brainstem are monoaminergic, either adrenergic or serotonergic. Both of these neuronal groups have an extensive menu of colocalized peptides. This suggests a complex system for fine tuning sympathetic output based on which substances are released that could depend on the firing frequency or pattern of firing. There seems to be at least some specificity of anatomical location and phenotype for particular sympathetic outflows; midline serotonergic neurons are involved in thermoregulatory outflow to brown adipose tissue and tail artery where RVLM adrenergic cells are innervating SPGNs controlling cardiovascular sympathetic outflows, however, keep in mind that at least some serotonergic neurons also innervate SPGNs involved in cardiovascular function if the PRV data are considered. It is still unknown whether the same or different medullary neurons innervate these different pools of SPGNs. Figure 1 offers an overview of the data reviewed here.
Figure 1.
Summary of data on neuronal phenotype, projections and axonal conduction velocity for presympathetic neurons. Thick lines represent fast conduction velocity (lightly myelinated axons), thin lines are slower conducting fibers (unmyelinated axons), dashed lines have unknown conduction velocity. Double pointing arrows represent neuronal populations with both cardiovascular and thermoregulatory SPGN targets. The larger circles represent the higher relative contribution to the spinal projection.
Abbreviations:
5HT, 5-hydoxytryptamine (serotonin); Cal, Calbindin; CART, cocaine- and amphetamine-regulated transcript; ENK, enkephalin; GAD, glutamic acid decarboxylase; GLY, Glycine; NPY, neuropeptide Y; PACAP, pituitary adenylate cyclase-activating polypeptide; RVLM, rostral ventrolateral medulla; RVMM, rostral ventromedial medulla; SPGNs, sympathetic preganglionic neurons; SubP, substance P; TRH, thyrotropin releasing hormone; VGLUT, vesicular glutamate transporter.
One drawback to functional phenotyping based solely on a cell's content is that a cell's function will depend on projection patterns (both input and output), although some projection patterns might be predicted by combinations of colocalized neuropeptides (e.g., C1 neurons expressing NPY tend to project to the hypothalamus rather than the spinal cord). However we are still a long way from being able to predict exact function based on the phenotype of a neuron.
Acknowledgments
This work was supported by grants from the National Institutes of Health: HL74011 and HL28785.
Abbreviations
- 5HT
5-hydoxytryptamine (serotonin)
- Cal
Calbindin
- CART
cocaine- and amphetamine-regulated transcript
- DBH
dopamine-beta-hydroxylase
- ENK
enkephalin
- EPSP
excitatory postsynaptic potential
- GAD
glutamic acid decarboxylase
- GLY
Glycine
- ir
immunoreactive
- IML
intermediolateral cell column
- IPSP
inhibitory postsynaptic potential
- NPY
neuropeptide Y
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PNMT
phenylethanolamine-N-methyltransferase
- PRV
pseudorabies virus
- RVLM
rostral ventrolateral medulla
- RVMM
rostral ventromedial medulla
- SPGNs
sympathetic preganglionic neurons
- SubP
substance P
- TH
tyrosine hydroxylase
- TRH
thyrotropin releasing hormone
- VGLUT
vesicular glutamate transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aicher SA, Reis DJ. Gigantocellular vasodepressor area is tonically active and distinct from caudal ventrolateral vasodepressor area. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;272:R731–R742. doi: 10.1152/ajpregu.1997.272.3.R731. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Reis DJ, Nicolae R, Milner TA. Monosynaptic projections from the medullary gigantocellular reticular formation to sympathetic preganglionic neurons in the thoracic spinal cord. J. Comp. Neurol. 1995;363:563–580. doi: 10.1002/cne.903630405. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Reis DJ, Ruggiero DA, Milner TA. Anatomical characterization of a novel reticulospinal vasodepresoor area in the rat medulla oblongata. Neurosci. 1994;60:761–779. doi: 10.1016/0306-4522(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J. Comp. Neurol. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- Anderson CR, McLachlan EM, Srb-Christie O. Distribution of sympathetic preganglionic neurons and monoaminergic nerve terminals in the spinal cord of the rat. J. Comp. Neurol. 1989;283:269–284. doi: 10.1002/cne.902830208. [DOI] [PubMed] [Google Scholar]
- Appel NM, Wessendorf MW, Elde RP. Thyrotropin-releasing hormone in spinal cord: coexistence with serotonin and with substance P in fibers and terminals apposing identified preganglionic sympathetic neurons. Brain Res. 1987;415(1):137–143. doi: 10.1016/0006-8993(87)90276-9. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral Afferents Directly Activate Catecholamine Neurons in the Solitary Tract Nucleus. J. Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman SB, Henry JL. Effect of substance P and thyrotropin-releasing hormone on sympathetic preganglionic neurones in the upper thoracic intermediolateral nucleus of the cat. Can. J Physiol Pharmacol. 1984;62:248–251. doi: 10.1139/y84-038. [DOI] [PubMed] [Google Scholar]
- Backman SB, Sequeira-Martinho H, Henry JL. Adrenal versus nonadrenal sympathetic preganglionic neurones in the lower thoracic intermediolateral nucleus of the cat: effects of serotonin, substance P, and thyrotropin-releasing hormone. Canadian J. Physiol. Pharmacol. 1990;68:1108–1118. doi: 10.1139/y90-166. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Zagon A, Smith AD. Electron microscopic evidence of a monosynaptic pathway between cells in the caudal raphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Exp. Brain Res. 1990;79:589–602. doi: 10.1007/BF00229327. [DOI] [PubMed] [Google Scholar]
- Bernstein-Goral H, Bohn MC. Phenylethanolamine N-methyltransferase-immunoreactive terminals synapse on adrenal preganglionic neurons in the rat spinal cord. Neurosci. 1989;32(2):521–538. doi: 10.1016/0306-4522(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Skagerberg G. Simultaneous use of retrograde fluorescent tracers and fluorescence histochemistry for convenient and precise mapping of monoaminergic projections and collateral arrangements in the CNS. J. Neurosci. Methods. 1979;1:261–277. doi: 10.1016/0165-0270(79)90038-4. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Oliver JR, Hodgson AH, Joh TH, Willoughby JO. Neuropeptide Y-like immunoreactive C1 neurons in the rostral ventrolateral medulla of the rabbit project to sympathetic preganglionic neurons in the spinal cord. J. Auton. Nerv. Syst. 1987;18:121–129. doi: 10.1016/0165-1838(87)90099-3. [DOI] [PubMed] [Google Scholar]
- Bolme P, Corrodi H, Fuxe K, Hokfelt T, Lidbrink P, Goldstein M. Possible involvement of central adrenaline neurons in vasomotor and respiratory control. Studies with clonidine and its interactions with piperoxane and yohimbine. Eur. J. Pharmacol. 1974;28:89–94. doi: 10.1016/0014-2999(74)90116-2. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Abbott LC. Quantitative re-evaluation of descending serotonergic and non- serotonergic projections from the medulla of the rodent: evidence for extensive co-existence of serotonin and peptides in the same spinally projecting neurons, but not from the nucleus raphe magnus. Brain Res. 1990;512(1):15–25. doi: 10.1016/0006-8993(90)91164-c. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Steinbusch HWM, Coulter JD. Serotonergic and peptidergic projections to the spinal cord demonstrated by a combined retrograde HRP histochemical and immunocytochemical staining method. Brain Res. 1981a;211:412–417. doi: 10.1016/0006-8993(81)90965-3. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: an immunohistochemical-retrograde transport study. Brain Res. 1981b;226:187–200. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Buller KM, Khanna S, Sibbald JR, Day TA. Central noradrenergic neurons signal via ATP to elicit vasopressin responses to haemorrhage. Neurosci. 1996;73:637–642. doi: 10.1016/0306-4522(96)00156-x. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Sartor DM, Verberne AJ, Llewellyn-Smith IJ. Cocaine- and amphetamine-regulated transcript in catecholamine and noncatecholamine presympathetic vasomotor neurons of rat rostral ventrolateral medulla. J. Comp. Neurol. 2004;476:19–31. doi: 10.1002/cne.20198. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, Enquist LW. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J. Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Fuxe K, HILLARP NA. Cellular localization of monoamines in the spinal cord. Acta Physiol. Scand. 1964;60:112–119. doi: 10.1111/j.1748-1716.1964.tb02874.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Magnusson T, ROSENGREN E. 5-hydroxytryptamine of the spinal cord normally and after transection. Experientia. 1963;19:354–359. doi: 10.1007/BF02152316. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Hemodynamic regulation of tyrosine hydroxylase messenger RNA in medullary catecholamine neurons: A c-fos-guided hybridization histochemical study. Neurosci. 1995;66:377–390. doi: 10.1016/0306-4522(94)00600-a. [DOI] [PubMed] [Google Scholar]
- Chen XL, Westfall TC. Depressor effect of intrathecal neuropeptide-Y (NPY) is mediated by Y(2)-subtype of NPY receptors. J. Cardiovasc. Pharmacol. 1993;21:720–724. doi: 10.1097/00005344-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Coote JH, Macleod VH. The influence of bulbospinal monoaminergic pathways on sympathetic nerve activity. J. Physiol. 1974;241:453–475. doi: 10.1113/jphysiol.1974.sp010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Macleod VH, Fleetwood-Walker S, Gilbey MP. The response of individual sympathetic preganglionic neurones to microelectrophoretically applied endogenous monoamines. Brain Res. 1981;215:135–145. doi: 10.1016/0006-8993(81)90497-2. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Comp. Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: An axonal transport and immunohistochemical study in the rat. Neurosci. 1994;58:635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. 11 experimentally induced changes in the intraneuronal amine levels of the bulbo spinal neuron systems. Acta Physiol. Scand. 1965;64:1–36. [PubMed] [Google Scholar]
- Dampney RA, McAllen RM. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J. Physiol. 1988;395:41–56. doi: 10.1113/jphysiol.1988.sp016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu J, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur. J. Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J. Neurosci. 2005;25:1063–1070. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Spyer KM, Gilbey MP. Stimulation within the rostral ventrolateral medulla can evoke monosynaptic GABAergic IPSPs in sympathetic preganglionic neurons in vitro. J. Neurophys. 1997;77:229–235. doi: 10.1152/jn.1997.77.1.229. [DOI] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J. Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Mo N. In vitro effects of substance P on neonatal rat sympathetic preganglionic neurones. J. Physiol. 1988;399:321–333. doi: 10.1113/jphysiol.1988.sp017083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Wu SY, Shen E, Miyazaki T, Dun SL, Ren C. Synaptic mechanisms in sympathetic preganglionic neurons. Can. J. Physiol. Pharmacol. 1992;70(Suppl):S86–S91. doi: 10.1139/y92-248. [DOI] [PubMed] [Google Scholar]
- Dun SL, Ng YK, Brailoiu GC, Ling EA, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide-immunoreactivity in adrenergic C1 neurons projecting to the intermediolateral cell column of the rat. J. Chem. Neuroanat. 2002;23:123–132. doi: 10.1016/s0891-0618(01)00147-8. [DOI] [PubMed] [Google Scholar]
- Dun SL, Brailoiu E, Hsieh WK, Lai CC, Yang J, Chang JK, Dun NJ. Expression and activity of cocaine- and amphetamine-regulated transcript peptide1-39 in the rat. Regul. Pept. 2007;140:47–54. doi: 10.1016/j.regpep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Farnham MM, Li Q, Goodchild AK, Pilowsky PM. PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1304–R1311. doi: 10.1152/ajpregu.00753.2007. [DOI] [PubMed] [Google Scholar]
- Franck J, Fried G, Brodin E. Substance P enhances the release of endogenous serotonin from rat ventral spinal cord. Eur. J Pharmacol. 1989;174:85–90. doi: 10.1016/0014-2999(89)90877-7. [DOI] [PubMed] [Google Scholar]
- Franz DN, Hare DB, McCloskey KL. Spinal sympathetic neurons: possible sites of opiate-withdrawal suppression by clonidine. Science. 1982;215:1643–1645. doi: 10.1126/science.6280276. [DOI] [PubMed] [Google Scholar]
- Geranton SM, Fratto V, Tochiki KK, Hunt SP. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol. Pain. 2008;4:35. doi: 10.1186/1744-8069-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey MP, McKenna KE, Schramm LP. Effects of substance P on sympathetic preganglionic neurones. Neurosci. Lett. 1983;41:157–159. doi: 10.1016/0304-3940(83)90239-2. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Llewellyn-Smith IJ, Sun QJ, Chalmers J, Cunningham AM, Pilowsky PM. Calbindin-immunoreactive neurons in the reticular formation of the rat brainstem: Catecholamine content and spinal projections. J. Comp. Neurol. 2000;424:547–562. doi: 10.1002/1096-9861(20000828)424:3<547::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL. Inhibition of sympathetic preganglionic discharges by epinephrine and and α-methylepinephrine. Brain Res. 1982;235:271–283. doi: 10.1016/0006-8993(82)91007-1. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hassessian H, Couture R, de CJ. Sympathoadrenal mechanisms underlying cardiovascular responses to intrathecal substance P in conscious rats. J Cardiovasc. Pharmacol. 1990;15:736–744. doi: 10.1097/00005344-199005000-00008. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Neil JJ, Massari VJ, Loewy AD. Substance P neurons project from the ventral medulla to the intermediolateral cell column and ventral horn in the rat. Brain Res. 1982;243:147–152. doi: 10.1016/0006-8993(82)91128-3. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, Seroogy K, Ulfhake B. Multiple messengers in descending serotonin neurons: localization and functional implications. J. Chem. Neuroanat. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Fuxe K, Goldstein M, Johansson O. Immunohistochemical evidence for the existence of adrenaline neurons in the rat brain. Brain Res. 1974;66:235–251. [Google Scholar]
- Holets V, Elde R. Sympathoadrenal preganglionic neurons: their distribution and relationship to chemically-coded fibers in the kitten intermediolateral cell column. J. Auton. Nerv. Syst. 1983;7:149–163. doi: 10.1016/0165-1838(83)90043-7. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Hwang LJ, Riley TA, Guyenet PG. Role of serotonin and catecholamines in sympathetic responses evoked by stimulation of rostral medulla. Am J Physiol Regul Integr Comp Physiol. 1994;266:R338–R352. doi: 10.1152/ajpregu.1994.266.2.R338. [DOI] [PubMed] [Google Scholar]
- Inokuchi H, Yoshimura M, Polosa C, Nishi S. Adrenergic receptors (alpha 1 and alpha 2) modulate different potassium conductances in sympathetic preganglionic neurons. Can. J. Physiol. Pharmacol. 1992;70(Suppl):S92–S97. doi: 10.1139/y92-249. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakajima S, Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J. Physiol. 1988;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverfeldt K, Serfozo P, Diaz AL, Bartfai T. Differential release of coexisting neurotransmitters: frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H]serotonin from tissue slices of rat ventral spinal cord. Acta Physiol. Scand. 1989;137:63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jansen ASP, Loewy AD. Neurons lying in the white matter of the upper cervical spinal cord project to the intermediolateral cell column. Neurosci. 1997;77:889–898. doi: 10.1016/s0306-4522(97)86660-2. [DOI] [PubMed] [Google Scholar]
- Jansen ASP, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system:basis of the fight-or flight response. Science. 1995a;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res. 1995b;683:1–24. doi: 10.1016/0006-8993(95)00276-v. [DOI] [PubMed] [Google Scholar]
- Johansson O, Hokfelt T, Pernow B, Jeffcoate SL, White N, Steinbusch HW, Verhofstad AA, Emson PC, Spindel E. Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P- and thyrotropin releasing hormone-like immunoreactivity in medullary neurons projecting to the spinal cord. Neurosci. 1981;6:1857–1881. doi: 10.1016/0306-4522(81)90028-2. [DOI] [PubMed] [Google Scholar]
- Johnson H, Ulfhake B, Dagerlind A, Bennett GW, Fone KC, Hokfelt T. The serotoninergic bulbospinal system and brainstem-spinal cord content of serotonin-, TRH-, and substance P-like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse. 1993;15:63–89. doi: 10.1002/syn.890150108. [DOI] [PubMed] [Google Scholar]
- Jones BE, Holmes CJ, Rodriguez-Veiga E, Mainville L. GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat. J. Comp. Neurol. 1991;313:349–367. doi: 10.1002/cne.903130210. [DOI] [PubMed] [Google Scholar]
- Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp. Brain Res. 2008;187:1–16. doi: 10.1007/s00221-008-1337-5. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J. Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukoff TL, Ciriello J, Calaresu FR. Segmental distribution of peptide- and 5HT-like immunoreactivity in nerve terminals and fibers of the thoracolumbar sympathetic nuclei of the cat. J. Comp. Neurol. 1985;240:103–116. doi: 10.1002/cne.902400108. [DOI] [PubMed] [Google Scholar]
- Kwiat GC, Basbaum AI. Organization of tyrosine hydroxylase-immunoreactive and serotonin-immunoreactive brainstem neurons with axon collaterals to the periaqueductal gray and the spinal cord in the rat. Brain Res. 1990;528:83–94. doi: 10.1016/0006-8993(90)90198-k. [DOI] [PubMed] [Google Scholar]
- Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens. Motor Res. 1992;9:157–173. doi: 10.3109/08990229209144768. [DOI] [PubMed] [Google Scholar]
- Lai CC, Wu SY, Lin HH, Dun NJ. Excitatory action of pituitary adenylate cyclase activating polypeptide on rat sympathetic preganglionic neurons in vivo and in vitro. Brain Res. 1997;748:189–194. doi: 10.1016/s0006-8993(96)01297-8. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J. Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Sermasi E, Coote JH. Excitatory and indirect inhibitory actions of 5-hydroxytryptamine on sympathetic preganglionic neurons in the neonate rat spinal cord invitro. Brain Res. 1993;610:267–275. doi: 10.1016/0006-8993(93)91410-t. [DOI] [PubMed] [Google Scholar]
- Li Q, Goodchild AK, Seyedabadi M, Pilowsky PM. Pre-protachykinin A mRNA is colocalized with tyrosine hydroxylase-immunoreactivity in bulbospinal neurons. Neurosci. 2005;136:205–216. doi: 10.1016/j.neuroscience.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: Morphological properties and relationship to C1 adrenergic neurons. Neurosci. 1995;69:601–618. doi: 10.1016/0306-4522(95)92652-z. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Arnolda LF, Pilowsky PM, Chalmers JP, Minson JB. GABA- and glutamate-immunoreactive synapses on sympathetic preganglionic neurons projecting to the superior cervical ganglion. J. Auton. Nerv. Syst. 1998;71:96–110. doi: 10.1016/s0165-1838(98)00069-1. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Minson JB, Pilowsky PM, Arnolda LF, Chalmers JP. The one hundred percent hypothesis: Glutamate or GABA in synapses on sympathetic preganglionic neurons. Clin. Exp. Hypertens. 1995;17:323–333. doi: 10.3109/10641969509087074. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Franklin MF, Haxhiu MA. CNS monoamine cell groups projecting to pancreatic vagal motor neurons: a transneuronal labeling study using pseudorabies virus. Brain Res. 1994;638:248–260. doi: 10.1016/0006-8993(94)90657-2. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T. Coexistence of peptides and classical neurotransmitters. TINS. 1983;6:325–333. [Google Scholar]
- Ma RC, Dun NJ. Excitation of lateral horn neurons of the neonatal rat spinal cord by 5-hydroxytryptamine. Dev. Brain Res. 1986;24:89–98. doi: 10.1016/0165-3806(86)90176-8. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DbetaH-saporin. Am. J. Physiol. 1999;277:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J. Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)(1A)/5-HT(7) receptors in the rat spinal cord. Neuropharmacol. 2008;54:487–496. doi: 10.1016/j.neuropharm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am. J. Physiol. 2003;285:H2734–H2748. doi: 10.1152/ajpheart.00155.2003. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Kachroo A, Sapru HN. Role of alpha1-adrenergic receptors in the intermediolateral column in mediating the pressor responses elicited by the stimulation of ventrolateral medullary pressor area. Brain Res. 1993;626:278–286. doi: 10.1016/0006-8993(93)90588-e. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu. Rev. Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Combs MR, Jones DJ. Characterization of 5-hydroxytryptamine1B receptors in rat spinal cord via [125I]iodocyanopindolol binding and inhibition of [3H]-5-hydroxytryptamine release. J Pharmacol. Exp Ther. 1992;260:614–626. [PubMed] [Google Scholar]
- Matsumoto M, Takayama K, Miura M. Distribution of glutamate- and GABAimmunoreactive neurons projecting to the vasomotor center of the intermediolateral nucleus of the lower thoracic cord of Wistar rats: a double-labeling study. Neurosci. Lett. 1994;174:165–168. doi: 10.1016/0304-3940(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Maxwell L, Maxwell DJ, Neilson M, Kerr R. A confocal microscopic survey of serotoninergic axons in the lumbar spinal cord of the rat: Co-localization with glutamate decarboxylase and neuropeptides. Neurosci. 1996;75:471–480. doi: 10.1016/0306-4522(96)00366-1. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Action and specificity of ventral medullary vasopressor neurons in the cat. Neurosci. 1986;18:51–59. doi: 10.1016/0306-4522(86)90178-8. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Dampney RA. The selectivity of descending vasomotor control by subretrofacial neurons. Prog. Brain Res. 1989;81:233–242. doi: 10.1016/s0079-6123(08)62013-0. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Dampney RA. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci. Lett. 1990;110:91–96. doi: 10.1016/0304-3940(90)90793-9. [DOI] [PubMed] [Google Scholar]
- McAllen RM, May CN, Campos RR. The supply of vasomotor drive to individual classes of sympathetic neuron. Clin. Exp. Hypertens. 1997;19:607–618. doi: 10.3109/10641969709083173. [DOI] [PubMed] [Google Scholar]
- McAllen RM, May CN, Shafton AD. Functional anatomy of sympathetic premotor cell groups in the medulla. Clin. Exp. Hypertens. 1995;17:209–221. doi: 10.3109/10641969509087066. [DOI] [PubMed] [Google Scholar]
- McCall RB. Serotonergic excitation of sympathetic preganglionic neurons: a microiontophoretic study. Brain Res. 1983;289:121–127. doi: 10.1016/0006-8993(83)90012-4. [DOI] [PubMed] [Google Scholar]
- McCall RB. Effects of putative neurotransmitters on sympathetic preganglionic neurons. Annu. Rev. Physiol. 1988;50:553–564. doi: 10.1146/annurev.ph.50.030188.003005. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Hokfelt T, Seroogy K, Oertel W, Verhofstad AA, Wu JY. Immunohistochemical evidence for colocalization of gamma- aminobutyric acid and serotonin in neurons of the ventral medulla oblongata projecting to the spinal cord. Brain Res. 1987;410:179–185. doi: 10.1016/s0006-8993(87)80043-4. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Hokfelt T, Verhofstad AA, Terenius L. Individual cells in the raphe nuclei of the medulla oblongata in rat that contain immunoreactivities for both serotonin and enkephalin project to the spinal cord. Exp. Brain Res. 1989;75:536–542. doi: 10.1007/BF00249904. [DOI] [PubMed] [Google Scholar]
- Milner TA, Pickel VM, Abate C, Joh TH, Reis DJ. Ultrastructural characterization of substance P-like immunoreactive neurons in the rostral ventrolateral medulla in relation to neurons containing catecholamine-synthesizing enzymes. J. Comp. Neurol. 1988;270:427–445. doi: 10.1002/cne.902700311. [DOI] [PubMed] [Google Scholar]
- Minson J, Llewellyn-Smith I, Neville A, Somogyi P, Chalmers J. Quantitative analysis of spinally projecting adrenaline- synthesising neurons of C1, C2 and C3 groups in rat medulla oblongata. J. Auton. Nerv. Syst. 1990;30:209–220. doi: 10.1016/0165-1838(90)90252-e. [DOI] [PubMed] [Google Scholar]
- Minson JB, Choy VJ, Chalmers JP. Bulbospinal serotonin neurons and hypotensive effects of methyldopa in the spontaneously hypertensive rat. J. Cardiovasc. Pharmacol. 1984;6:312–317. doi: 10.1097/00005344-198403000-00016. [DOI] [PubMed] [Google Scholar]
- Miura M, Takayama K, Okada J. Distribution of glutamate- and GABA-immunoreactive neurons projecting to the cardioacceleratory center of the intermediolateral nucleus of the thoracic spinal cord of SHR and WKY rats: a double-labeling study. Brain Res. 1994;638:139–150. doi: 10.1016/0006-8993(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol. Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1763–R1775. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J. Neurosci. 1988;8:1286–1301. doi: 10.1523/JNEUROSCI.08-04-01286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 2004a;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Wu SX, Fujiyama F, Okamoto K, Hioki H, Kaneko T. Independent inputs by VGLUT2- and VGLUT3-positive glutamatergic terminals onto rat sympathetic preganglionic neurons. NeuroReport. 2004b;15:431–436. doi: 10.1097/00001756-200403010-00010. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Arvidsson U, Hokfelt T. Serotonin-like, substance P-like and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neurosci. 1992;48(3):545–560. doi: 10.1016/0306-4522(92)90401-m. [DOI] [PubMed] [Google Scholar]
- Pernow J, Schwieler J, Kahan T, Hjemdahl P, Oberle J, Wallin BG, Lundberg JM. Influence of sympathetic discharge pattern on norepinephrine and neuropeptide Y release. Am. J. Physiol. 1989;257(3P2):H866–H872. doi: 10.1152/ajpheart.1989.257.3.H866. [DOI] [PubMed] [Google Scholar]
- Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J. Comp. Neurol. 2001;432:20–34. doi: 10.1002/cne.1086. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Bohn MC. Cl-3 adrenergic medullary neurones project to the paraventricular thalamic nucleusin the rat. Neurosci. Lett. 1994;176:67–70. doi: 10.1016/0304-3940(94)90873-7. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Spanswick D, Logan SD. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J. Physiol. 1994;480:109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J. Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Llewellyn-Smith IJ, Minson JB, Arnolda LF, Chalmers JP. Substance P and serotonergic inputs to sympathetic preganglionic neurons. Clin. Exp. Hypertens. 1995;17:335–344. doi: 10.3109/10641969509087075. [DOI] [PubMed] [Google Scholar]
- Polson JW, Halliday GM, McAllen RM, Coleman MJ, Dampney RA. Rostrocaudal differences in morphology and neurotransmitter content of cells in the subretrofacial vasomotor nucleus. J. Auton. Nerv. Syst. 1992;38:117–137. doi: 10.1016/0165-1838(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain. Res. Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54. doi: 10.1016/s0006-8993(98)00655-6. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Cravo SL, Golanov E, Gomez R, Anwar M, Reis DJ. Adrenergic and non-adrenergic spinal projections of a cardiovascular-active pressor area of medulla oblongata: Quantitative topographic analysis. Brain Res. 1994;663:107–120. doi: 10.1016/0006-8993(94)90468-5. [DOI] [PubMed] [Google Scholar]
- Sasek CA, Wessendorf MW, Helke CJ. Evidence for co-existence of thyrotropin-releasing hormone, substance P and serotonin in ventral medullary neurons that project to the intermediolateral cell column in the rat. Neurosci. 1990;35:105–119. doi: 10.1016/0306-4522(90)90125-n. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Bohn MC. Glucocorticoid receptor-immunoreactivity in C1, C2, and C3 adrenergic neurons that project to the hypothalamus or to the spinal cord in the rat. J. Comp. Neurol. 1989;285(1):107–116. doi: 10.1002/cne.902850109. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J. Comp. Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-D H-saporin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R729–R742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Scruggs P, Lai C, Scruggs JE, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide potentiates spinal glutamatergic sympathoexcitation in anesthetized rats. Regul. Pept. 2005;127:79–85. doi: 10.1016/j.regpep.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen ASP, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Stornetta R, Adam N, Guyenet PG. Neuropeptide Y (NPY) mRNA is co-localized in catecholamine (CA) synthesizing neurons in rat brain. Faseb J. 1990;4:A882–A882. [Google Scholar]
- Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat. J. Comp. Neurol. 1999;415:482–500. doi: 10.1002/(sici)1096-9861(19991227)415:4<482::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J. Comp. Neurol. 1999;407:367–380. [PubMed] [Google Scholar]
- Stornetta RL, Huangfu D, Rosin DL, Lynch KR, Guyenet PG. Alpha-2 adrenergic receptors. Immunohistochemical localization and role in mediating inhibition of adrenergic RVLM presympathetic neurons by catecholamines and clonidine. Ann. NY Acad. Sci. 1995;763:541–551. doi: 10.1111/j.1749-6632.1995.tb32448.x. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J. Comp. Neurol. 2004;479:257–270. doi: 10.1002/cne.20332. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp. Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Schreihofer AM, Pelaez NM, Sevigny CP, Guyenet PG. Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. J. Comp. Neurol. 2001;435:111–126. doi: 10.1002/cne.1196. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J. Comp. Neurol. 2002a;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J. Comp. Neurol. 2002b;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989a;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989b;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- Sun MK, Young BS, Hackett JT, Guyenet PG. Reticulospinal pacemaker neurons of the rat rostral ventrolateral medulla with putative sympathoexcitatory function: an intracellular study in vitro. Brain Res. 1988a;442:229–239. doi: 10.1016/0006-8993(88)91508-9. [DOI] [PubMed] [Google Scholar]
- Sun MK, Young BS, Hackett JT, Guyenet PG. Rostral ventrolateral medullary neurons with intrinsic pacemaker properties are not catecholaminergic. Brain Res. 1988b;451:345–349. doi: 10.1016/0006-8993(88)90781-0. [DOI] [PubMed] [Google Scholar]
- Sved AF. PNMT-containing catecholaminergic neurons are not necessarily adrenergic. Brain Res. 1989;481:113–118. doi: 10.1016/0006-8993(89)90490-3. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Dunwiddie TV, Williams JT. Opioid inhibition in locus coeruleus. J. Neurophys. 1995;74:518–528. doi: 10.1152/jn.1995.74.2.519. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Lin HC, Wang SD, Tung CS. Immunohistochemical study of catecholamine enzymes and neuropeptide Y (NPY) in the rostral ventrolateral medulla and bulbospinal projection. J. Comp. Neurol. 1993;334:294–303. doi: 10.1002/cne.903340210. [DOI] [PubMed] [Google Scholar]
- Verberne AJM, Stornetta RL, Guyenet PG. Properties of C1 and other ventrolateral medullary neurones with hypothalamic projections in the rat. J. Physiol. 1999;517:477–494. doi: 10.1111/j.1469-7793.1999.0477t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. TINS. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR. Peptide Cotransmitter Release from Motorneuron B16 in Aplysia californica: Costorage, Corelease, and Functional Implications. J. Neurosci. 2000;20:2036–2042. doi: 10.1523/JNEUROSCI.20-05-02036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager-Page SA, Rosenbaum G, Veale WL, Davison JS. Spinal and peripheral modulation of gastric acid secretion and arterial pressure by neuropeptide Y, peptide YY, and pancreatic polypeptide in rats. Peptides. 1993;14:1299–1308. doi: 10.1016/0196-9781(93)90190-r. [DOI] [PubMed] [Google Scholar]
- Westfall TC, Martin J, Chen X, Ciarleglio A, Carpentier S, Henderson K, Knuepfer M, Beinfeld M, Naes L. Cardiovascular effects and modulation of noradrenergic neurotransmission following central and peripheral administration of neuropeptide Y. Synapse. 1988;2(3):299–307. doi: 10.1002/syn.890020320. [DOI] [PubMed] [Google Scholar]
- Wu SY, Dun NJ. Potentiation of NMDA Currents by Pituitary Adenylate Cyclase Activating Polypeptide in Neonatal Rat Sympathetic Preganglionic Neurons. J. Neurophysiol. 1997;78:1175–1179. doi: 10.1152/jn.1997.78.2.1175. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neurosci. 1999;90:1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur. J. Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Polosa C, Nishi S. Noradrenaline-induced afterdepolarization in cat sympathetic preganglionic neurons in vitro. J. Neurophys. 1987a;57:1314–1324. doi: 10.1152/jn.1987.57.5.1314. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Polosa C, Nishi S. Slow EPSP and the depolarizing action of noradrenaline on sympathetic preganglionic neurons. Brain Res. 1987b;414:138–142. doi: 10.1016/0006-8993(87)91334-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Polosa C, Nishi S. Multiple actions of noradrenaline on sympathetic preganglionic neurons of the cat studied in the spinal cord slice. Prog. Brain Res. 1989;81:181–190. doi: 10.1016/s0079-6123(08)62008-7. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neurosci. 1989;31:105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res. Rev. 1998;26:136–147. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]