Introduction
Dimethacrylate monomers of various types are commonly used to form the matrix phase of dental composite materials. Traditional dental composites are reinforced with a filler phase consisting of silanized glass particles of various sizes and shapes. Polymerization of these acrylic resin composites is usually effected by free radical photopolymerization via visible light irradiation. These resin based composites are subject to two significant problems that arise from the rapid photopolymerization of the resin matrix phase: polymerization shrinkage (PS) and stress development (SD) in the polymeric composite, which are related to the chemical structure of matrix resin and its degree of conversion (DC) on photopolymerization.
Recent research in our laboratories has focused on similar problems in bioactive resin-based composites that utilize a unique calcium phosphate, amorphous calcium phosphate, ACP, as the filler phase rather than the conventional silanized glass fillers (1). This type of composite is capable of providing a sustained release of calcium and phosphate ions in aqueous milieus such as exists in the oral cavity (2). Significantly, this type of composite has the potential for repairing and preserving contiguous mineralized tissues such as enamel and dentin (3). While there have been some promising improvements recently (3–6) with regard to mechanical properties, the less than optimal PS and SD, along with DC are still considered shortcomings of these as well as conventional glass-filled composites.
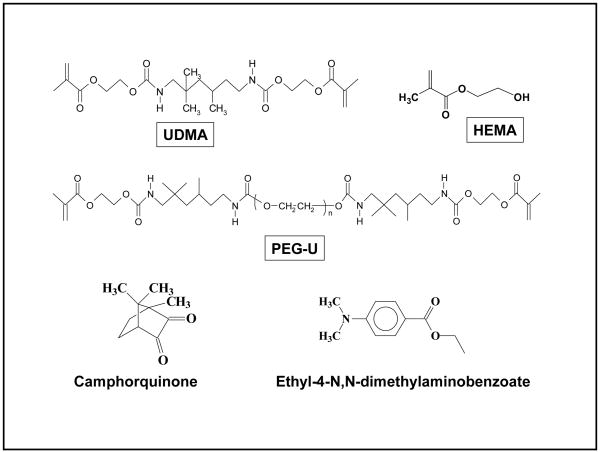
This study was designed to determine the effects of a new high molecular mass oligomeric urethane dimethacrylate co-monomer (PEG-U) on PS, SD and DC. In the past, urethane dimethacrylate (UDMA), a commonly used dental monomer, has been blended with 2-hydroxyethyl methacrylate (HEMA) in formulating ACP composites, see Fig. 1 for structures of UDMA, HEMA and PEG-U. Specifically, the aim of this study was to assess the effects of the UDMA resin matrix composition on DC, PS, SD and the biaxial flexure strength (BFS) of ACP composites when PEG-U was used in place of HEMA.
Fig. 1.
Chemical structure of monomers and photo-initiator system.
Experimental
Formulation of the resins
Four types of experimental resins, photoactivated with camphorquinone and ethyl-4-N, N-dimethylaminobenzoate were formulated from the commercially available UDMA, PEG-U and HEMA (Table 1). Chemical structures of the monomers and the components of the photoinitiatior system are shown in Fig. 1.
Table 1.
Composition of resins (% mass fraction; * based on the equivalent molar concentration of UDMA comonomer).
| Resin/monomer | UDMA | PEG-U | HEMA |
|---|---|---|---|
| UDMA | 99.00 | - | - |
| PEG-U | - | 99.00 | - |
| UDMA/PEG-U | 74.25 | 24.75 | - |
| UDMA/HEMA | 92.00 | - | 7.00* |
Synthesis and characterization of ACP filler
Zirconia-hybridized ACP (Zr-ACP) was synthesized as previously described (3). Its amorphous state was verified by powder X-ray diffraction (XRD: Rigaku X-ray diffractometer, Rigaku/USA Inc., Danvers, MA, USA) and Fourier-transform spectroscopy (FTIR: Nicolet Magna-IR FTIR System 550 spectrophotometer, Nicolet Instrument Corporation, Madison, WI, USA) before the ACP was utilized to make the composite specimens. Morphological/topological features of Zr-ACP were examined by scanning electron microscopy (SEM; JEOL 35C instrument, JEOL Inc., Peabody, MA, USA). Particle size distribution (PSD) of the filler (dispersed in isopropanol and ultrasonicated for 10 min) was determined by gravitational and centrifugal sedimentation analyzer (SA-CP3 model; Shimadzu Scientific Instruments, Inc., Columbia, MD, USA).
Preparation of the composites
Composite pastes were made from mixing the resins (60 % mass fraction) and Zr-ACP filler (40 % mass fraction) by hand spatulation. The homogenized pastes were kept under a moderate vacuum (2.7 kPa) overnight to eliminate the air entrained during mixing. For the BFS and the near infrared (NIR) DC measurements, the pastes were molded into disks by filling the circular openings of flat Teflon molds, covering each side of the mold with a Mylar film plus a glass slide, and then clamping the assembly together with spring clips. The disks were photo-polymerized by irradiating sequentially each face of the mold assembly for 120 s with visible light (Triad 2000, Dentsply International, York, PA, USA).
Evaluation of composites
The BFS measurements were performed on dry cured specimens [diameter: (15.1 ± 0.2) mm; thickness: (1.4 ± 0.1) mm; stored in air at 23 °C for 24 h)] by using a computer-controlled Universal Testing Machine (Instron 5500R, Instron Corp., Canton, MA, USA; crosshead speed: 0.5 mm/min) operated by Testworks4 software. BFS values were calculated according to ASTM F394-78 (7).
Near-infrared (NIR) spectroscopy was utilized to determine DC of composites by monitoring the reduction in the 6165 cm−1 methacrylate=CH2 absorption band (8). NIR spectra were acquired before photo-cure, immediately after and at 24 h post-cure. Triplicate measurements were performed for each experimental group. Use of an internal reference was not required, provided that the thicknesses of uncured (monomer) and cured specimen (polymer) have been measured. DC was calculated from the decrease in integrated peak area/sample thickness values between the polymer and monomer.
The PS of composite specimens was measured by a computer-controlled mercury dilatometer (PRC-ADAF, Gaithersburg, MD, USA) (9). Composite pastes are irradiated twice, initially for 60 s followed by a second irradiation of 30 s after 60 min. Data were acquired for a total of 90 min. A minimum of triplicate runs were performed for each experimental group. The PS (vol %) was calculated based on the known mass of the sample [(100 ± 10) mg)] and its density. The latter was determined by means of the Archimedean displacement principle using an attachment to a microbalance (Sartorius YDK01 Density Determination Kit; Sartorius AG, Goettingen, Germany).
The SD of the composites was determined by utilizing a computer-interfaced tensometer developed by PRC-ADAF (10). The deflection of the cantilever beam was measured with a linear variable differential transformer, the tensile force was calculated from a calibration constant (3.9 N/μm) and a beam length (12.5 cm), and the shrinkage stress obtained by dividing the measured tensile force with the cross sectional area of the sample.
Experimental data were analyzed by ANOVA (α=0.05). Significant differences between experimental groups were determined by all-pair-wise multiple comparisons (two-tail t-test; unequal variances). One standard deviation is taken as a measure of standard uncertainty of the measurements.
Results and Discussion
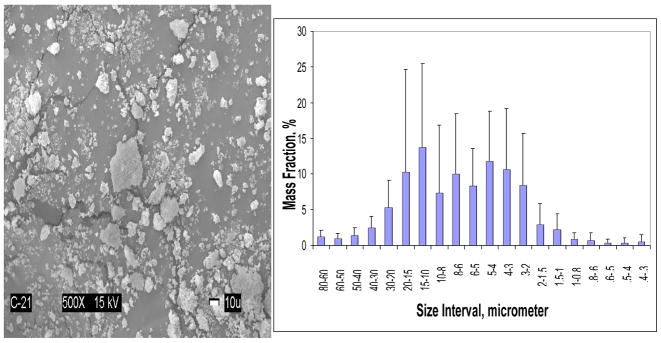
As-made Zr-ACP utilized in the study had heterogeneous PSD (Fig. 2) with the median diameter dm = (5.9 ± 0.7) μm and the specific surface area, SSA = (0.5 ± 0.1) m2/g. The results of the physicochemical evaluation of the composites formulated such ACP and different resin matrices are summarized in Table 2.
Fig. 2.
SEM image (left) and PSD (right) of Zr-ACP filler utilized in the study. Error bars indicate one standard deviation.
Table 2.
Mean values of DC, PS, SD and the BFS of Zr-ACP UDMA, PEG-U, UDMA/PEG-U and UDMA/HEMA composites. One standard deviation of the measurement is given in parentheses. Number of experiments/group, n ≥3.
| Parameter | UDMA | PEG-U | UDMA/PEG-U | UDMA/HEMA |
|---|---|---|---|---|
| DC (%) imm. 24 h |
62.0 (3.4) 68.3 (1.6) |
94.6 (0.6) 96.3 (0.6) |
74.5 (0.8) 79.7 (0.9) |
72.7 (0.6) 76.0 (0.3) |
| PS (% vol) | 5.3 (0.3) | 5.1(0.3) | 4.4 (0.4) | 4.2 (0.2) |
| SD (MPa) | 3.3 (0.3) | 3.3 (0.1) | 3.4 (0.1) | 4.5 (0.1) |
| BFS (MPa) | 76.0 (6.0) | 40.0 (1.9) | 65.4 (16.9) | 75.6 (14.3) |
The DC of composite resins decreased in the following order: PEG-U > UDMA/PEG-U > UDMA/HEMA > UDMA. With binary resin matrices, higher levels of DC attained in PEG-U containing matrix compared to the HEMA-containing matrix were statistically significant. Introducing the PEG-U into resin formulation appears desirable in order to attain the high DC and, in turn, maintain high biocompatibility of the material. These enhanced DC levels obtained in PEG-U matrices are attributed to its long, flexible structure between the vinyl groups. UDMA/HEMA also had an enhanced DC compared to the unblended UDMA matrix. This is because HEMA is a monofunctional monomer and would be expected to enhance DC through copolymerization with UDMA without contributing pendant vinyl groups as can occur with difunctional comonomers.
The level of shrinkage observed in UDMA and PEG-U composites was higher than the PS of UDMA/PEG-U or UDMA/HEMA composites but for different reasons. UDMA has a lower relative molecular mass than PEG-U and also has a much lower DC than PEG-U. PS is indirectly related to relative molecular mass and directly to DC. There was no difference in the level of shrinkage stress that developed in UDMA, PEG-U and UDMA/PEG-U matrices. However, higher SD values were obtained in UDMA/HEMA composites. The higher DC attained in UDMA/PEG-U specimens would imply that this material would have a higher SD. The fact that the SD was lower for the UDMA/PEG-U composition is probably due to the higher relative molecular mass and the more flexible nature of PEG-U oligomer compared to less flexible poly(HEMA) segments in the matrix.
The dry BFS values of all UDMA-based composites ranged between 65.4 MPa and 76.0 MPa; the difference between the UDMA/PEG-U and UDMA/HEMA composites was not statistically significant.
Conclusions
Introducing PEG-U into UDMA based matrices improves vinyl conversion (and presumably biocompatibility) while not adversely affecting the polymerization shrinkage, stress development and mechanical strength of their ACP composites. Less polymerization stress of PEG-U formulations and the expected improved biocompatibility of such materials may lead to better clinical performance.
Acknowledgments
This work was supported by the National Institute of Standards and Technology, Food and Drug Administration, American Dental Association Foundation, National Institute of Health/National Institute of Standards and Technology Interagency Agreement Y1-DE-1021-04 and the grant DE13169-06 from the National Institute of Dental and Craniofacial Research. We gratefully acknowledge donation of the monomers from Esstech, Essington, PA.
Footnotes
Disclaimer: Certain commercial materials and equipment are identified in this article to specify the experimental procedure. In no instance does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, Food and Drug Administration or American Dental Association Foundation or that the material or equipment identified is necessarily the best available for the purpose.
References
- 1.Eanes ED. Calcium Phosphates in Biological and Industrial Systems. 1998. pp. 21–39. [Google Scholar]
- 2.Skrtic D, et al. J Dent Res. 1996;79(5):1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 3.Skrtic D, et al. J Res Natl Inst Stand Technol. 2003;108:167–182. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skrtic D, et al. Biomaterials. 2004;25:11411–1140. [Google Scholar]
- 5.Antonucci JM, Skrtic D. J Bioact Comp Polym. 2005;20:29–49. [Google Scholar]
- 6.Lee SY, et al. J Biomed Mater Res. 2005 in press. [Google Scholar]
- 7.ASTM F394-78, re-approved 1991.
- 8.Stansbury JW, Dickens S. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 9.Reed B, et al. J Dent Res. 1996;75:290. [Google Scholar]
- 10.Lu H, et al. Mater Sci Mater Med. 2004;15:1097–1103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]