Abstract
Myotilinopathies are a group of muscle disorders caused by mutations in the MYOT gene. It was first described in two families suffering from limb girdle muscle dystrophy type 1 (LGMD 1A), and later identified in a subset of dominant or sporadic patients suffering from myofibrillar myopathy, as well as in a family with spheroid body myopathy. Disease phenotypes associated with MYOT mutations are clinically heterogeneous and include pure LGMD forms as well as late-onset distal myopathies. We report here on a 53-year-old male suffering from a unique clinical profile characterized by generalized symmetrical increase in muscle bulk leading to a Herculean appearance. Muscle weakness and stiffness in the lower extremities were the patient’s main complaints. Muscle MRI showed extensive fatty infiltration in the thigh and leg muscles and a muscle biopsy showed a myofibrillar myopathy with prominent protein aggregates. Gene sequencing revealed a Ser55Phe missense mutation in the myotilin gene. The mutation was identified in his older brother, who presented a mild hypertrophic appearance and had a myopathic pattern in EMG, despite not presenting any of the complaints of the proband and having normal muscle strength. This finding, and his deceased father and paternal aunt’s similar gait disorders, suggest that this is in fact a new autosomal dominant kindred. The present observations further expand the spectrum of clinical manifestations associated with mutations in the myotilin gene.
Keywords: Myotilin, Myotilinopathy, MYOT, Phenotype, Pseudo-hypertrophy, Herculean appearance, Muscle MRI, S55F
1. Introduction
Myotilin is a 57-kDa component of sarcomeric Z-discs exclusively expressed in skeletal and cardiac muscle [1]. Myotilin interacts with several other Z-disc proteins including alpha-actinin, filamin C, F-actin and FATZ. These interactions play a major role in the dynamic molecular events mediating myofibrillar assembly in normal and diseased skeletal muscle [1–4]. Myotilin is encoded by a single gene on chromosome 5q31 [1,5]. The coding sequence encompasses 10 exons and codes for 498 amino acids. Seven missense mutations in the MYOT gene are currently known [6–10]. They are all clustered in exon 2, a region which has been shown to be a hot spot for mutations. A wide spectrum of clinical manifestations associated with myotilin mutations has been reported. These include autosomal dominant LGMD syndromes in combination with dysarthria (LGMD 1A) [6,7,11], and late onset sporadic or dominant distal myopathies [8,9,12–14]. Muscle biopsies performed in these latter cases have demonstrated a myofibrillar myopathy [8,9]. Still other cases may present with mixed proximal and distal phenotypes [8,9,14]. Cardiomyopathy, respiratory insufficiency, and peripheral neuropathy may occasionally be additional features in patients [8,9]. Muscle atrophy has been described in some cases [9,12,13]; however, generalized muscle pseudo-hypertrophy has been never observed in myotilinopathy. Here we report on a 53-year-old patient presenting with a distinct clinical phenotype characterized by generalized muscle pseudo-hypertrophy, stiffness and mild muscle weakness due to a myotilin mutation.
2. Case report
A 53-year-old man was referred to our Neuromuscular Unit for evaluation of slowly progressive gait difficulty over the previous 12 years. Two years before admission, the patient noticed progressive muscular weakness in the lower limbs, and to a lesser extent in the upper extremities, as well as muscle pain and stiffness when walking. He was unable to walk for more than 20m without resting, mimicking intermittent claudication syndrome. He also had severe difficulties in climbing and descending stairs, rising from a chair and getting in and out of vehicles. The patient had never done sports or bodybuilding and had never taken hormones or anabolic drugs.
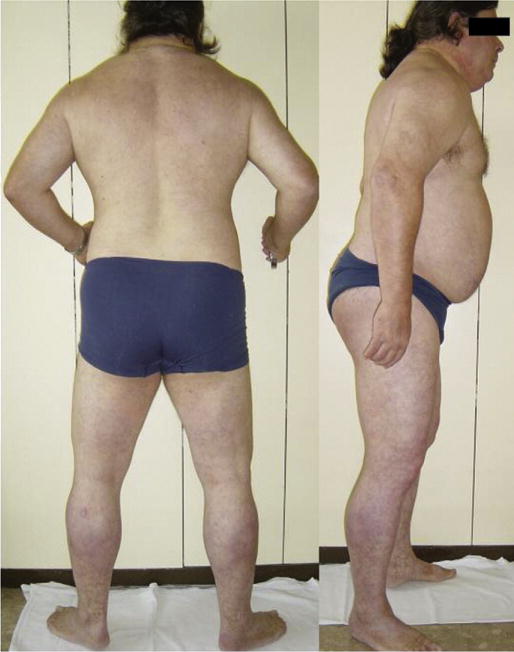
Physical examination revealed a patient with a Herculean appearance with a symmetrical increase in muscle bulk involving the neck, trunk and extremities (Fig. 1). He could not stand with his feet together and the distance between the medial malleoli necessary to stand without falling was 32 cm. He was able to walk without support but with considerable staggering, and had difficulties in half-turning. When walking, the trunk was slightly flexed forwards and the speed was markedly reduced. The muscles felt stiff on palpation. There was no clinicalmyotonia or muscular edema. Ankle jerks were absent. Bilateral Achilles tendon retraction and incipient elbow retraction were also evident. There was no facial weakness, dysarthria or macroglossia. Livedo reticularis was observed as most marked in the lower limbs. Muscle strength (MRC scale) was 4/5 in the deltoids, biceps brachialis and pectoralis muscles; 4+/5 in the neck flexor–extensors, wrist and finger extensor and interosseous muscles; 5/5 in the triceps, wrist flexors, and finger flexors. In the lower limbs, muscle strength was 4/5 in the gluteus maximus, iliopsoas, tibialis anterior, peroneal and toe extensor muscles, 4+/5 in the quadriceps, and 5/5 in the hip adductor and plantar flexor muscles.
Fig. 1.
Rear and side view of the patient showing generalized skeletal muscle enlargement and pseudo-hypertrophy.
Sensory and motor nerve conduction studies were normal. EMG examination showed a myopathic pattern with spontaneous activity at rest with positive sharp waves, fibrillation potentials and complex repetitive discharges with a diffuse distribution but which was most marked in the vastus medialis, tibialis anterior, deltoids and extensor digitorum communis. CK levels were within normal levels. Thyroid function was normal. No anticardiolipin antibodies were present. No monoclonal gammapathy was detected. The electrocardiogram showed non-specific abnormalities consisting of asymmetric T-waves in aVL, V5 and V6. Echocardiogram, brain and spinal MRI were normal.
A brother of the index case, aged 55, had suffered an acute myocardial infarction at 52 years of age. Despite presenting a similar Herculean appearance to his brother, although to a lesser extent (160 cm tall, weight 80 kg, neck perimeter 45 cm, thigh muscles 50 cm, calf muscles 46 cm), the examination showed normal muscle strength and normal CK levels. Nerve conduction studies were normal, and electromyography of the deltoid, vastus medialis, rectus femoris and tibialis anterior muscles showed a myopathic pattern, but no complex repetitive discharges. The patient’s healthy 75-year-old mother had no signs of neuromuscular disease. However, the patient’s father, who had died from Alzheimer’s disease at 71 years old, presented a gait disorder similar to the proband, as did a paternal aunt, about whom we only know that she had an EMG examination consistent with a myopathy.
3. Methods
3.1. Muscle MRI
Magnetic resonance imaging was performed on a 1.5 T whole body MRI system (Magnetom Vision, Siemens Medical Solutions) using the standard body coil including an axial T1-weighted MRI image (TR 483, TE 12) and a STIR MRI image (TR 5000, TE 29).
3.2. Muscle biopsy
An open muscle biopsy was obtained from the vastus lateralis of the patient’s right thigh, with severe fat infiltration observed. Suture of the fascia lata was difficult due to the tension in the pseudo-hypertrophic thigh muscles. The sample was processed according to the methods described elsewhere [9]. Filamin C immunohistochemistry using the monoclonal anti-filamin C antibody RR90 was also performed [15].
3.3. Molecular genetic analysis
Informed consent for genetic analysis was obtained from the proband and his brother. Genomic DNA was obtained from peripheral leukocytes and analyzed for myotilin mutation using the primers and conditions described elsewhere [9].
4. Results
4.1. Muscle MRI
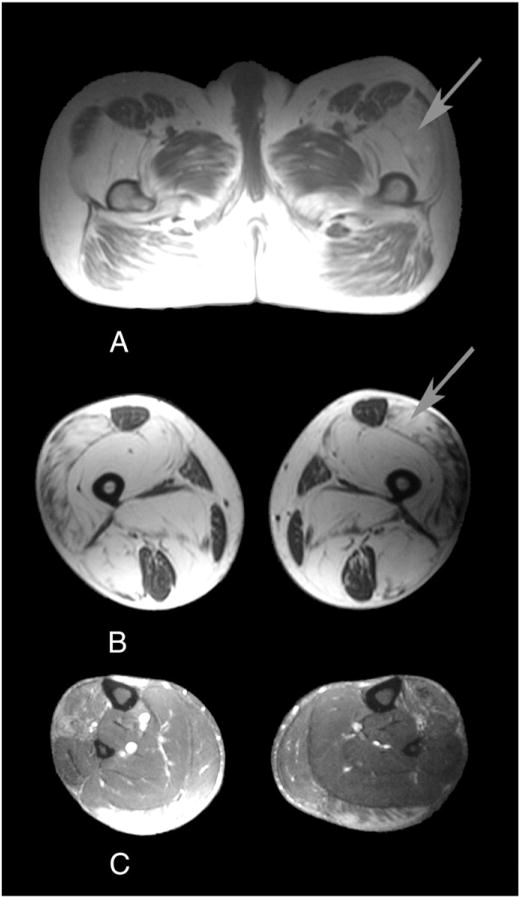
MRI showed severe fatty infiltration involving the vastus intermedius, vastus medialis, adductor magnus, semimembranosus, biceps femoris, and to a lesser extent, the vastus lateralis. The rectus femoris, gracilis, semitendinosus, and sartorius muscles were preserved but contained minor areas of fatty infiltration. At mid-leg level, fatty infiltration was observed on the anterior tibialis, lateral gastrocnemious, and to a lesser extent, the medial gastrocnemious. The remaining muscle groups of the leg, including the soleus muscles, were well-preserved or showed minor abnormalities. This fatty infiltration was also pseudo-hypertrophic, leading to a bilateral symmetric increase in the volume of the thighs and legs (Fig. 2).
Fig. 2.
MRI at the pelvis (A), mid-thigh (B), and mid-leg level (C) showing severe fatty replacement of the glutei, vastus intermedius, vastus medialis, adductor magnus, semimembranosus, and biceps femoris, and less severe changes in the vastus lateralis. The rectus femoris, gracilis, sartorius and semi-tendinosus are well preserved. At mid-leg level, fatty infiltration is observed in the lateral gastrocnemius, medial gastrocnemius and anterior tibialis. The soleus muscle is well preserved. The fatty infiltration causes a bilateral symmetrical increase in the volume of limbs (arrowed).
4.2. Muscle biopsy
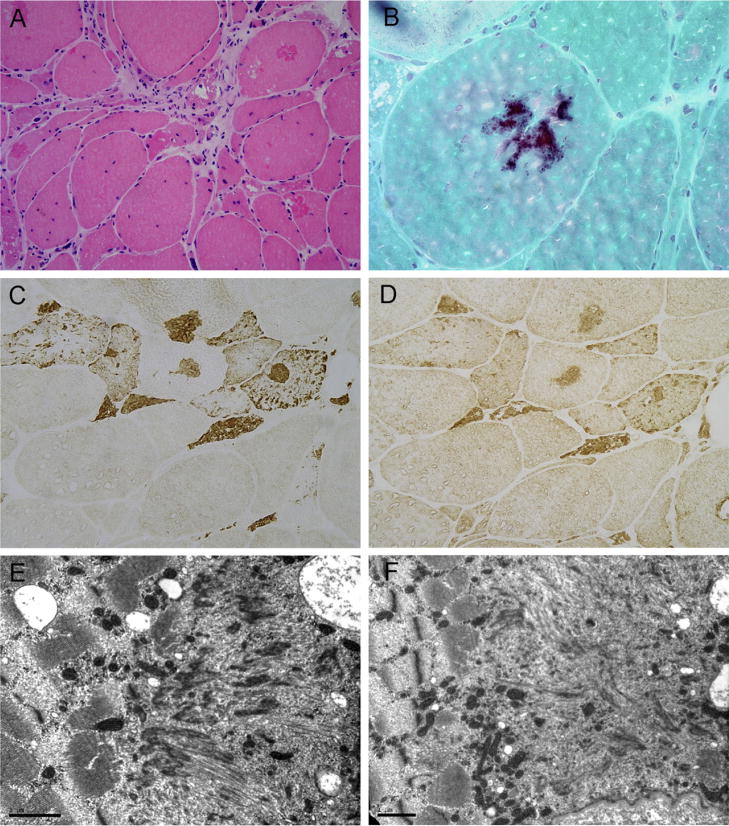
A muscle biopsy taken from the vastus lateralis revealed a marked variation in the fiber size, with some atrophic fibers of both histochemical types often clustered in small groups, intermediate-size fibers and several large round fibers. There were increased numbers of internal nuclei, endomysial fibrosis, and areas replaced by fatty tissue. Muscle fiber necrosis and phagocytosis were occasionally seen. No inflammatory infiltrates were observed. Large numbers of fibers containing vacuoles were seen, most of which were unrimmed. A large number of fibers contained prominent polymorphous hyaline inclusions which stained dark-blue to purple with the modified trichrome stain, and were eosinophilic on hematoxylin and eosin staining, with some displaying strong congophilia. No congophilic material was observed around the intramuscular vessels or in the endomysium. Small cytoplasmic bodies were also present in many fibers. Oxidative and ATPase activity was reduced in the fiber areas occupied by the inclusions. There was type I fiber predominance.
Immunohistochemical studies revealed prominent myotilin, desmin, filamin C, dystrophin and ubiquitin immunoreactivity (Fig. 3). EM examination showed areas of major myofibrillar abnormalities that included electrondense material of Z-disc origin as well as fine granular and filamentous material. In addition, autophagic vacuoles, clusters of mitochondria and some residual polymorphic bodies were observed (Fig. 3).
Fig. 3.
Muscle biopsy showing a wide variation in the fiber size, central nuclei and mild endomysial fibrosis. Several muscle fibers contain cytoplasmic inclusions, which stain pink on hematoxylin and eosin (A), and dark purple with the modified trichrome stain (B). Some of the fibers contain rimmed and unrimmed vacuoles (A). The inclusions show strong myotilin (C) and filamin C (D) immunoreactivity. Under EM, large areas of sarcomeric disorganization are replaced by electrondense material of Z-disc origin, remnants of filaments and fine granular material (E and F). A cluster of mitochondria is seen in F. A ×200; B ×600; C and D ×400. Bar in E and F=1 μm.
4.3. Molecular genetic analysis
Sequence analysis identified a c.444C>T missense mutation in exon 2 of the myotilin gene, predicted to result in a change of residue 55 from serine to phenylalanine (Ser55Phe). The same mutation was found in the patient’s brother. No irregularities in this region were found on sequencing 100 Spanish control individuals.
5. Discussion
We have described a patient suffering from generalized muscle pseudo-hypertrophy and stiffness caused by a mutation in the myotilin gene. The genealogical background suggests an autosomal dominant transmission as previously reported in kindreds with the Ser55Phe MYOT mutation.
Although the case seems sporadic in nature, the presence of an asymptomatic brother who nevertheless carried the mutation and who to date remains asymptomatic, with only a pathological EMG, as well as the gait disorders in the proband’s deceased father and paternal aunt, suggest otherwise. This clinical intra-familial heterogeneity has previously been reported in myotilinopathy pedigrees. Investigation of the previous three generations on both sides of the family also shows that there is no consanguinity. Furthermore, possible consanguinity was deemed extremely unlikely, considering that the mother and father came from villages 700 kilometers apart in rural Spain in the 1940s.
The family insisted after repeated questioning that the father did not have a hypertrophic build, although no conclusive full body photographs of him were available. There is a theoretical possibility that there are two pathological processes at work — a myotilin defect and pseudohypertrophy. However, this has yet to be ascertained.
Myotilinopathy was originally described in two families suffering from LGMD 1A [6,7]. Affected patients in both families presented between the ages of 18 and 58 with proximal weakness in the lower extremities, which later spread to involve the distalmuscles. Dysarthria was a prominent symptomin somecases. Amuscle biopsy performed in one affected member showed variation in fiber size, fiber splitting, a large number of rimmed vacuoles and patches of Z-line streaming [6,7,11]. No immunocytochemical studies were performed. More recently, mutations in myotilin gene were found in a subset of patients suffering from myofibrillar myopathy [8,9]. In these later cases, the age of onset of the disease varied between 42 and 77 years old; distal weakness in the lower extremities is the most common mode of presentation, although some cases it may present with proximal weakness, or with mixed proximal and distal weakness [8,9]. The Ser55Phe myotilin mutation identified in the patient described here was previously found in a LGMD1A family [7] as well as in several sporadic or familial cases presenting with a late-onset distal myopathy [8,9,12,13], and in two sporadic cases presenting with late-onset proximal weakness [9]. Cardiopathy was absent except for a single case [9]. Three additional myotilin mutations (S60C, S60F and S91I) have been found in patients presenting with progressive muscle weakness in either the proximal or the distal muscles, with decreased reflexes in the legs and various degrees of cardiac involvement [8,9,14]. Finally, an S39F MYOT mutation has been shown to be the cause of spheroid body myopathy, a disorder which has clinical and pathological features overlapping with LGMD 1A and MFM [11,16]. Irrespective of the mode of presentation, muscle weakness in myotilinopathy may be accompanied by muscle atrophy alone or in combination with stiffness and myalgia in some patients [8,9,12–14]. Painful enlargement of leg muscles has been observed in some cases; however, generalized muscle pseudo-hypertrophy has never been described in myotilinopathy.
Muscle MRI performed on our patient showed severe fatty infiltration of several muscle groups in the thigh and leg, thus indicating that the increase in muscle bulk was a result of fatty replacement rather than a true muscle hypertrophy. The pattern of muscle involvement observed in the thigh muscles of the patient reported here mirrors the abnormalities previously found in several other myotilinopathy cases [8,12–13]. However, unlike previously published cases, the soleus muscle, which has been shown to be the first muscle involved in myotilinopathy, was well preserved. Interestingly, this preservation of the soleus muscles present in our patient was described in transgenic mice expressing the myotilin T57I mutation [19]. The reasons behind this sparing are not yet fully known.
In summary, we have described generalized muscle pseudo-hypertrophy and stiffness as a novel phenotypic manifestation of myotilinopathy. Generalized muscle pseudo-hypertrophy as seen in our case may be observed in other clinical conditions such as hypothyroid myopathy and amyloid myopathy [17,18]. Myotilinopathy should be therefore considered in the differential diagnosis of patients presenting with generalized muscle pseudo-hypertrophy.
Acknowledgments
JG was supported by a Spanish Fondo de Investigaciones Sanitarias grant (FIS 02/0648). MO was supported by FIS grant PI051213. We wish to thank Dolores Moreno for her excellent technical assistance.
References
- 1.Salmikangas P, Mykkänen OM, Grönholm M, Heiska L, Kere J, Carpén O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–36. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- 2.Salmikangas P, van der Ven PFM, Lalowski M, et al. Myotilin, the limb-girdle dystrophy 1A (LGMD1A) protein, cross-links actin filaments and controls sarcomere assembly. Hum Mol Genet. 2003;12:189–203. 170. doi: 10.1093/hmg/ddg020. [DOI] [PubMed] [Google Scholar]
- 3.von Nandelstadh P, Grönholm M, Moza M, Lamberg A, Savilahti H, Carpén O. Actin-organising properties of the muscular dystrophy protein myotilin. Exp Cell Res. 2005;310:131–9. doi: 10.1016/j.yexcr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Gontier Y, Taivainen A, Fontao L, et al. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci. 2005;118:3739–49. doi: 10.1242/jcs.02484. [DOI] [PubMed] [Google Scholar]
- 5.Yamaoka LH, Westbrook CA, Speer MC, et al. Development of a microsatellite genetic map spanning 5q31-q33 and subsequent placement of the LGMD1A locus between D5S178 and IL9. Neuromuscul Disord. 1994;4:471–5. doi: 10.1016/0960-8966(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 6.Hauser MA, Horreigan SK, Salmikangas P, et al. Myotilin is mutated in limb girdle muscular dystrophy 1A. Hum Mol Genet. 2000;14:2141–7. doi: 10.1093/hmg/9.14.2141. [DOI] [PubMed] [Google Scholar]
- 7.Hauser MA, Conde CB, Kowaljow V, et al. Myotilin mutation found in second pedigree with LGMD1A. Am J Hum Genet. 2002;71:1428–32. doi: 10.1086/344532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–71. doi: 10.1212/01.wnl.0000123576.74801.75. Erratum in: Neurology 2004;63:405. [DOI] [PubMed] [Google Scholar]
- 9.Olive M, Goldfarb LG, Shatunov A, Fisher D, Ferrer I. Myotilinopathy: refining the clinical and myopathological phenotype. Brain. 2005;128:2315–26. doi: 10.1093/brain/awh576. [DOI] [PubMed] [Google Scholar]
- 10.Foroud T, Pankratz N, Batchman AP, et al. A mutation in myotilin causes spheroid body myopathy. Neurology. 2005;65:1936–40. doi: 10.1212/01.wnl.0000188872.28149.9a. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist JM, Pericak-Vance M, Silverman L, Roses AD. Clinical and genetic investigation in autosomal dominant limb-girdle muscular dystrophy. Neurology. 1988;38:5–9. doi: 10.1212/wnl.38.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Fischer D, Clamen CS, Olivé M, et al. Different early pathogenesis inmyotilinopathy compared to primary desminopathy. Neuromuscul Disord. 2006;16:361–7. doi: 10.1016/j.nmd.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Berciano J, Gallardo E, Dominguez-Perles R, et al. Autosomal dominant distal myopathy with a myotilin S55F mutation: sorting out the phenotype. J Neurol Neurosurg Psychiatry. 2008;79:205–8. doi: 10.1136/jnnp.2007.125435. [DOI] [PubMed] [Google Scholar]
- 14.Pénnison-Besnier I, Talvinen K, Dumez C, et al. Myotilinopathy in a family with late onset myopathy. Neuromusc Disord. 2006;16:427–31. doi: 10.1016/j.nmd.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.van der Ven PF, Obermann WM, Lemke B, Gautel M, Weber K, Fürst DO. Characterization of muscle filamin isoforms suggests a possible role of gamma-filamin/ABP-L in sarcomeric Z-disc formation. Cell Motil Cytoskeleton. 2000;45:149–62. doi: 10.1002/(SICI)1097-0169(200002)45:2<149::AID-CM6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Goebel HH, Muller J, Gillen HW, Merritt AD. Autosomal dominant “spheroid body myopathy”. Muscle Nerve. 1978;1:14–26. doi: 10.1002/mus.880010104. [DOI] [PubMed] [Google Scholar]
- 17.Klein I, Parker M, Shebert R, Ayyar DR, Levey GS. Hypothyroidism presenting as muscle stiffness and pseudohypertrophy: Hoffmann’s syndrome. Am J Med. 1981;70:891–4. doi: 10.1016/0002-9343(81)90550-7. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker JN, Hashimoto K, Quinones M. Skeletal muscle pseudohypertrophy in primary amyloidosis. Neurology. 1977;27:47–54. doi: 10.1212/wnl.27.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Garvey SM, Miller SE, Claflin DR, Faulkner JA, Hauser MA. Transgenic mice expressing the myotilin T57I mutation unite the pathology associated with LGMD1A and MFM. Hum Mol Genet. 2006;15:2348–62. doi: 10.1093/hmg/ddl160. [DOI] [PubMed] [Google Scholar]