Abstract
Context
Obesity has been implicated as a risk factor for pancreatic cancer.
Objective
To demonstrate the association of excess body weight across an age cohort and the risk, age of onset, and overall survival of patients with pancreatic cancer.
Design, Setting, and Participants
A case-control study of 841 patients with pancreatic adenocarcinoma and 754 healthy individuals frequency matched by age, race, and sex. The study was conducted at a university cancer center in the United States from 2004 to 2008. Height and body weight histories were collected by personal interview starting at ages 14 to 19 years and over 10-year intervals progressing to the year prior to recruitment in the study.
Main Outcome Measures
The associations between patients’ body mass index (BMI) and risk of pancreatic cancer, age at onset, and overall survival were examined by unconditional logistic regression, linear regression, and Cox proportional hazard regression models, respectively.
Results
Individuals who were overweight (a BMI of 25-29.9) from the ages of 14 to 39 years (highest odds ratio [OR], 1.67; 95% confidence interval [CI], 1.20-2.34) or obese (a BMI ≥30) from the ages of 20 to 49 years (highest OR, 2.58; 95% CI, 1.70-3.90) had an associated increased risk of pancreatic cancer, independent of diabetes status. The association was stronger in men (adjusted OR, 1.80; 95% CI, 1.45-2.23) by mean BMI from the ages of 14 to 59 years than in women (adjusted OR, 1.32; 95% CI, 1.02-1.70) and in ever smokers (adjusted OR, 1.75; 95% CI, 1.37-2.22) than in never smokers (adjusted OR, 1.46; 95% CI, 1.16-1.84). The population-attributable risk percentage of pancreatic cancer based on the mean BMI from the ages of 14 to 59 years was 10.3% for never smokers and 21.3% for ever smokers. Individuals who were overweight or obese from the ages of 20 to 49 years had an earlier onset of pancreatic cancer by 2 to 6 years (median age of onset was 64 years for patients with normal weight, 61 years for overweight patients [P=.02], and 59 years for obese patients [P<.001]). Compared with those with normal body weight and after adjusting for all clinical factors, individuals who were overweight or obese from the ages of 30 to 79 years or in the year prior to recruitment had reduced overall survival of pancreatic cancer regardless of disease stage and tumor resection status (overweight patients: hazard ratio, 1.26 [95% CI, 0.94-1.69], P=.04; obese patients: hazard ratio, 1.86 [95% CI, 1.35-2.56], P<.001).
Conclusions
Overweight or obesity during early adulthood was associated with a greater risk of pancreatic cancer and a younger age of disease onset. Obesity at an older age was associated with a lower overall survival in patients with pancreatic cancer.
PANCREATIC CANCER IS THE fourth leading cause of cancer-related death for both men and women in the United States. Understanding the etiology and illuminating the risk factors identifying high-risk individuals are essential to the primary prevention of this often rapidly fatal disease.
As the prevalence of overweight (defined as a body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] of 25-29.9) and obesity (a BMI ≥30) have rapidly increased during the last 2 decades, accumulating evidence has emerged that excess body weight is a risk factor for pancreatic cancer. Several recent cohort and case-control studies have reported elevated risks of pancreatic cancer in obese individuals compared with individuals with a normal weight (relative risks, 1.2-3.0).1-7 A recent meta-analysis of 21 independent prospective studies involving 3.5 million individuals and 8062 patients with pancreatic cancer reported a relative risk of pancreatic cancer per 5-unit increase in BMI of 1.16 (95% confidence interval [CI], 1.06-1.17) in men and 1.10 (95% CI, 1.02-1.19) in women.8 It has been estimated that the population-attributable risk percentage of obesity-associated pancreatic cancer is 26.9% for the US population.9
However, to our knowledge, no study has explicitly reported the association between excess body weight across an individual's life span and the risk of pancreatic cancer or identified at which ages the key predisposing weight change usually occurs. It is also unknown whether BMI influences the age at onset or overall survival in patients with pancreatic cancer.
We conducted a large-scale case-control study to determine the associations between BMI over a lifetime and pancreatic cancer risk, age at onset, and overall patient survival.
METHODS
Study Population
The study population was drawn from an ongoing hospital-based, case-control study conducted at the University of Texas M. D. Anderson Cancer Center that began in January 2004. Patients with pathologically confirmed pancreatic ductal adenocarcinoma were prospectively identified and consecutively recruited by reviewing daily the clinical schedules of all known medical oncologists and surgeons who see patients with pancreatic cancer and by identifying those on their initial visit to the gastrointestinal clinic. Of 841 patients, 673 were newly diagnosed cases (80%) and received their pathological diagnosis at M. D. Anderson Cancer Center and 168 patients (20%) received a pathological diagnosis and treatment prior to their M. D. Anderson Cancer Center visit. With the physician's approval, all patients with either confirmed or suspected pancreatic adenocarcinoma were approached for consent.
The 754 controls were healthy individuals selected from among the spouses, relatives, and friends accompanying patients with various types of cancer seen at the same institute. Controls were not genetically related to their accompanying patients and were not chosen from among those accompanying patients with gastrointestinal, lung, or head and neck cancer (to avoid over-matching on exposure). Patients and controls were frequency matched by age (±5 years), sex, and self-reported race. All study participants were US residents, had no history of cancer (except non–melanoma skin cancer), and were able to communicate in English.
The M. D. Anderson institutional review board approved the study and written informed consent was obtained from each individual before a personal interview was conducted and a DNA sample was collected. The study design and patient population has been described in detail.10
According to the M. D. Anderson tumor registry, a total of 2207 patients with pancreatic cancer, including 1242 with pathologically confirmed adenocarcinoma without concurrent or previous cancers, were registered between May 31, 2004, and June 30, 2008. Among the 1242 adenocarcinoma cases, as of the end of June 2008, 1002 eligible patients were approached for interview and 841 (83.9%) agreed to be enrolled in the study. Of these 1002 eligible patients, 57 (35.4%) were not included due to patient or physician refusal, 73 (45.4%) due to emotional stress and severity of illness, and 31 (19.3%) due to limited time to conduct the interview. The remaining 240 patients were not approached because they were either international patients (who were not eligible for the study) or patients who were only seeking a pathological diagnosis but not treatment so they did not see a medical oncologist or surgeon. Descriptive analysis indicated no significant difference between the approached and the not approached patients in the distribution of male sex (56% vs 58.3%, respectively), mean (SD) age of pancreatic cancer diagnosis (61.2 [0.4] years vs 61.4 [0.5] years), well or moderate differentiated tumor grade (24.3% vs 22.4%), and advanced disease stage (76% vs 72%).
Controls were recruited from diagnostic radiology clinics of the M. D. Anderson Cancer Center. A short structured questionnaire was used to screen for potential controls on the basis of the eligibility criteria. Analysis of the answers received on the short questionnaire indicated that 83.6% of those questioned agreed to participate in clinical research. A comparison of those recruited as controls and those who refused to participate revealed no significant differences in age, sex, race or ethnicity, educational level, personal history of cancer, or the accompanied patient's cancer type.
Data Collection
A structured questionnaire was used to collect demographic information and known or potential risk factors (eg, cigarette smoking status, alcohol consumption, family history of cancer, and medical history). The questionnaire was administered by personal interview. Each individual was asked about his or her usual and current height in inches and weight in pounds starting at ages 14 to 19 years and over 10-year intervals (20-29, 30-39, 40-49, 50-59, 60-69, and 70-79 years) progressing to the year prior to recruitment. The BMI for each individual was then calculated using the usual height and the weight at each age period.
Clinical information was systematically abstracted from patients’ medical records using an established abstraction form by trained individuals with a medical background. Information collected included the date of first pathological diagnosis, clinical stage of the tumor (localized, locally advanced, metastatic), tumor resection status, serum carbohydrate antigen 19-9 level at diagnosis, and date of last follow-up or death. Among patients who received tumor resection, surgical margin, node status, and tumor grade also were evaluated. Clinical disease stage was defined on the basis of the patient's initial computed tomographic images and endoscopic ultrasound report as stated in the American Joint Committee on Cancer Staging Manual using the TNM staging system.11 Patients without distant metastasis but with resectable or unresectable tumors were classified, respectively, in the groups of localized or locally advanced disease. Treatment modalities except tumor resection were not considered in the survival analysis because of the minimal effect on survival and the large heterogeneity.
Performance status was not assessed because this information was not available for patients who started their treatment before they came to the M. D. Anderson Cancer Center. Dates of death were obtained and cross-checked using at least 1 of the following sources: inpatient medical records, the M. D. Anderson tumor registry, or the Social Security Death Index.12 Survival analysis was performed in 609 patients who had at least 1 year of follow-up at the time of data analysis (July 2008; ie, those who were diagnosed before July 2007). The median follow-up was 22.1 months (95% CI, 20.2-24.0 months).
Statistical Analysis
The distribution of categorical variables was compared between patients and controls using the Pearson χ2 test. Ever smokers were defined as individuals who had smoked more than 100 cigarettes in their lifetime. Ever drinkers of alcohol were defined as individuals who had consumed at least 4 alcoholic drinks of beer, wine, or hard liquor each month for 6 months in their lifetime. Family history of cancer was restricted to first-degree relatives. The association of pancreatic cancer with any factor was analyzed using multiple factor unconditional logistic regression with adjustments for age, race, sex, smoking status, alcohol consumption, diabetes status, and family history of cancer.
Body mass index was analyzed as both a continuous and categorical variable. The mean BMI from the ages of 14 to 59 years was calculated using the values at each age period. To control for the effect of cancer-related weight loss among patients, the BMI at the age period of recruitment to the study was excluded from the calculation. Body mass index was categorized according to the World Health Organization's standard ranges: normal body weight (18.5-24.9), overweight (25-29.9), and obesity (≥30). The odds ratios (ORs) and 95% CIs of pancreatic cancer in association with BMI at each age period and the mean BMIs from the ages of 14 to 59 years were estimated using unconditional logistic regression with adjustment for age (continuous), race, sex, smoking (ever vs never), alcohol consumption (yes or no), history of diabetes (yes or no), and family history of cancer (yes or no). The association of BMI and risk of pancreatic cancer also was analyzed by sex and smoking strata. Because patients with a cancer diagnosis at an early age were not relevant to the analysis of BMI at a later age, the relationship between case and control status and BMI for the older age groups was conditional on the individual living to that decade.
To overcome the lag-time effect, the association between ages of starting to be overweight or obese with risk of pancreatic cancer also was analyzed using logistic regression models. In this study, the population-attributable risk percentage (PAR%) of pancreatic cancer was calculated in relation to being overweight or obese (BMI ≥25) as follows:
in which OR is the adjusted OR for the relationship between being overweight or obese and having pancreatic cancer and Pe is the prevalence of being overweight or obese (BMI ≥25) in the control population.
The mean age of disease onset by BMI status was analyzed among patients using analysis of covariance. Linear regression models were used to estimate the mean difference in age of onset for BMI after adjusting for other factors that are associated with age of onset in this study population, such as diabetes and alcohol consumption.
Overall survival time was calculated from the date of pathological diagnosis to the date of death or last follow-up visit. Data of living patients were censored by their last follow-up date at the time of data analysis. The overall survival curve by BMI was described using the Kaplan-Meier method and log-rank test. The association of BMI and overall survival was analyzed using multivariate Cox proportional hazard models with adjustment for sex, race, stage, tumor resection status, diabetes status, and serum carbohydrate antigen 19-9 level at diagnosis. Tumor grade, margin status, and node status also were included in the Cox regression model for patients with resected tumors.
All statistical analyses were performed using SPSS version 15.0 (SPSS, Cary, North Carolina) and Stata version 10 (StataCorp, College Station, Texas) software with 2-sided tests. P<.05 was considered statistically significant.
RESULTS
The study involved 841 patients with pancreatic adenocarcinoma and 754 healthy controls. The characteristics of the study population are provided in Table 1. Compared with patients, controls were underrepresented in being older than 70 years, having female sex, and having black or Hispanic race. Ever smoking cigarettes, history of diabetes, family history of cancer among first-degree relatives, and alcohol consumption were significantly associated with increased risk of pancreatic cancer (Table 1).
Table 1.
Distribution of Selected Characteristics Among Patients and Controls
| No. (%)a |
||||
|---|---|---|---|---|
| Characteristic | Patients (n = 841) | Controls (n = 754) | AOR (95% CI)b | P Value |
| Age at recruitment, y | ||||
| Mean (95% CI) |
61.7 (61.0-62.4) |
60.8 (60.1-61.5) |
1.01 (1.00-1.11) |
.17 |
| <40 |
16 (1.9) |
15 (2.0) |
|
|
| 40-49 |
85 (10.1) |
85 (11.3) |
|
|
| 50-59 |
228 (27.1) |
233 (30.9) |
|
|
| 60-69 |
318 (37.8) |
271 (35.9) |
|
|
| ≥70 |
194 (23.1) |
150 (19.9) |
|
|
| Sex | ||||
| Female |
345 (41.0) |
282 (37.4) |
1 [Reference] |
|
| Male |
496 (59.0) |
472 (62.6) |
0.72 (0.58-0.91) |
.005 |
| Race/ethnicity | ||||
| Non-Hispanic white |
725 (86.2) |
697 (92.4) |
1 [Reference] |
|
| Hispanic |
52 (6.2) |
37 (4.9) |
1.24 (0.78-1.95) |
.36 |
| Black |
54 (6.4) |
16 (2.1) |
3.03 (1.68-5.45) |
<.001 |
| Otherc |
10 (1.2) |
4 (0.5) |
2.65 (0.80-8.72) |
.11 |
| Family history of cancerd | ||||
| No |
297 (35.3) |
364 (48.3) |
1 [Reference] |
|
| Yes |
533 (63.4) |
386 (51.2) |
1.70 (1.38-2.09) |
<.001 |
| History of diabetes | ||||
| No |
620 (73.7) |
658 (87.3) |
1 [Reference] |
|
| Yes |
221 (26.3) |
96 (12.7) |
2.36 (1.79-3.10) |
<.001 |
| Smoking status | ||||
| Never |
385 (45.8) |
400 (53.1) |
1 [Reference] |
|
| Ever |
456 (54.2) |
354 (46.9) |
1.34 (1.08-1.66) |
.008 |
| Education level | ||||
| ≤Bachelor's degree |
679 (80.7) |
612 (81.2) |
1 [Reference] |
|
| Advanced degree |
162 (19.2) |
142 (18.8) |
1.19 (0.95-1.50) |
.14 |
| Alcohol consumptione | ||||
| Never |
346 (41.1) |
338 (44.8) |
1 [Reference] |
|
| Ever | 493 (58.6) | 415 (55.0) | 1.31 (1.04-1.64) | .02 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
Unless otherwise indicated. Percentages may not equal 100% due to rounding.
Obtained from logistic regression model including age (continuous), sex, race, family history of cancer, smoking status, education level, and alcohol consumption.
Indicates American Indians and Asians.
This was among first-degree relatives only. One patient and 3 controls had been adopted and this information was not available.
Data were missing from 2 patients and 1 control.
Association of BMI and Risk of Pancreatic Cancer
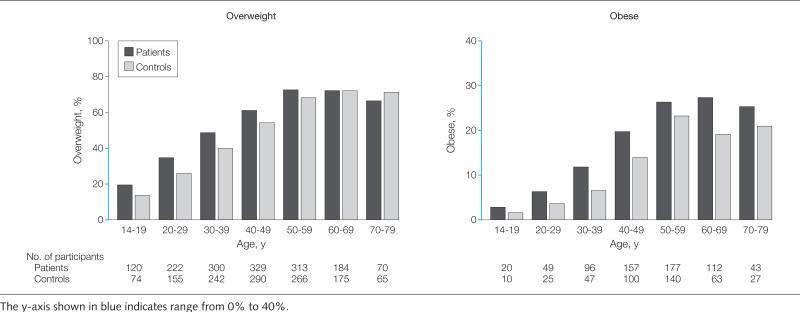
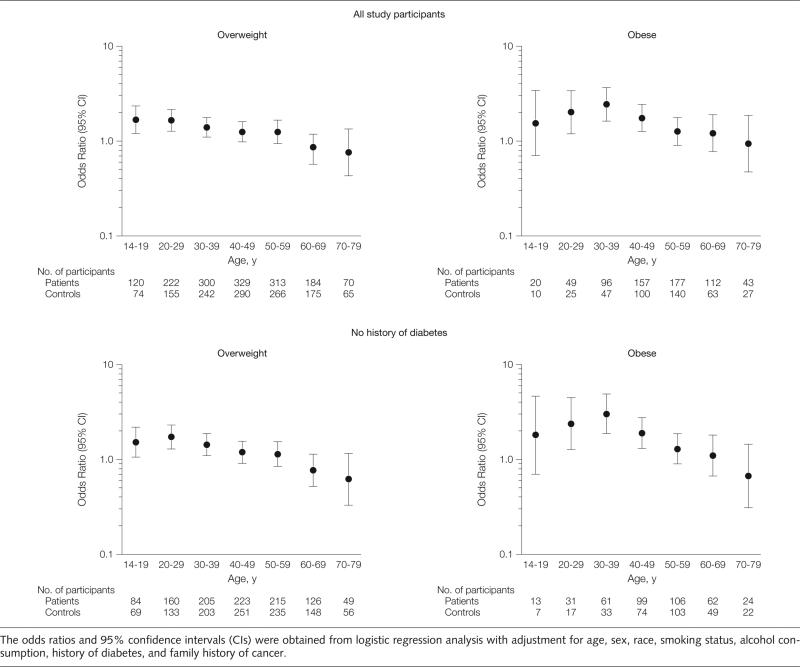
A linear increase with age in the prevalence of overweight and obesity was seen in both patients and controls. The slope of weight increase by age was not statistically different between patients and controls (P = .32; Figure 1). The duration (number of decades having a BMI >25) of being overweight was significantly longer among patients (mean [SD], 4.11 [2.03] decades) than among controls (mean [SD], 3.89 [2.22] decades) (P = .03). Using categorical variables in the analysis, being overweight from the ages of 14 to 39 years and obese from the ages of 20 to 49 years also were significantly associated with an increased risk of pancreatic cancer regardless of diabetes status (Figure 2). The highest OR of 3.03 (95% CI, 1.88-4.90) was detected for obesity from the ages of 30 to 39 years among individuals without diabetes. The risk leveled off for weight increase after ages 40 to 49 years. Body mass indices from the ages of 60 to 79 years and in the year prior to recruitment were not associated with or were inversely associated with the risk of pancreatic cancer.
Figure 1.
Frequency of Overweight and Obesity at Various Age Periods
Figure 2.
Associations With Risk of Pancreatic Cancer Among All Study Participants and Among Those Without a History of Diabetes
The mean (SD) BMI from the ages of 14 to 59 years was 24.70 (3.76) for patients and 23.73 (3.25) for controls (P<.001). The OR of pancreatic cancer per 5-unit increase in mean BMI was 1.55 (95% CI, 1.32-1.84; P < .001) among all study participants and was 1.66 (95% CI, 1.37-2.01; P < .001) among individuals without diabetes. The association between mean BMI (per 5-unit increase) and risk of pancreatic cancer was stronger in men (OR, 1.80; 95% CI, 1.45-2.23) than in women (OR, 1.32; 95% CI, 1.02-1.70) (P for interaction=.02). The association was statistically significant for each age cohort from 14 to 69 years in men but only from ages 14 to 39 years in women (Table 2). The estimated association of mean BMI (per 5-unit increase) with cancer risk also was slightly stronger in ever smokers (OR, 1.75 [95% CI, 1.37-2.22]; P for interaction=.23) than in never smokers (OR, 1.46 [95% CI, 1.16-1.84]; P for interaction=.83). Among controls, 139 never smokers (34.8%) and 128 ever smokers (36.2%) had a history of being overweight or obese (BMI ≥25) earlier in life (from the ages of 14-59 years). The adjusted OR of pancreatic cancer was 1.33 (95% CI, 0.96-1.84) in never smokers and 1.74 (95% CI, 1.26-2.39) in ever smokers. Given these parameters and under the assumption that obesity is associated with risk of pancreatic cancer independently from other significant risk factors, it was estimated that 10.3% of never smokers and 21.3% of ever smokers had pancreatic cancer attributable to being overweight or obese at an early age prior to cancer diagnosis (ie, from the ages of 14-59 years).
Table 2.
Association Between Body Mass Index and Risk of Pancreatic Cancera
| All Participants |
Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. |
No. |
No. |
||||||||
| Patients | Controls | AOR (95% CI)b | P Value | Patients | Controls | AOR (95%CI) | Patients | Controls | AOR (95%CI) | |
| Age, y | ||||||||||
| Meanc |
841 |
753 |
1.55 (1.32-1.84) |
<.001 |
496 |
472 |
1.80 (1.45-2.23) |
345 |
281 |
1.32 (1.02-1.70) |
| 14-19 |
841 |
751 |
1.50 (1.26-1.77) |
<.001 |
496 |
471 |
1.51 (1.22-1.85) |
345 |
280 |
1.51 (1.13-2.02) |
| 20-29 |
840 |
751 |
1.53 (1.30-1.79) |
<.001 |
496 |
471 |
1.60 (1.31-1.96) |
344 |
280 |
1.46 (1.12-1.89) |
| 30-39 |
836 |
751 |
1.48 (1.28-1.71) |
<.001 |
496 |
472 |
1.65 (1.36-2.01) |
340 |
279 |
1.30 (1.05-1.62) |
| 40-49 |
809 |
732 |
1.25 (1.10-1.42) |
.001 |
479 |
457 |
1.39 (1.16-1.65) |
330 |
275 |
1.14 (0.95-1.38) |
| 50-59 |
680 |
608 |
1.16 (1.02-1.32) |
.02 |
401 |
376 |
1.34 (1.12-1.60) |
279 |
232 |
1.00 (0.83-1.21) |
| 60-69 |
413 |
331 |
1.08 (0.91-1.28) |
.41 |
237 |
211 |
1.30 (1.01-1.66) |
176 |
120 |
0.92 (0.71-1.18) |
| No Diabetes | Ever Smokers | Never Smokers | ||||||||
| Meanc |
620 |
657 |
1.66 (1.37-2.01) |
<.001 |
456 |
354 |
1.75 (1.37-2.22) |
385 |
399 |
1.46 (1.16-1.84) |
| 14-19 |
620 |
656 |
1.54 (1.27-1.85) |
<.001 |
456 |
353 |
1.61 (1.27-2.03) |
385 |
398 |
1.45 (1.14-1.86) |
| 20-29 |
620 |
656 |
1.71 (1.36-2.06) |
<.001 |
456 |
353 |
1.71 (1.36-2.16) |
384 |
398 |
1.44 (1.15-1.80) |
| 30-39 |
616 |
655 |
1.61 (1.37-1.91) |
<.001 |
454 |
353 |
1.70 (1.37-2.12) |
382 |
398 |
1.37 (1.12-1.67) |
| 40-49 |
595 |
637 |
1.28 (1.10-1.48) |
.008 |
446 |
351 |
1.32 (1.10-1.59) |
363 |
381 |
1.22 (1.02-1.46) |
| 50-59 |
488 |
525 |
1.16 (0.99-1.34) |
.04 |
375 |
301 |
1.18 (0.99-1.41) |
305 |
307 |
1.17 (0.96-1.42) |
| 60-69 | 287 | 281 | 1.00 (0.87-1.21) | .97 | 218 | 176 | 1.11 (0.87-1.42) | 195 | 155 | 1.05 (0.83-1.34) |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
Body mass index was calculated as weight in kilograms divided by height in meters squared. Data on body mass index at early age was missing from 3 controls and 1 patient.
The AOR of pancreatic cancer per 5-unit increase in body mass index was estimated by a logistic regression model that included age, race, sex, smoking status, diabetes status, alcohol consumption, and family history of cancer. Sex and smoking status were omitted from the model in the analyses of sex and smoking strata.
Indicates a mean body mass index from the ages of 14 to 59 years.
An analysis on starting age of being overweight or obese in association with cancer risk was performed (Table 3). Using individuals who were never overweight throughout their lives as the reference group, being overweight from the ages of 14 to 29 years and being obese from the ages of 20 to 39 years were significantly associated with increased risk for pancreatic cancer. Individuals who started gaining extra weight at or after age 40 years did not show significantly increased risk of pancreatic cancer. Weight change in each age period from the previous period did not show a significant association with the risk of pancreatic cancer. However, a significant association with risk of pancreatic cancer was observed with increasing BMI from the ages of 30 to 39 years compared with from the ages of 14 to 19 years (mean [SD] BMI increase of 3.10 [3.04] for controls and 3.44 [3.00] for patients; P=.03). In addition, 140 controls (18.6%) compared with 219 patients (26.0%) had BMI increases of more than 5 units (P = .003); after adjusting for other factors, the OR of pancreatic cancer associated with this change was 1.65 (95% CI, 1.16-2.34; P=.005).
Table 3.
Age of Onset of Overweight or Obesity and Pancreatic Cancer
| No. |
P Value | Mean (SD) Age of Pancreatic Cancer Diagnosis, y | P Valueb | |||
|---|---|---|---|---|---|---|
| Patients | Controls | AOR (95% CI)a | ||||
| Overweight | ||||||
| Never |
212 |
225 |
1 [Reference] |
|
63.1 (11.1) |
|
| Age, yc | ||||||
| 14-19 |
143 |
85 |
2.03 (1.41-2.92) |
<.001 |
59.4 (10.4) |
.01 |
| 20-29 |
138 |
102 |
1.65 (1.16-2.36) |
.006 |
60.6 (9.4) |
.39 |
| 30-39 |
137 |
110 |
1.27 (0.90-1.79) |
.17 |
60.8 (10.9) |
.56 |
| 40-49 |
114 |
129 |
0.91 (0.65-1.27) |
.58 |
62.0 (9.4) |
>.99 |
| 50-59 |
97 |
104 |
0.95 (0.67-1.35) |
.78 |
64.8 (7.3) |
>.99 |
| Obese | ||||||
| Never |
212 |
225 |
1 [Reference] |
|
63.1 (11.1) |
|
| Age, yc | ||||||
| 14-19 |
20 |
10 |
1.83 (0.81-4.15) |
.15 |
57.3 (13.3) |
.28 |
| 20-29 |
35 |
19 |
2.17 (1.15-4.07) |
.02 |
56.0 (10.2) |
.003 |
| 30-39 |
49 |
26 |
2.31 (1.32-4.02) |
.003 |
58.3 (10.1) |
.06 |
| 40-49 |
81 |
58 |
1.47 (0.97-2.24) |
.07 |
60.3 (9.8) |
.71 |
| 50-59 | 64 | 83 | 0.68 (0.45-1.02) | .06 | 62.3 (5.9) | >.99 |
Abbreviations: CI, confidence interval; AOR, adjusted odds ratio.
Model included age, sex, race, smoking status, alcohol consumption, diabetes status, and family history of cancer.
Based on analysis of variance with Bonferroni comparison with the never overweight group.
Age when overweight or obesity started.
Association of BMI and Age of Disease Onset
In this study population, being overweight from the ages of 14 to 39 years and obese from the ages of 20 to 49 years were significantly associated with a younger age of pancreatic cancer diagnosis (Table 4). The median age of diagnosis (by mean BMI from the ages of 20-49 years) was 64 years for patients with normal weight, 61 years for overweight patients (P=.02), and 59 years for obese patients (P<.001). The mean difference in age of disease onset between overweight or obese patients compared with those with normal body weight remained statistically significant after adjusting for other factors associated with age of onset (eg, sex, diabetes, and alcohol consumption; Table 4). Smoking was not significantly associated with age of disease onset in the entire study population but was associated with a 3-year earlier cancer diagnosis in women. Among women, the median age of diagnosis was 64 years (interquartile range, 56-72 years) for never smokers and 61 years (interquartile range, 55-68 years) for ever smokers.
Table 4.
Associations Between Body Mass Index and Age of Onset of Pancreatic Cancera
| Age of Pancreatic Cancer Onset, y |
||||||
|---|---|---|---|---|---|---|
| Age Period | No. of Patientsb | Median (IQR)c | Mean (SD) | P Valued | Mean Difference (95% Cl)e | P Valued |
| 14-19 y | ||||||
| Normal weight |
579 |
63 (55 to 70) |
62.4 (10.1) |
|
|
|
| Overweight |
120 |
60 (54 to 66) |
59.7 (9.9) |
.004 |
–2.57 (–4.63 to –0.53) |
.01 |
| Obese |
20 |
56 (49 to 66) |
57.3 (13.3) |
.18 |
–5.37 (–9.88 to –0.86) |
.02 |
| 20-29 y | ||||||
| Normal weight |
511 |
63 (55 to 70) |
62.5 (10.4) |
|
|
|
| Overweight |
222 |
62 (54 to 67) |
60.9 (9.4) |
.006 |
–1.56 (–3.23 to 0.11) |
.07 |
| Obese |
49 |
59 (51 to 65) |
56.9 (10.7) |
<.001 |
–5.74 (–8.83 to –2.84) |
<.001 |
| 30-39 y | ||||||
| Normal weight |
417 |
64 (57 to 70) |
63.2 (10.0) |
|
|
|
| Overweight |
300 |
61 (54 to 68) |
61.3 (9.8) |
.01 |
–2.18 (–3.72 to –0.64) |
.006 |
| Obese |
96 |
59 (50 to 65) |
57.6 (9.9) |
<.001 |
–6.02 (–8.27 to –3.79) |
<.001 |
| 40-49 y | ||||||
| Normal weight |
310 |
65 (58 to 71) |
64.4 (9.3) |
|
|
|
| Overweight |
329 |
62 (55 to 68) |
62.2 (9.1) |
.004 |
–2.73 (–4.23 to –1.23) |
<.001 |
| Obese |
157 |
60 (53 to 66) |
60.1 (9.1) |
<.001 |
–5.03 (–6.88 to –3.20) |
<.001 |
| 50-59 y | ||||||
| Normal weight |
184 |
68 (61 to 73) |
67.2 (8.0) |
|
|
|
| Overweight |
313 |
65 (60 to 70) |
65.2 (7.4) |
.008 |
–2.26 (–3.68 to –0.83) |
.002 |
| Obese | 177 | 62 (58 to 68) | 63.3 (7.0) | <.001 | –4.25 (–5.88 to –2.60) | <.001 |
Abbreviations: CI, confidence interval; IQR, interquartile range.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
The numbers do not add up to totals because individuals with a body mass index of less than 18.5 were not included in the analyses.
The IQR is the 25th and 75th percentile.
Calculated by linear regression.
Estimated by linear regression with age as a dependent variable and body mass index (<25 vs ≥25 or <25 vs ≥30) as an independent variable with adjustment for sex and diabetes status.
When the association of age of obesity or overweight onset with the age of cancer diagnosis was examined, obesity from the ages of 20 to 29 years was significantly associated with a 7-year earlier age of cancer onset. The mean (SD) age of diagnosis was 63.1 (11.1) years for the never overweight group and 56.0 (10.2) years for those who were obese from the ages of 20 to 29 years (P=.003; Table 3).
Association of BMI and Overall Survival
Obesity among individuals from the ages of 30 to 79 years and within the year prior to recruitment in the study was significantly associated with reduced overall survival in patients with pancreatic cancer (Table 5). Obesity during the year prior to recruitment remained a significant predictor of shorter survival after adjusting for other clinical predictors. The association of obesity and overall survival was stronger among patients with resected tumors (hazard ratio [HR], 3.35 [95% CI, 1.50-7.49]; P=.003) than among those with unresected tumors (HR, 1.64 [95% CI, 1.15-2.33]; P = .006). Even for patients with metastatic disease, the association of obesity in the year prior to recruitment and overall survival was statistically significant (HR, 1.57 [95% CI, 1.03-2.40]; P=.04).
Table 5.
Body Mass Index at Different Age Periods and Overall Survival of Patients With Pancreatic Cancera
| No. of Patients | No. of Deaths | Survival, Median (IQR), mob | P Valuec | Adjusted HR (95% CI)d | P Value | |
|---|---|---|---|---|---|---|
| Age period, y | ||||||
| 14-19 to 20-29 | ||||||
| Normal weight |
415 |
241 |
14.7 (8.8-27.5) |
|
1 [Reference] |
|
| Overweight |
127 |
77 |
15.1 (7.5-26.4) |
.05 |
1.15 (0.85-1.56) |
.29 |
| Obese |
15 |
8 |
15.2 (9.8-23.8) |
.28 |
0.86 (0.38-1.96) |
.72 |
| 30-39 to 50-59 | ||||||
| Normal weight |
237 |
123 |
16.9 (9.8-30.1) |
|
1 [Reference] |
|
| Overweight |
252 |
155 |
13.9 (8.5-29.0) |
.11 |
1.19 (0.90-1.59) |
.22 |
| Obese |
117 |
73 |
13.5 (7.5-23.8) |
.02 |
1.75 (1.23-2.50) |
.002 |
| 60-69 to 70-79 | ||||||
| Normal weight |
83 |
43 |
16.5 (7.7-30.4) |
|
1 [Reference] |
|
| Overweight |
119 |
65 |
15.3 (8.3-18.6) |
.83 |
0.99 (0.63-1.58) |
.99 |
| Obese |
87 |
63 |
11.4 (7.6-16.8) |
.003 |
1.76 (1.09-2.84) |
.02 |
| All ages for year prior to recruitment | ||||||
| Normal weight |
191 |
101 |
18.2 (9.9-32.6) |
|
1 [Reference] |
|
| Overweight |
255 |
146 |
13.7 (8.1-28.0) |
.04 |
1.26 (0.94-1.69) |
.04 |
| Obese |
163 |
105 |
13.5 (8.4-19.7) |
<.001 |
1.86 (1.35-2.56) |
<.001 |
| Resected tumorse | ||||||
| Normal weight |
46 |
15 |
35.0 (30.2-39.9) |
|
1 [Reference] |
|
| Overweight |
59 |
19 |
32.3 (27.7-36.9) |
.52 |
1.44 (0.70-2.96) |
.32 |
| Obese |
33 |
17 |
24.6 (18.8-30.4) |
.006 |
3.35 (1.50-7.49) |
.003 |
| Unresected tumors | ||||||
| Normal weight |
145 |
86 |
14.0 (7.8-23.3) |
|
1 [Reference] |
|
| Overweight |
196 |
127 |
14.5 (6.3-17.8) |
.04 |
1.22 (0.88-1.69) |
.24 |
| Obese |
130 |
88 |
12.1 (7.2-16.7) |
.007 |
1.64 (1.15-2.33) |
.006 |
| Metastatic disease | ||||||
| Normal weight |
82 |
47 |
12.1 (7.0-22.9) |
|
1 [Reference] |
|
| Overweight |
133 |
85 |
11.5 (5.8-17.7) |
.16 |
1.05 (0.72-1.51) |
.81 |
| Obese | 71 | 50 | 8.8 (5.4-13.2) | .001 | 1.57 (1.03-2.40) | .04 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, interquartile range.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
The IQR is the 25th and 75th percentile.
Calculated using the log-rank test.
Model included tumor stage, tumor resection, diabetes status, and serum carbohydrate antigen 19-9 level at diagnosis (when appropriate).
Median survival time could not be calculated so mean and 95% CI is presented.
COMMENT
The current study found excess body weight (ie, a BMI ≥25 in early adulthood) to be more strongly associated with risk of pancreatic cancer than a subsequent increase in BMI. Also, being overweight or obese, particularly in early adulthood, resulted in a younger age at onset of pancreatic cancer. Furthermore, being obese at an older age or shortly before the cancer diagnosis was associated with a reduced overall survival time. These observations provide strong evidence supporting an important role of excess body weight in the development and progression of pancreatic cancer.
In most previously reported studies on obesity and pancreatic cancer risk, BMI was calculated based on either baseline body weight or usual adult body weight, with few studies focused on the body weight during different periods of adulthood.1-8,13-16 One of the major strengths of the current study is the detailed information on lifetime body weight changes, allowing examination of the association of BMI at different ages with the eventual development of the disease. Our data have shown the increasing frequencies of being overweight or obese with age and the significant association of pancreatic cancer risk with being overweight for individuals from the ages of 14 to 39 years and being obese from the ages of 20 to 49 years, independent of having diabetes. Notably, the strongest association between obesity and pancreatic cancer was seen in those who were overweight or obese from the ages of 30 to 39 years, especially in those who had a BMI increase of more than 5 units over that of BMI from the ages of 14 to 19 years. Even though the prevalence of overweight and obesity continued to increase until ages 70 to 79 years, the increased risk of pancreatic cancer with weight gain leveled off for gains coming after ages 40 to 49 years. Furthermore, when the starting age of overweight or obesity was considered, being overweight starting at ages 14 to 19 years or 20 to 29 years and being obese starting at ages 20 to 29 years or 30 to 39 years showed the strongest association with risk of pancreatic cancer. These observations have great public health implications because it implies that weight gain in young adults is associated with a greater risk of pancreatic cancer than in older adults. The inverse relationship of BMI within the year of cancer diagnosis with risk of pancreatic cancer was likely due to cancer-related weight loss among patients. However, the trend of decreasing risk after the ages of 40 to 49 years could not be explained by the same reason because BMI at the age period of the cancer diagnosis or recruitment to the study was excluded from the risk analysis.
In US adults aged 25 to 74 years, the rate of major weight gain over 10 years (BMI gain of 5 units) was highest at ages 25 to 34 years.17,18 Previous studies have shown that excess weight gain in early adulthood adversely affects the development of cardiovascular disease risk factors such as hypertension, dyslipidemia, and diabetes.19-21 Our observations suggest that excess weight gain in early adulthood may also be associated with increased risk for obesity-related cancers. The stronger association of the disease with weight gain in earlier adulthood as opposed to later adulthood might be explained by the longer duration of exposure to cumulative excessive body fat in the earlier gainers.22 Because few individuals who are overweight or obese at a younger age return to a normal weight later in life, it is unknown whether the risk of pancreatic cancer could be reduced by successful weight control during middle age. Thus, weight control at younger ages should be one of the primary strategies for the prevention of pancreatic cancer. Furthermore, previous inconsistent observations on the relationship of obesity and risk of pancreatic cancer could be partially explained by the variations in when and how BMI was measured. It is possible that the risk of pancreatic cancer could be underestimated when BMI was evaluated at age 50 years or older.
We observed a higher risk of obesity-associated pancreatic cancer in men than in women, which is consistent with previous findings from a number of cohort and case-control studies.6,7,15,23-27 Other sex-associated factors such as calorie intake, body fat distribution, and physical activity could modify the association between BMI and risk of pancreatic cancer. These factors need to be examined in future analyses to understand the observed sex difference. We also observed a greater attributable risk of pancreatic cancer in ever smokers (21.3%) with a mean BMI of 25 or greater at ages 14 to 59 years than in never smokers (10.3%). Body mass index and smoking are known independent risk factors for pancreatic cancer. It is conceivable that being overweight or obese confers a favorable environment for tumor development by providing growth-promoting hormones, cytokines, and metabolic changes. Rapid cell turnovers caused by tumor promoters would increase the chance of fixing DNA damage caused by tobacco carcinogens into gene mutations, a key step in tumorigenesis.
Previous studies on the age of pancreatic cancer onset were mostly conducted among familial cases, and smoking was the only modifiable risk factor that had been associated with younger age at onset.28,29 For the first time, to our knowledge, our study has demonstrated a striking linear inverse relationship between BMI and age at diagnosis of sporadic pancreatic cancer while controlling for other factors. This observation in pancreatic cancer mirrors the observations in type 2 diabetes in which obesity was found to be associated with a younger age of onset.21,30
Obesity has been associated with increased mortality from pancreatic cancer.1 In clinical investigations, higher BMI has been related to reductions in both overall and recurrence-free survival,31 increased risk for secondary tumor,32 and tumor recurrence or progression33 in various types of cancer. For pancreatic cancer, some studies have reported a higher frequency of complication after tumor resection among obese patients.34,35 However, the current study found a significant association of obesity and overall survival in patients with pancreatic cancer, independent of tumor stage or resection status. Because pancreatic cancer is such a rapidly fatal malignancy and most patients die of the cancer, the comorbidity associated with obesity, such as cardiovascular disease, could not explain the reduced overall survival rate.
Insulin resistance may serve as a common mechanism for the observed associations between obesity and increased risk, earlier onset, and reduced survival in patients with pancreatic cancer. In obesity, the adipose tissues act as an endocrine organ in regulating the release of free-fatty acids, cytokines, and hormones, which leads to the development of insulin resistance and compensatory chronic hyperinsulinemia. An increased insulin level and the resulting higher level of bioavailable insulin–like growth factor 1 could promote cellular proliferation and inhibit apoptosis, thus contributing to tumorigenesis.9,36 In addition to insulin and insulin-like growth factors, increased oxidative stress caused by hyperglycemia may initiate DNA damage pathways, and in turn, tumor initiation at younger age. The inflammatory responses regulated by adipocytokines and other growth hormones may enhance angiogenesis and cell adhesion, thus rapid tumor progression and metastasis.
The strengths of this study included the minimum bias for disease misclassification, the large number of patients, and the detailed information on lifetime BMI and clinical outcome. One weakness was the limited generalizability because the entire study population came from a single tertiary referral hospital, whose population was younger, included fewer minorities, and was better educated than the general population. Another concern was that information on body weight was self-reported, potentially allowing under-reporting or overreporting to occur. Even though previous studies have investigated the validity of self-reported past body weights and have found a high level of accuracy compared with measured weight,5,37-39 our study is still subject to the inherent recall bias associated with a case-control design. In addition, the uncontrolled confounding effect of physical activity, energy in-take, and other unknown factors could also bias the risk estimates. The controls in our study differ demographically (by age, race, and sex) from the patients. Even though all of these factors were adjusted for in the data analysis, the unknown confounding associated with these factors could not be considered. While our observations require confirmation, they provide support for a role of excess body weight in the development and progression of pancreatic cancer.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health grants CA98380 and CA101936 (SPORE P20) and a multidisciplinary research program grant from M. D. Anderson Cancer Center.
Role of the Sponsor: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, and approval of the manuscript.
Footnotes
Financial Disclosures: None reported.
REFERENCES
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Samanic C, Gridley WH, Chow J, Lubin RN, Hoover RN, Fraumeni JF., Jr Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Permert J, Hakansson N, Näslund I, Bergkvist L, Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93(11):1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93(9):1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity,obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286(8):921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 6.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health–AARP Diet and Health Cohort. Am J Epidemiol. 2008;167(5):586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 7.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large US cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(2):459–466. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer. 2007;120(9):1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 10.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102(12):2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; New York, NY: 2002. Exocrine pancreas. pp. 157–164. [Google Scholar]
- 12.Beine J. [May 18, 2009];The Social Security Death Index: a genealogy records guide. http://www.deathindexes.com/ssdi.html.
- 13.Berrington de Gonzalez A, Sweetland S, Spencer EA. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003;89(3):519–523. doi: 10.1038/sj.bjc.6601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberle CA, Bracci PM, Holly EA. Anthropometric factors and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control. 2005;16(10):1235–1244. doi: 10.1007/s10552-005-0354-y. [DOI] [PubMed] [Google Scholar]
- 15.Fryzek JP, Schenk M, Kinnard M, Greenson JK, Garabrant DH. The association of body mass index and pancreatic cancer in residents of southeastern Michigan, 1996–1999. Am J Epidemiol. 2005;162(3):222–228. doi: 10.1093/aje/kwi183. [DOI] [PubMed] [Google Scholar]
- 16.Silverman DT, Swanson CA, Gridley G, et al. Dietary and nutritional factors and pancreatic cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1998;90(22):1710–1719. doi: 10.1093/jnci/90.22.1710. [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 18.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 19.Berkey CS, Gardner J, Colditz GA. Blood pressure in adolescence and early adulthood related to obesity and birth size. Obes Res. 1998;6(3):187–195. doi: 10.1002/j.1550-8528.1998.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy, the CARDIA Study: Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271(22):1747–1751. [PubMed] [Google Scholar]
- 21.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84(2):427–433. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- 22.Michaud DS, Fuchs CS. Obesity and pancreatic cancer: overall evidence and latency period. Cancer Epidemiol Biomarkers Prev. 2005;14(11 pt 1):2678–2679. doi: 10.1158/1055-9965.EPI-05-0428. [DOI] [PubMed] [Google Scholar]
- 23.Berrington de González A, Spencer EA, Bueno-de-Mesquita HB, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15(5):879–885. doi: 10.1158/1055-9965.EPI-05-0800. [DOI] [PubMed] [Google Scholar]
- 24.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17(7):901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 25.Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control. 2007;18(2):165–175. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Kikuchi S, Tamakoshi A, et al. JACC Study Group Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer. 2007;120(12):2665–2671. doi: 10.1002/ijc.22614. [DOI] [PubMed] [Google Scholar]
- 27.Hanley AJ, Johnson KC, Villeneuve PJ, Mao Y, Canadian Cancer Registries Epidemiology Research Group Physical activity, anthropometric factors and risk of pancreatic cancer: results from the Canadian enhanced cancer surveillance system. Int J Cancer. 2001;94(1):140–147. doi: 10.1002/ijc.1446. [DOI] [PubMed] [Google Scholar]
- 28.McWilliams RR, Bamlet WR, Rabe KG, Olson JE, de Andrade M, Petersen GM. Association of family history of specific cancers with a younger age of onset of pancreatic adenocarcinoma. Clin Gastroenterol Hepatol. 2006;4(9):1143–1147. doi: 10.1016/j.cgh.2006.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James TA, Sheldon DG, Rajput A, et al. Risk factors associated with earlier age of onset in familial pancreatic carcinoma. Cancer. 2004;101(12):2722–2726. doi: 10.1002/cncr.20700. [DOI] [PubMed] [Google Scholar]
- 30.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24(9):1522–1527. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 31.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14(6):1718–1725. doi: 10.1158/1078-0432.CCR-07-1479. [DOI] [PubMed] [Google Scholar]
- 32.Efstathiou JA, Bae K, Shipley WU, et al. Obesity and mortality in men with locally advanced prostate cancer: analysis of RTOG 85-31. Cancer. 2007;110(12):2691–2699. doi: 10.1002/cncr.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SM, Lim MK, Jung KW, et al. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol. 2007;25(30):4835–4843. doi: 10.1200/JCO.2006.10.3416. [DOI] [PubMed] [Google Scholar]
- 34.Fleming JB, Gonzalez RJ, Petzel MQ, et al. Influence of obesity on cancer-related outcomes after pancreatectomy for pancreatic adenocarcinoma. Arch Surg. 2009;144(3):216–221. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 35.Noun R, Riachy E, Ghorra C, et al. The impact of obesity on surgical outcome after pancreaticoduodenectomy. J Pancreas (Online) 2008;9(4):468–476. [PubMed] [Google Scholar]
- 36.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132(6):2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 37.Stunkard AJ, Albaum JM. The accuracy of self-reported weights. Am J Clin Nutr. 1981;34(8):1593–1599. doi: 10.1093/ajcn/34.8.1593. [DOI] [PubMed] [Google Scholar]
- 38.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132(6):1156–1163. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 39.Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr. 1991;53(6):1493–1498. doi: 10.1093/ajcn/53.6.1493. [DOI] [PubMed] [Google Scholar]