Abstract
The role of epithelial-mesenchymal transition (EMT) in metastasis remains to be controversial. EMT has been postulated as an absolute requirement for tumor invasion and metastasis. Three different models including incomplete EMT, mesenchymal-epithelial transition (MET), and collective migration have been proposed for the role of EMT in cancer invasion and metastasis. However, skepticism remains as to whether EMT truly occurs during caner progression, and if it does, whether it plays an indispensible role in metastasis. Our recent findings suggest that EMT cells are responsible for degrading the surrounding matrix to enable invasion and intravasation of both EMT and non-EMT cells. Only non-EMT cell that have entered the blood stream are able to reestablish colonies in the secondary sites. Here we discuss an alternative model for the role of EMT in cancer metastasis in which EMT and non-EMT cells cooperate to complete the entire process of spontaneous metastasis.
Epithelial-mesenchymal transition (EMT) and cancer metastasis
EMT was first recognized as a central differentiation process in early embryogenic morphogenesis (1). It is a coordinated molecular and cellular change defined as a reduction in cell-cell adhesion, apical-basolateral polarity and epithelial markers as well as an acquisition of motility, spindle-cell shape, and mesenchymal markers. The definition of EMT in embryo development, which includes an ordered series of transcriptional events and a switch in cell fate, has not been strictly followed in cancer research where this term has been more liberally refereed as a recognizable change in cellular phenotype characterized as loss of cell junctions and gain of migratory behaviors (2).
This more inclusive EMT process has been proposed and supported by numerous publications to be a potent mechanism that enhances the detachment of cancer cells from primary tumors. However, it is still controversial as to whether transformation of a non-invasive tumor into a metastatic tumor truly represents an EMT, and if it is, how important it is in the process of cancer metastasis (3, 4). The main argument for the lack of a role of EMT in cancer is that metastases appear histopathologically similar to the primary tumors from which they are derived. To resolve this apparent contradiction, a mesenchymal-epithelial transition (MET) process in the metastatic sites has been postulated as part of the process of metastatic tumor formation (5). MET is an attractive hypothesis that can explain the histopathological similarity between primary and metastatic tumors. In support of the MET hypothesis, dynamic expression of E-cadherin (CDH1) in cancer progression has been documented. However, direct experimental data supporting MET in cancer metastasis is still lacking. For example, Graff et al demonstrated that the DNA methylation status of the CDH1 promoter varies at different stage of the metastatic process. In primary breast cancers, the tumor cells that underwent transient hypermethylation and repression of CDH1 are more invasive and metastatic. Subsequently, demethylation of the CDH1 promoter occurs and E-cadherin is re-expressed during metastases (6). Unfortunately, the clonal identities of these metastatic cancer cells have not been determined. There is still a lack of convincing experimental data to support the notion that MET is an integral part of metastasis. Dumont et al have also reported that de novo methylation during cancer progression has a deterministic rather than stochastic pattern (7). As such, the model of sequential EMT and MET has yet to be further tested. There is also strong evidence that tumor invasion can occur in the absence of EMT. For example, Wicki et al have shown that podoplanin promotes breast cancer cell invasion through downregulation of the activities of small Rho family GTPases, which results in remodeling of the actin cytoskeleton but does not alter the expression and localization of EMT markers (8).
On the other hand, tumors are highly diverse population of cells that exhibit a wide range of phenotypes from stem cell-like cells to well-differentiated cells (3). There are several lines of evidence suggesting that many invasive and metastatic carcinomas have not undergone a complete transition to a mesenchymal phenotype or even lack signs of EMT and that invasive carcinomas do not invade adjacent connective tissue as individual mesenchymal-like cells. These carcinoma cells invade as multicellular aggregates or clusters (9). Accordingly, a cell cooperativity theory has been proposed and experimentally proven to play an important role in caner metastasis (10). In view of the contention that cancer EMT is a result of reactivation of a normal embryonic development program, the cell cooperativity theory also has developmental root as such cooperation also occurs during normal development of embryos (11).
Similarly, a “class action” theory has been proposed in which successive waves of homogenous and heterogeneous circulating cancer cells form a premetastatic niche that promotes colonization of metastases (12). This premetastatic niche hypothesis is in agreement with the observation that cancers can remain dormant for years or even decades before overt progression and metastasis (13). It is also consistent with the report that EMT plays an important role in generation of cancer cells with stem cell-like characteristics (13).
EMT regulators
A variety of extracellular signals have been shown to trigger transition of epithelial cells into mesenchymal or mesenchymal-like cells during embryogenesis and in tumorigenesis. TGF-β, EGF family members, FGFs, HGF and IGFs have all been shown to induce EMT in an autocrine or paracrine manner (14). TGF-β was the first EMT inducer described in normal mammary epithelial cells by signaling through its receptor serine/threonine kinase complex. It remains to be the main and the best-characterized inducer of EMT phenotype in a variety of biological and pathophysiological conditions. TGF-β has an important tumor suppressor function at early stage of tumorigenesis by inducing apoptosis and cell cycle arrest. However, it acts as a positive modulator of tumor progression in the late phase of tumorigenesis. This tumor promotional function of TGF-β, which is consistent with its EMT-induction activity, plays an important role in tumor progression including invasion and metastasis. TGF-β-mediated signaling during EMT involves both gene expression-dependent and -independent pathways. The type II receptor of TGF-β, upon activation by TGF-β binding, interacts with occludin, a component of the tight junction, and phosporylates Par6 protein. This direct protein-protein interaction and the subsequent phosphorylation of Par6 protein recruits Smurf1 thereby leading to ubiquitin-dependent degradation of RhoA (15), a small GTPase family member responsible for the maintenance of apico-basal polarity and junctional stability. This degradation mechanism of RhoA by TGF-β results in loss of polarity of epithelial cells, one of the key events of EMT. Besides direct alteration of cell surface protein complex structure through activated receptor, TGF-β signaling also induces expression of Slug that inhibits gene expression of desmoplakin and plakoglobin, the two desmosomal plague proteins. This will lead to disassembly of desmosomes, a type of junction complexes specialized for cell-to-cell adhesion. The consequence of this series of events is the loss of epithelial cell adjoining and detachment of tumor cells from the primary tumor tissues.
TGF-β also induces the expression of E-cadherin (CDH1) repressors including E12/47, Twist 1, Twist 2, ZEB 1 (δEF 1 or AREB 6), ZEB 2 (SIP 1), Snail 1 (Snail), Snail 2 (Slug), KLF 8, FOXC 2, and Goosecoid (16). These transcriptional repressors bind to the E-boxes at the CDH1 promoter and repress E-cadherin expression. One of the hallmarks of EMT is the functional loss of E-cadherin that is essential for intercellular adhesion junctions.
EMT of HCPC-1 cells induced by p12CDK2-AP1
p12Cdk2ap1(p12, doc-1) was first identified and isolated from the hamster normal oral keratinocytes as a putative tumor suppressor gene by subtractive hybridization (17). p12 expression is induced by TGF-β (18) and serves as its down-stream effector to mediate the growth inhibitory activity of TGF-β by interacting with DNA polymerase α/primase and CDK2. In our recent study, we demonstrated that p12 also mediates TGF-β-induced EMT of hamster cheek pouch carcinoma-1 (HCPC-1) cells (19). Moreover, overexpression of p12 directly induces EMT of HCPC-1 cells (19), at least according to the more inclusive definition of EMT in cancer biology (2). Ectopic expression of p12 in HCPC-1 cells induced morphological change from polygonal to fibroblastoid structure, accompanied by a complete loss of E-cadherin and desmoplakin expression and the gain of vimentin and N-cadherin expression. Twist 2, an E-cadherin repressor, was upregulated in p12-transfected cells. Although the mechanism by which Twist 2 suppresses CDH1 expression is not clear, its function in p12-induced EMT was confirmed by morphological reversion of EMT cells after treatment with Twist 2 siRNA (19).
Twist proteins are essential for proper gastrulation, mesoderm formation and neural crest migration during development, which represents a typical EMT event. Twist 2 expression is detected in cranial mesenchymal cells around the nose and pharyngeal arches and tongue of mouse embryos, and is progressively restricted to the superficial regions in tongue and jaws during embryonic development (20), indicating its involvement in maintenance of mesenchymal lineage. Twist 2 is overexpressed in a large variety of human primary tumors and cancer cell lines. Ectopic expression of Twist 2 induces EMT of MDCK cells by repressing expression of E-cadherin, α-catenin, occludin and claudin-7, and promoting expression of vimentin and N-cadherin (21). Twist 2 knockout mice have severe growth retardation with atrophic dermis, thymus, liver and fat tissues (22). p12 knockout mice died during gestation but two survived mice have been shown to exhibit craniofacial defects with a short snout and a round forehead compared to wild type animals (23). These results indicate that p12 and Twist 2 are linked to EMT and to the maintenance of mesenchymal lineage during embryogenesis. Therefore, p12-induced, Twist 2-mediated EMT is not merely an isolated experimental phenomenon but has physiological and pathological relevance.
Cooperativity between EMT and non-EMT cells
Besides the overt morphological and molecular changes, overexpression of p12 also results in behavioral changes of HPCP-1 cells. The p12 transfectants have a clearly enhanced motility both in vitro and in vivo, and have acquired the ability to invade into the surrounding tissues from the primary tumors grown at subcutaneous regions. Both non-EMT (vector transfectants) and EMT (p12 transfectants) cells were able to establish ectopic tumors with a 100% tumor take rate and a similar growth curve when they were inoculated subcutaneously into athymic mice. However, only the EMT cells showed invasive fronts, penetrated into the surrounding muscle tissues, and intravasated into the blood vessels. EMT cells were detected inside the capillaries around the primary tumor site and in the blood stream. However, no non-EMT cells were detected inside the blood vessels or in the circulation, indicating that they either failed to invade into the surrounding tissues or failed to intravasate. It is thus clear that EMT of HCPC-1 cells enhances their invasive behaviors, consistent with numerous previous reports of the property of EMT cells. However, no metastasis was detected in both groups of the animals even when the primary tumors had grown to 20% of the body weight, at which time the animals had to be sacrificed.
Metastasis of carcinoma is a complex process including detachment of tumor cells from primary tumors, invasion through the basement membranes and the local mesenchymal tissues, intravasation into blood or lymphatic vessels, survival in the circulation and from immune clearance, lodging and extravasation in distant organs, and proliferation at the secondary sites. It is possible to model various stage of the metastatic cascade by varying the site of inoculation of cancer cells. The growth and development of metastases from the orthotopic or subcutaneous site requires tumor cells to complete the entire metastatic process. Intravenous injection can bypass the initial stages of metastasis and measures the ability of the cells to survive in the blood circulation, extravasation, and formation of metastatic deposits at a secondary site. Surprisingly, EMT HCPC-1 cells failed to establish lung metastases even when they were directly inoculated into the blood stream by tail vein injection. However, overt lung metastases formed from non-EMT cells when they were injected via tail vein. These results indicate that p12-induced EMT is accompanied with a decreased ability to establish metastatic tumors in the lung although they have enhanced migratory and local invasive phenotypes. This is somewhat in contrast to the main stream theory that EMT cells have increased ability of metastasis.
Re-expression of E-cadherin by transfection of a CMV promoter-controlled CDH1 cDNA changed the morphology of EMT cells from the fibroblastoid structure back to polygonal ones. Therefore, MET did occur from the viewpoint of cell morphology. However, E-cadherin re-expressed MET cells also failed to establish lung metastasis when they were directly injected into the tail vein of athymic mice. It is notable that the ability to establish and to grow xenograft tumors in the subcutaneous region was not different among the non-EMT, EMT, and MET cells.
More surprisingly, lung metastases formed when a mixture of non-EMT and EMT cells were co-inoculated subcutaneously. In this experiment, non-EMT and EMT cells were labeled with DsRed and GFP, respectively, so that the origin of the metastatic tumors could be determined by fluorescence and IHC. In the primary tumors, non-EMT and EMT cells coexisted with an enrichment of non-EMT cells in the center and EMT cells in the edges. The edges of the primary tumors had invasive appearances. They invaded into the muscle and fat tissues, in a way very similar to the tumors derived from EMT cells alone. Importantly, both EMT and non-EMT cells were detected in the blood stream, indicating that intravasation of both cell types occurred under this circumstance. Since inoculation of non-EMT cells alone failed to intravasate, this result demonstrated a cooperation between non-EMT and EMT cells in the process of local invasion and intravasation, adding another line of evidence to the cell cooperativity theory in cancer metastasis (10). The metastatic tumors in the lung were entirely composed of non-EMT cells. No EMT cells were detected in the lung tissue either by IHC for GFP protein or by PCR for its DNA. Since both cell types were detected in the blood stream with a similar half life, the likely reason for the failure of EMT cells to establish metastasis may be related to their inability to adhere to the lung vasculature or to extravasate, rather than a difference in escaping immune clearance. These results demonstrate that at least in HCPC-1 cells, p12-induced EMT is not sufficient for metastasis, and cooperation of non-EMT cells is necessary.
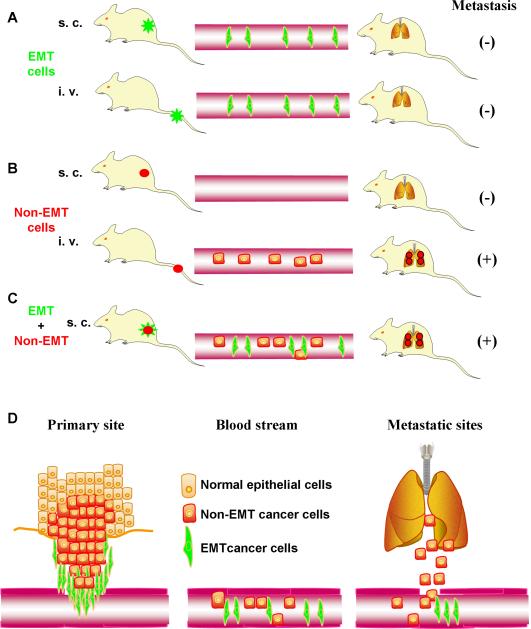
These findings are summarized in Fig. 1. Cancer cells with an EMT phenotype can invade into adjacent connective tissues, and intravasaste. But they are unable to form metastatic nodules in the lung even when they are directly injected into the blood circulation (Fig. 1A). Cancer cells without EMT phenotype cannot invade into adjacent connective tissue but they have the ability to form metastatic nodules in the lung when they are intravenously injected (Fig.1B). More importantly, cancer cells with a mixed EMT and non-EMT phenotype can complete the entire process of spontaneous metastasis (Fig. 1C). Based on these findings, an additional model can be proposed for the role of EMT in cancer metastasis (Fig. 1D). EMT and non-EMT cells cooperate to complete the spontaneous metastasis process. EMT cells, with enhanced migratory and invasive phenotype, are responsible for degrading the surrounding matrix and penetrating into the local tissues and blood or lymphatic vessels thereby leading the way to intravasation. Non-EMT cells, migrated either together with EMT cells, or immediately following them to enter the blood or lymphatic streams. Both cell types survive in the circulation but EMT cells fail to lodge to the vessel way at the secondary site, probably due to its reduced adhesive properties. Circulating non-EMT cells, with an unaltered adhesive phenotype, will be able to attach to the vessel wall, extravasate, and reestablish colonies in the secondary sites.
Fig 1. A novel model for the role of EMT in cancer progression and metastasis.
A, EMT invade into the blood stream but do not establish lung metastasis. B, Non-EMT cells do not invade but establish lung metastasis once it gets into the blood stream. C, Cooperation between non-EMT and EMT cells complete the entire metastatic process. D, Model of cell cooperativity. Cancer cells with an EMT phenotype invade into the surrounding tissues, enable non-EMT cells to migrate and intravasate so that both cell types enter the circulation. However, only non-EMT cells are able to regrow in the distant organs and establish metastasis.
Although the significance and relevance of this model in cancer therapy are not clear at present, these findings support the cell coperativity as well as the premetastasis niche theories, and suggest that a linear conversion of cancer cells through the successive EMT and MET processes for establishing metastasis may not be entirely correct.
Acknowledgments
Grant support: NIH grant CA10044 (T. Tsuji), CA10524 (G.-f. Hu), and the Alexander and Margaret Stewart Trust (G.-f. Hu).
References
- 1.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Cowin P, Welch DR. Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia. 2007;12:99–102. doi: 10.1007/s10911-007-9041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 4.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion -1. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG. Methylation patterns of the E-cadherin 5' CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem. 2000;275:2727–32. doi: 10.1074/jbc.275.4.2727. [DOI] [PubMed] [Google Scholar]
- 7.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A. 2008;105:14867–72. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–72. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 10.Lyons JG, Lobo E, Martorana AM, Myerscough MR. Clonal diversity in carcinomas: its implications for tumour progression and the contribution made to it by epithelial-mesenchymal transitions. Clin Exp Metastasis. 2008;25:665–77. doi: 10.1007/s10585-007-9134-2. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MS, D'Amico LA. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol. 1996;180:184–98. doi: 10.1006/dbio.1996.0294. [DOI] [PubMed] [Google Scholar]
- 12.Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 14.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–9. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 16.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 17.Todd R, McBride J, Tsuji T, Donoff RB, Nagai M, Chou MY, Chiang T, Wong DT. Deleted in oral cancer-1 (doc-1), a novel oral tumor suppressor gene. FASEB J. 1995;9:1362–70. doi: 10.1096/fasebj.9.13.7557027. [DOI] [PubMed] [Google Scholar]
- 18.Hu MG, Hu GF, Kim Y, Tsuji T, McBride J, Hinds P, Wong DT. Role of p12(CDK2-AP1) in transforming growth factor-beta1-mediated growth suppression. Cancer Res. 2004;64:490–9. doi: 10.1158/0008-5472.can-03-2284. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–86. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 21.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–80. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, McBride J, Kimlin L, Pae EK, Deshpande A, Wong DT. Targeted inactivation of p12, CDK2 associating protein 1, leads to early embryonic lethality. PLoS ONE. 2009;4:e4518. doi: 10.1371/journal.pone.0004518. [DOI] [PMC free article] [PubMed] [Google Scholar]