Abstract
Objectives
Previous research among HIV-infected individuals suggests that spiritual well-being is inversely related to psychological distress and rates of disease progression. Use of amantram, a spiritual word or phrase repeated frequently and silently throughout the day, has been associated with decreased psychological distress and increased spiritual well-being. This study compared the effects of 2 interventions — aspiritually-based mantram intervention versus anattention-matched controlgroup — on faith/assurance and average salivary cortisol levels among HIV-infected individuals.
Methods
Using arandomized design, HIV-infected adults were assigned to the intervention (n=36) or control condition (n=35). Faith scores and saliva (collected at 7 a.m., 11 a.m.,4 p.m., and 9 p.m.) were assessed at preintervention, postintervention, and 5-week follow-up. Path analyses tested competing models that specify both concurrent and sequential relationships between faith and average daily cortisol levels while comparing groups.
Results
Faithlevels increased among mantram participants from pre- to post intervention. Greater faithat preintervention was significantly associated with lower average cortisol at postintervention in the mantram group but not in the controls. The associations between faith at postintervention and cortisol levels at 5-week follow-up were significant among both groups but weaker than the pre- to postintervention association identified in the mantram group.
Conclusions
These results suggest the presence of lagged or antecedent consequent relationships between faith and cortisol, which may be enhanced by mantram use. Decreased cortisol could potentially benefit immune functioning among HIV-infected individuals.
Keywords: Complementary therapies, Cortisol, HIV/AIDS, Meditation, Mind-body relaxation technique, Psychoneuroimmunology, Spirituality
INTRODUCTION
Human Immunodeficiency Virus (HIV) disease is marked by a progressive loss of immune functioning, leading to opportunistic infections. Elevated levels of cortisol, a marker of stress-related neuroendocrine activity with substantial immunosuppressive and modulatory capabilities, can deleteriously affect immune system functioning among HIV-infected individuals [1]. For example, cortisol secretion can stimulate HIV viral replication [2] and affect various immunologic measures pertinent to HIV pathogenesis and disease progression [3–7].
With the implementation of highly active antiretroviral therapy (HAART), HIV infection has become a chronic condition that can be managed with rigorous attention to medication adherence and healthy lifestyle patterns [8, 9]. Research suggests that individuals who respond to the stress of living with HIV by employing maladaptive coping strategies (e.g., avoidance coping, anxious rumination, hopelessness, or substance abuse) [10, 11] are more likely to experience increased symptoms of psychological distress [11–13], poorer medication adherence [14], and higher viral load [14]. Previous research suggests that psychological distress indices may be associated with subsequent changes in biomarkers of HIV health [15]. Conversely, there is growing evidence to support a direct relationship between spiritual or religious coping and decreased levels of stress [16, 17] and depression [18], as well as slower disease progression in HIV [19]. Moreover, a review of the existing evidence for the impact of psychosocial factors on HIV progression suggests that it may take some time for psychosocial distress (or, potentially, interventions that counteract distress) to affect biology [20]. This implies a need for interventions to examine not only cross-sectional associations between psychosocial factors and biological outcomes, but also time-lagged effects.
Religion and spirituality may affect health and well-being in HIV [21–23]. Religion is defined as an organized system of beliefs and practices to enhance one's relationship to the sacred in the midst of a structured community [21], whereas spirituality is considered less formal and is based on a more “personal search for the sacred” [24 p. 35]. Spirituality is viewed as distinct from religion [25] and has been shown to moderate the effects of stress, regardless of religious affiliation [26]. People living with HIV often identify themselves more as “spiritual” than “religious,” [27] and spiritually-based coping strategies have been particularly beneficial to many HIV-infected individuals in reducing psychological distress [4, 19, 22, 28–39]. As measures of spirituality become more refined, there is a growing interest in assessing the efficacy of spiritual interventions to improve health status of people with HIV [40–42].
Mantram repetition is an ancient traditional practice of repeating a spiritual word or phrase, sometimes called a holy name [43, 44], continuously throughout the day [45–47]. It has been associated with decreased symptoms of psychological distress and increased quality of life and spiritual well-being in veterans and healthcare employees [43],[48–50]. In a randomized clinical trial previously conducted, HIV-infected adults who completed a 5-week mantram intervention program reported significant increases in spiritual faith/assurance and decreases in trait-anger compared to an attention-matched educational control condition [40]. Perceived stress and depression were measured but no group differences were found [40]. Practicing mantram repetition frequently throughout the day and integrating it into one's lifestyle appeared to enhance spiritual faith by increasing awareness of, or a sense of connectedness with, the sacred [43, 44, 51]. For certain patients, mantram repetition may be easier to implement as compared to types of meditation or relaxation techniques that require a quiet environment, closed eyes, particular postures, or lengthy sitting periods. HIV research has demonstrated that people who report experiencing a positive relationship to God or a higher power tend to have better health status and greater longevity, whereas people who feel judged or punished by God tend to have worse health status [52]. If the spiritual components of an intervention are the key efficacious elements, then one would expect to see a direct relationship between measures of spiritual faith and relevant health outcomes, including biomarkers of immune function.
The primary aim of this paper was to compare the effects of a spiritually-based mantram intervention with those of an attention-matched educational control intervention on average daily levels of salivary cortisol among HIV-infected adults. A dataset from prior research was used for this analysis which also included salivary cortisol samples. Because the prior study demonstrated that mantram repetition increased faith/assurance (40) and having a more positive view of God predicts better immune function over time (19, 52), we chose to explore relationships between levels of faith and salivary cortisol over time in two groups. We hypothesized there would be an inverse relationship between faith and cortisol levels at the same or subsequent time-point, and that the magnitude of this relationship would be stronger in the mantram intervention group as compared to the education control group. To establish the time-course of the relationships, we evaluated competing path analytical models.
METHODS AND MATERIALS
Procedure
This study was approved by the University of California, San Diego Human Subjects Protections Program and the Veterans Affairs (VA) San Diego Healthcare System Research and Development Committee. Participants from the community were recruited from February 2003 through December 2004 using flyers and provider referrals. Inclusion criteria were (1) literate in English (2) diagnosed with HIV ≥6 months, (3) from 18 to 65 years old, and (4) clean and sober from drug/alcohol abuse for ≥6 months. Exclusion criteria consisted of (1) cognitive impairment with a score of ≤25 on the Mini Mental Status Exam [53], (2) a medical condition or use of medications that affect cortisol levels; (3) bereavement in past 3 months; (4) learning a new alternative/complementary practice in past 3 months; (5) current practice of other forms of mantram repetition; and (6) acute infection or a recent change in highly active anti-retroviral therapy (HAART) regimen.
Sample size was based on a power analysis indicating that a sample of 35 participants per group was necessary to detect a medium sized effect. To assure that groups had equivalent health status, participants were block randomized by project coordinator for HAART (yes/no) and T-helper or CD4 cell counts (<200 or ≥200) using computer generated numbers and concealed envelopes. Participants were blinded to assignment, but group facilitators could not be.
Ninety-three participants were enrolled. Prior to the first group meetings and baseline cortisol collection, 7 dropped from the intervention and 9 from the control group due to sickness (n=3), schedule changes (n=3), or no shows (n=10). After the groups began, 2 from each group were lost to follow-up and 1 from each group had unusable cortisol samples, leaving 36 in the intervention and 35 in the control group for the present analysis.
Participants
The study included 71 participants, 57 (80%) men and 14 (20%) women, with a mean age of 43.5±6.77 years (range, 29–51 years). Using chi-square and t-tests, there were no significant differences between groups on demographics or health status at pre-intervention except for years living with HIV (see Table 1). The control group averaged 11.3±6.45 years living with HIV, compared to the intervention group that averaged 8.26±4.98 years (t(1.69)=2.24, p=.028). There was a trend for individuals in the intervention group to report higher levels of employment, with 47% of the intervention participants, as compared to 20% of controls, working more than 20 hours/week (χ2[2]=5.96, p=.051). Groups were equivalent for the remaining variables including health status measured by CD4 counts, faith/assurance and frequency of religious practice.
Table 1.
Demographic Characteristics by Group at Baseline (N = 71)
| Variable (Range) | Mantram (n=36) X (SD) | Control (n=35) X (SD) | Both (N=71) X (SD) | t-test (df) | p |
|---|---|---|---|---|---|
| Age (29–57) | 43.3 (7.13) | 43.9 (6.46) | 43.5 (6.77) | 0.38 (69) | .70 |
| Years with HIV (0.5–24) | 8.3 (4.98) | 11.3 (6.45) | 9.7 (5.91) | 2.24 (69) | .03 |
| CD4 (8–1,269) | 540.3 (320.00) | 433.8 (215.00) | 487.8 (276.64) | −1.64 (69) | .11 |
| n (%) | n (%) | n (%) | χ2(df) | p | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 28 (77.8) | 29 (82.9) | 57 (80.0) | .003 (1) | .96 |
| Female | 8 (22.2) | 6 (17.1) | 14 (20.0) | ||
| Ethnicity (χ2 white/non-white) | |||||
| White | 20 (55.6) | 21 (60.0) | 41 (57.7) | .27 (1) | .61 |
| Black | 11(30.5) | 7 (20.0) | 18 (25.4) | ||
| Hispanic | 5 (13.9) | 6 (17.1) | 11 (15.5) | ||
| American Indian/Alaskan | 0 (0.0) | 1 (2.9) | 1 (1.4) | ||
| Marital Status | |||||
| Never married/partnered | 21 (58.3) | 26 (74.3) | 35 (49.3) | 2.01 (1) | .16 |
| Ever married/partnered | 15 (41.7) | 9 (25.7) | 36 (50.7) | ||
| Education | |||||
| High school or less | 9 (25.0) | 13 (37.1) | 22 (30.9) | 2.45 (2) | .29 |
| Some college/no degree | 14 (38.9) | 14 (40.0) | 28 (39.4) | ||
| College degree or higher | 13 (36.1) | 8 (22.9) | 21 (29.7) | ||
| Employment | |||||
| none | 11 (30.6) | 15 (42.9) | 26 (36.6) | 5.96 (2) | .06 |
| <20 hrs/wk | 8 (22.2) | 13 (37.1) | 21 (29.6) | ||
| ≥20 hrs/wk | 17 (47.2) | 7 (20.0) | 24 (33.8) | ||
| Frequency of Religious Practice | |||||
| Not Religious | 14 (38.8) | 15 (42.8) | 29 (40.8) | .03 (2) | .99 |
| Infrequently | 7 (19.4) | 6 (17.1) | 13 (18.4) | ||
| Frequently | 15 (41.8) | 14 (40.1) | 29 (40.8) | ||
| CD4 | |||||
| <200 | 6 (16.7) | 5 (14.3) | 11 (15.5) | .08 (1) | .79 |
| ≥200 | 30 (83.3) | 30 (83.7) | 60 (84.5) | ||
| HIV Viral Load | |||||
| ≤400 | 24 (66.7) | 17 (48.6) | 41 (57.7) | .12 (1) | .10 |
| >400 | 12 (33.3) | 18 (51.4) | 30 (42.3) | ||
| HAART* | |||||
| Yes | 33 (71.7) | 32 (68.1) | 35 (49.2) | .15 (1) | .70 |
HAART=Highly Active Anti-Retroviral Therapy
Intervention and Control Groups
The time frame of both the intervention and control groups was the same: 5 weekly face-to-face classes (90 minutes/week) followed by 4 weekly phone calls and a follow-up meeting at Week 10. Both groups were facilitated by the same two master's-prepared psychiatric nurses.
The manualized mantram intervention consisted of skills training on how to choose and regularly use a mantram to manage stressors. Participants were taught by lecture, discussion, and homework assignments. They were given The Mantram Handbook [46] with weekly readings and a list of recommended mantrams to choose from. Participants were encouraged to practice repeating the mantram as much as possible, especially during times that were not particularly stressful, such as before sleep or while waiting in lines. As such, mantram repetition would be associated with a state of physiological calmness, and later, during stressful times, could be implemented to interrupt the stress response. Other strategies taught included “one-pointed attention,” the practice of increasing one's ability to focus on the mantram and engage in only one task at a time. Another concept, “slowing down” both mentally and behaviorally, was taught as a means of making wiser choices, setting priorities, decreasing stress from hurried behavior, and allowing time to employ the mantram in stressful moments in order to decrease reactivity. A more detailed description of the intervention can be found in Bormann and colleagues [40, 43, 45].
The control group watched educational videotapes on HIV medications, treatment issues, wasting syndrome, and nutrition. Following each video, the co-facilitators led group discussions related to the video and avoided teaching or reinforcing any stress management or coping skills.
Quality Control
To assure that groups were delivered in a standardized way, mantram group instructors followed written guidelines, and researchers collected audio-taped recordings of both groups at each meeting. These tapes were reviewed by an expert psychiatric nurse who was familiar with the content of both groups. Inconsistencies were noted, and group leaders were contacted immediately to correct any deficiencies before the next group meetings.
Measurement
Faith was measured using the Functional Assessment of Chronic Illness Therapy Spiritual Well-Being–Expanded (FACIT-SpEx version 4) scale, a 23-item measure that assesses 3 aspects of spiritual well-being: a sense of (1) faith/assurance, (2) meaning/purpose in life, and (3) other spiritual concerns such as connectedness, compassion, and forgiveness [54–56]. Each item is scored on a 5-point scale ranging from 0 (not at all) to 4 (very much). The total score ranges from 0 to 92.
The faith/assurance subscale was selected as a primary target of investigation for the present analysis because it had previously shown the most significant improvements in the intervention group [40]. Another reason was based on the finding that having a more positive view of God predicts better immune function as measured by CD4 counts in people with HIV (19, 53). The faith subscale contains 4 items: “I find comfort in my faith or spiritual beliefs”; “I find strength in my faith or spiritual beliefs”; “My illness has strengthened my faith or spiritual beliefs”; and “I know that whatever happens with my illness, things will be okay.” Scores on the faith/assurance subscale range from 0 to 16. In the current study, Cronbach's alpha was .94 for total FACIT score and .94 for faith/assurance subscale, indicating both scaled scores demonstrated high reliability in this sample.
Salivary Cortisol Collection
To collect saliva samples, participants were given Salivette kits (Sarstedt, Illinois) and asked to collect 4 samples throughout the day (7 A.M., 11 A.M., 4 P.M., and 9 P.M), just prior to each study group meeting (pre-intervention, post-intervention, and follow-up). They were instructed to rinse their mouths with water, roll the cotton stick around in their mouths for 3 minutes until saturated with saliva, and then place the cotton into the top chamber of the tube. Participants were asked to freeze the tubes and bring them to the next assessment. Samples were labeled with the patient's ID number, date, time, and assessment number. The Salivette tubes containing the cotton swabs were centrifuged at 2000 rpm in a refrigerated centrifuge and were stored at −80° C until the day of assay. Researchers measured salivary cortisols by radioimmunoassay using the Coat-a-Count RIA kit from Diagnostic Products Corporation (DPC), Los Angeles, CA. Cortisol levels in saliva were measured by a modification of the DPC RIA method to improve sensitivity with a limit of ≥;0.05 μg/dL. The intra- and inter-assay coefficients of variation for saliva cortisol were 3.0% and 6.4% respectively.
Design
A randomized controlled trial with a two group (intervention versus control) by three time (pre-intervention, post-intervention, and 5-week follow-up) design was conducted to evaluate the relationship of faith on neuroendocrine activity, as indicated by salivary cortisol levels. Scores from the faith/assurance subscale of the FACIT and average levels of daily cortisol (CRT) were assessed at 3 times over the course of a 10-week intervention. In order to block randomize and insure both groups had equivalent health status, CD4 counts were collected approximately 10 days prior to the first group meetings. The instructions for saliva sample collection were given at that time so specimens could be collected the day prior to the first group meetings.
To establish a more reliable representation of the overall degree of neuroendocrine activation, a mean daily cortisol level was calculated for each assessment (i.e., CRT1, CRT2, CRT3), representing the average of 4 samples collected throughout the day [57].
Data Analysis
Data were analyzed using intent to treat. For participants with missing data, a maximum-likelihood approach was employed to assign values based on group assignment using imputation with the Expectation-Maximization algorithm in SPSS. Three participants who had missing saliva samples for one entire day and two participants with unusable saliva samples were not included, leaving a total sample of 71 for analysis.
Independent t-tests were conducted to ensure that the intervention (n=36) and control groups (n=35) did not significantly differ at baseline on raw faith or average daily cortisol levels. For all subsequent analyses (i.e., those conducted in EQS 6.1 for Windows), cortisol values were natural log-transformed to improve their distributional properties. Pearson r bivariate correlations were used to assess concurrent and prospective relationships between faith and cortisol. We used path analysis (described in detail in the next section) to test the first hypothesis regarding faith-cortisol relationships, and employed model comparison strategies to determine the temporal structure of these relationships. Multigroup comparisons were used to test the second hypothesis regarding group differences.
Model Comparison within Path Analytical Models
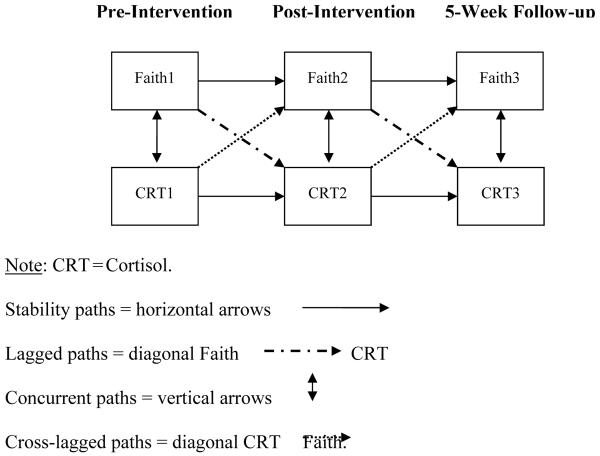
To best characterize the relationship between faith and average daily levels of salivary cortisol, per hypothesis 1, we utilized path analytical modeling techniques involving an iterative procedure of model comparison [58]. Four models were tested using EQS 6.1 for Windows. To begin, a target model was specified that included all pathways of interest (Figure 1). Four types of paths were specified: (1) Stability paths (i.e., relations between repeated measures of the same variable at adjacent assessments, for example, CRT1→CRT2); (2) lagged or “antecedent-consequent” paths from faith at one assessment to cortisol at the subsequent assessment (e.g., Faith1→CRT2); (3) concurrent paths, specified as the covariance between faith and CRT at the same assessment (e.g., Faith1→Faith2); and (4) cross-lagged paths from cortisol at one assessment to faith at the subsequent assessment (e.g., Faith1→CRT2).
Figure 1.
The Target Model Including All Paths: Stability, Lagged, Cross-Lagged, & Concurrent
To evaluate the primary hypothesis that the best fit model would contain both lagged and stability coefficients, but not concurrent or cross-lagged paths, a subtractive procedure involving 3 comparison tests of nested models was conducted following standard procedures of model comparison [59]. The sequence of comparisons was as follows: (1) The target model was compared to a model that omitted the cross-lagged paths; (2) the new model without cross-lagged paths was compared to a model omitting the concurrent paths; and (3) the model with both the lagged and stability paths was compared to a model with only the stability paths. Model comparison tests were conducted by comparing the target model to a nested model using the scaled difference chi-square statistic, ΔS-Bχ 2, per the methods outlined by Satorra and Bentler [60]. A nonsignificant ΔS-Bχ2 indicated no significant difference in the fit of the 2 models. In that case, the more parsimonious model, or the one specifying the fewest number of paths, was considered to provide better representation of the data [58]. The more parsimonious model then became the new point of reference (i.e., the new target model in subsequent model comparison tests). Thus, if the lagged model indeed provided the best fit, it was expected that the first 2 comparison tests would be nonsignificant, while the last would be significant.
The models were analyzed in a multigroup format, allowing separate estimates of all paths in the mantram and control groups. As Mardia's coefficient exceeded 5, we report results based on robust standard errors, which provide the Satorra-Benter Scaled χ2 (S-Bχ2) [61] and Comparative Fit Index (CFI) estimates, correcting for multivariate nonnormality [60]. Since the best-fit model was determined, group equivalence on the remaining paths of interest was evaluated using LaGrange Multiplier constraints of group invariance. The LaGrange Multiplier tests provided a statistical test of the second hypothesis, namely, whether the relationship between faith and cortisol was significantly stronger (i.e., a significantly larger path coefficient) in the mantram group compared to the control condition. Lastly, the path coefficients based on robust standard errors and means relevant to the final model were presented.
RESULTS
Analysis of Attrition
To compare drop-outs with those in the analysis, χ2 and t-tests were performed on demographics, CD4, HAART, faith and frequency of religious practice variables. Those who dropped out prior to the first group meetings (n=16) could not provide cortisol samples for comparison. Non-white participants formed a significantly higher proportion of those who dropped out of the study compared to those who completed (68% non-white versus 32% white; χ2 [1]=4.52, p=.033). There were no baseline differences in age, gender, employment, CD4 count, HAART, faith, or frequency of religious practices between those who completed and those who dropped out of the study.
Bivariate Correlations between Faith and Cortisol Across Timepoints
To examine the bivariate relationships among the faith/assurance and cortisol variables at different timepoints, correlations using Pearson r were performed as shown Table 2. There were significant inverse relationships between pre-intervention faith and post-intervention cortisol in the mantram group and significant inverse relationships between post-intervention faith and follow-up cortisol in the mantram group. Neither of these relationships was significant in the control group.
Table 2.
Pearson r Correlations by Groups† among Faith and Cortisol Variables across Timepoints
| Faith1 | Faith2 | Faith3 | CRT1 | CRT2 | CRT3 | |
|---|---|---|---|---|---|---|
| Faith1 | -- | .68*** | .70*** | −.13 | −.43** | −.29 |
| Faith2 | .80*** | -- | .74*** | −.30 | −.28 | −.37* |
| Faith3 | .74*** | .86*** | -- | −.04 | −.24 | −.37* |
| CRT1 | −.04 | .06 | −.04 | -- | .39* | .49** |
| CRT2 | .09 | .08 | .04 | .56*** | -- | .37* |
| CRT3 | −.27 | −.16 | −.15 | .58*** | .68*** | -- |
†Mantram group results = top half of table
†Control group results = bottom half of table
CRT = daily average salivary cortisol taken at 7a, 11a, 4p, 9p
T1 =Pre-intervention, T2=Post-intervention, T3=5-week follow-up
p≤ .05
p≤ .01
p≤ .01
Group Comparisons of Faith/Assurance and Average Daily Cortisol Levels
The unadjusted group means and standard deviations for faith/assurance and untransformed average daily cortisol levels at each of the 3 time-points are provided in Table 3. Prior to the intervention, there were no group differences in faith or average daily cortisol levels; however, the mantram group exhibited significantly higher levels of faith compared to the control group at the end of the intervention (T2) and at the 5-week follow-up (T3) (see Table 3).
Table 3.
Comparison of Unadjusted Faith/Assurance and Cortisol Levels by Group and Time
| Mantram (n=36), M(SD) | Education (n=35), M(SD) | T-Test (df) | p-Value | |
|---|---|---|---|---|
| FaithT1 | 10.66 (4.53) | 9.63 (4.70) | −0.94 (69.00) | .351 |
| FaithT2 | 12.21 (3.74) | 10.05 (5.22) | −2.00 (61.54) | .050* |
| FaithT3 | 12.08 (3.94) | 9.86 (5.21) | −2.02 (63.29) | .047* |
| CRT1, μg/dl | .2154 (.1342) | .2365 (.1276) | 0.68 (69.00) | .499 |
| CRT2, μg/dl | .2121 (.0963) | .2083 (.1056) | −0.16 (69.00) | .873 |
| CRT3, μg/dl | .1521 (.0742) | .1750 (.0890) | 1.18 (69.00) | .242 |
Faith=faith/assurance
CRT=average cortisol level
p-values ≤ .05 considered significant
Note: Cortisol values are untransformed.
M=mean, SD=standard deviation of the mean, df=degrees of freedom, T1=pre-Intervention, T2=post-Intervention, T3=5-week follow-up
Model Comparisons
The results of the model comparison tests are presented in Table 4. Using a cutoff of .9 for the Comparative Fit Index (CFI) [62], in which a higher value represents a better descriptive fit, the lagged model (which included lagged and stability paths but no cross-lagged or concurrent paths) provided the best fit for the data. The S-Bχ2 test of overall model fit was significant for all models; however, as this test is highly influenced by sample size, preference is typically given to the CFI in characterizing model fit. In support of the primary hypothesis that faith scores at one time-point would be related to cortisol levels at the same or subsequent time-point, the ΔS-Bχ2 tests comparing the first and second, as well as the second and third models, were nonsignificant (Table 4). However, in the last comparison, the lagged model provided a significantly better fit (p=.009) than a nested model without lagged paths. Thus, the lagged model appeared to provide the best representation of the data. As an additional comparison of interest, we returned to Model 2 (see Model 5 in Table 4), and removed the lagged paths rather than the concurrent paths. The resulting model with concurrent and stability paths provided a significantly poorer fit, again confirming the lagged model as the best fit model. Thus, the first hypothesis was partially upheld in that the lagged paths provided a good fit, and partially falsified in that the concurrent paths did not substantially improve the fit.
Table 4.
Model Fit and Comparison Tests Based on Robust Standard Errors
| Model (N=71) | CFI | S-Bχ2 (df) | P-value | ΔS-Bχ2 (Δdf) | ΔP-value |
|---|---|---|---|---|---|
| 1. Target | .919 | 24.241 (8) | .0021 | N/A | N/A |
| 2. Concurrent & Lagged | .914 | 29.215 (12) | .0037 | 4.152 (4) | .3858 |
| 3. Lagged | .923 | 33.370 (18) | .0151 | 2.791 (6) | .8346 |
| 4. Stability | .879 | 46.258 (22) | .0018 | 12.631 (4) | .0132* |
| 5. Concurrent (vs. Model 2) | .871 | 41.772 (16) | .0004 | 12.559 (4) | .0136* |
CFI=Comparative Fit Index
Significant by p≤.001
Note: These models estimate all parameters separately for each group.
Group Differences in the Relations between Faith and Cortisol
Subsequently, as a test of the second hypothesis, multigroup comparisons were conducted to evaluate whether path coefficients differed as a function of group. These paths were tested by constraining model parameters (i.e., path coefficients or variable means) to be invariant between groups. A significant LaGrange Chi-Square test indicated that the relationship between the 2 variables of interest (or the variables' means when investigating mean constraints) was significantly different in the mantram versus the control group, while controlling for all other factors in the model. These constraints were tested in 3 stages: (1) Stability coefficients, (2) lagged path coefficients, and (3) group means on all 6 observed variables. At each stage, significant constraints were freed before proceeding to the next stage. Tests of the constraints are provided in Table 5; the path coefficients for the final model are presented in Figure 2. Adjusted means are not presented, as the unadjusted means are already given in Table 3.
Table 5.
LaGrange Constraints and Tests of Group Invariance
| Constraint | LaGrange χ2 (df=1 for all) | p-value |
|---|---|---|
| 1. Stability Paths | ||
| CRT1, CRT2 | 1.655 | .198 |
| CRT2, CRT3 | 6.079 | .014* |
| FaithT1, FaithT2 | 4.308 | .038* |
| FaithT2, FaithT3 | .369 | .544 |
| 2. Lagged Paths | ||
| FaithT1, CRT2 | 5.071 | .024* |
| FaithT2, CRT3 | .282 | .595 |
| 3. Means Equivalence | ||
| FaithT1 | .846 | .358 |
| FaithT2 | 5.850 | .016* |
| FaithT3 | .398 | .528 |
| CRT1 | .648 | .421 |
| CRT2 | 4.866 | .027* |
| CRT3 | 4.638 | .031* |
CRT=Average Cortisol Level
Faith=Faith/Assurance
Significant by p ≤ .05.
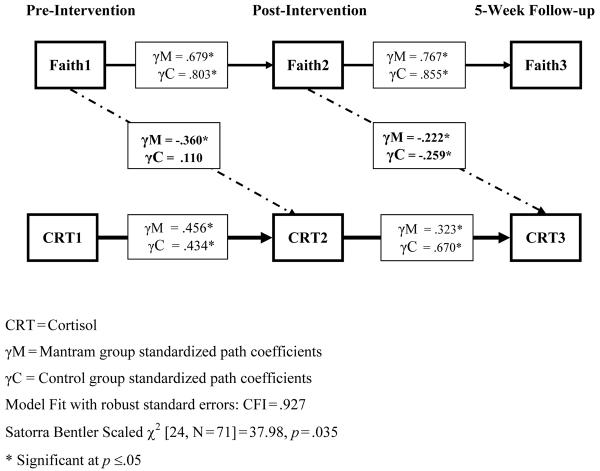
Figure 2.
Lagged Effects of Faith on Cortisol are Enhanced in the Mantram Group from Pre- to Post-Intervention.
The final model provided a good fit for the data: CFI=.927; Satorra Bentler Scaled χ2 [24, N=71]=37.98, p=.035. In the mantram group, there was a significant association between Faith1 and CRT2, such that higher faith at pre-intervention was associated with lower cortisol at post-intervention. Regarding group differences in the strength of the faith-cortisol association (Hypothesis 2), we found no significant association in the control group during the course of the intervention. However, there was a significant association in both groups between Faith2 and CRT3, indicating that the level of faith at post-intervention was related to lower average cortisol at 5-week follow-up in both groups. Both faith and cortisol exhibited high stability in both groups across all assessments.
Follow-up Analyses
To evaluate whether the lagged effects were independent of other relevant factors, follow-up analyses were run using multiple regression analyses, including group differences in employment and years with HIV as factors. HAART was additionally entered, as HAART may affect cortisol metabolic pathways [63]. Age and ethnicity (coded as white versus non-white) were included as demographics. As the first lagged effect differed between groups, we ran a separate hierarchical regression analysis for each group, entering the independent variables in the following manner: block 1=years with HIV, employment, ethnicity, age, HAART; block 2=CRT1; block 3= Faith1. CRT2 was the dependent variable. As expected, in the control group, Faith1 was not significantly associated with CRT2. In the mantram group, however, Faith1 remained significantly associated with CRT2 (β=−.387, t(27)=−2.264, p=.032), controlling for all covariates.
We ran a similar regression analysis that examined the effects of Faith2 on CRT3, replacing CRT1 with CRT2 (per stability coefficients) and controlling for the other covariates. The groups were combined for this analysis, as they did not significantly differ on this lagged effect. Again, Faith2 was significantly associated with CRT3, controlling for all covariates (β=−.238, t(63)=−2.170, p=.034). Notably, HAART was not significantly related to cortisol in any analysis. In summary, the lagged effects of faith on cortisol were maintained when controlling for numerous other factors. Participants in the mantram intervention increased their faith levels during the course of the intervention and maintained those levels at 5-week follow-up.
One potential alternative method of assessing cortisol function is to evaluate the steepness of the diurnal cortisol slope (i.e., operationalized here as the amount of decrease from morning to evening cortisol levels). Given the existing literature does not clearly delineate the best method, we reran the final model utilizing a change score (morning minus evening levels on a given day) to evaluate whether faith at one time-point would be positively associated with the magnitude of the diurnal decline in cortisol at the subsequent time-point. This analysis revealed no significant relationships in either group between faith at one time-point and the diurnal slope of cortisol at a subsequent time-point (all p's>.05), while controlling for all covariates.
DISCUSSION
The overall pattern of findings suggests that HIV-positive individuals who had a stronger sense of faith or felt that “no matter what happened, everything would be okay” in living with HIV, exhibited lower levels of neuroendocrine activation at subsequent time points, as indicated by average daily cortisol levels. These results build upon previous research findings that indicate that positive views of God in people diagnosed with HIV predict higher CD4 counts [19, 30] and improved immune function [52]. Moreover, these findings suggest that mantram participants with higher levels of faith exhibited the steepest declines in neuroendocrine activity, as measured by salivary cortisol. Thus, if one accepts the premise that decreasing cortisol levels may facilitate improved immune function among HIV-infected individuals, as some research has suggested [2, 19, 52], the present findings could indicate that faith has beneficial effects on neuroendocrine-related immune activity, and that a spiritually-based mantram intervention may enhance this relationship.
Despite the fact that faith levels between the groups at baseline did not significantly differ, individuals in the mantram group with greater faith levels prior to the intervention experienced decreased average daily salivary cortisol levels 5 weeks later (at post-intervention). Interestingly, both groups exhibited an inverse association between faith and cortisol between post-intervention and follow-up. Nevertheless, the largest effect size between faith/assurance and cortisol was found from pre- to post-intervention in the mantram group. Moreover, cortisol was significantly more changeable from post-intervention to follow-up (i.e., Table 5, p=.014) in the control group relative to the mantram group (Figure 2), which supports greater long-term benefits of the mantram group. Although speculative, this may suggest that effects from the education control condition may have also influenced cortisol levels at the follow-up. This is not unexpected, as increases in social support in HIV-positive individuals have previously been reported to partially mediate the effect of a stress reduction intervention on physiological parameters [7]. Alternative models exploring concurrent and cross-lagged relationships provided a significantly worse fit for the data. These findings build upon a prior cross-sectional study correlating spirituality with lower cortisol levels in people with HIV/AIDS [30]. Taken as a whole, the results suggest the presence of lagged relationships between faith and cortisol, where higher faith has a beneficial effect on subsequent stress-hormone activity. These results also introduce the possibility that the mantram intervention may have amplified or enhanced the relationship between faith and cortisol.
Other stress management intervention studies have also reported beneficial neuroendocrine effects. Cognitive-behavioral stress management reduced 24-hour urinary free cortisol output after the 10-week program among symptomatic HIV-infected gay men [64]. Previous research on the impact of psychosocial factors on HIV progression has suggested the possibility that longitudinal studies investigating temporal relationships are needed [65]. This study extends previous work by suggesting the presence of a time-lag between intervention and cortisol changes. Furthermore, a spiritually-based group intervention using mantram repetition may serve the dual function of increasing a sense of faith and reducing physiological biomarkers of stress over time, which may have additional health benefits.
Although the drop-out rate of 30% in this study may seem high, it is comparable to other HIV+ community-based intervention studies [66, 67]. There were no differences on the faith variable between those who completed and those who dropped out. We were unable to compare cortisol levels between the drop-outs and completers, however, because drop-outs left before providing baseline cortisol samples.
Psychological stress responses may contribute to variations in HIV disease course [65]. The risk of HIV disease stage progression is doubled with every increase in one severe stressor [20, 65]. Stress-related activation of the HPA axis that results in cortisol secretion can stimulate HIV viral replication [2] and affect various immunologic measures pertinent to HIV pathogenesis and disease progression [3–5, 7, 68]. Therefore, reducing stress hormones with the mantram intervention may have health benefits in HIV-positive individuals.
Although this study's sample size may be a limiting factor, the ratio of participants to observed variables surpasses a minimum 10:1 ratio [69], and modeling of correlations between repeated measures (i.e., the stability paths) generally provides substantial increases in power [70]. Moreover, the primary problem with small samples and non-normality is a tendency to over-reject the correct model [62], which is not of concern here, given that results support the primary hypotheses. In contrast, the diurnal cortisol model (i.e., decrease from morning to evening) did not yield significant results, which could have been affected by both statistical and compliance limitations. Specifically, change scores have been criticized as less reliable due to the management of the error components [71]. Future studies with larger samples might ameliorate such statistical concerns associated with change scores by utilizing multilevel modeling techniques to form diurnal slopes. However, a minimum of 30 groups is recommended to adequately power higher-order, cross-level interactions (i.e., the current finding of group differences in slope over time) [72]. Since the current study had only two groups (intervention versus control), we elected to err on the side of caution in these initial evaluations and did not include multilevel analyses due to sample size concerns. Additionally, previous research suggests that compliance issues may confound diurnal assessments, [73] which may have contributed to the lack of significance in the diurnal analysis.
The smaller sample could be one reason that HAART treatment was not associated with cortisol in the current findings. However, previous studies have revealed contradictory results [63, 74, 75], and this study was not in a position to examine whether various types of HAART regimes may have exerted differential neuroendocrine effects, as some evidence suggests [63]. The use of a repeated measures design also helped to improve power despite the sample size. As for generalizability, this sample represents HIV-infected patients in southern California and findings may differ in other geographic areas.
In conclusion, faith increased with the mantram intervention and was inversely associated with the stress hormone cortisol levels at the end of the intervention. Previous studies indicate that spirituality may have health benefits, particularly in HIV-positive individuals [4, 19, 28–30, 34–39]. Furthermore, reductions in stress-related activation of the HPA axis and cortisol secretion may reduce stress hormone stimulation of HIV viral replication and various immunologic measures pertinent to HIV pathogenesis. If confirmed, these findings suggest that spiritually-based mantram practice may benefit health in HIV-positive individuals. However, larger-scale studies with morbidity and mortality outcome measures need to be performed to determine if mantram repetition intervention effects are replicable and clinically relevant.
ACKNOWLEDGEMENTS
This study was conducted with funding from the National Center of Complementary and Alternative Medicine, National Institutes of Health (NCCAM/NIH) (R21 AT 01159-01A1) and support from Department of Veterans Affairs, VA San Diego Healthcare System; San Diego Veterans Medical Research Foundation; University of California San Diego (UCSD) General Clinical Research Center (#1637), National Center for Research Resources/NIH (M01RR008); UCSD Center for AIDS Research (CFAR 5P30 AI 36214) and the UCSD Antiretroviral Research Center (AVRC). Thanks to Patricia Bone and Wendy Belding for study implementation; Sheryl Becker and Ann Kelly as group facilitators; Laureen Pada and Madeline Gershwin for quality control; Walter Boyle for IT support and to Barbara Gray for assistance with the manuscript. The authors report no conflicts of interest. The views in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kumar M, Kumar A, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress. 2003;6(3):167–172. doi: 10.1080/10253890310001605376. [DOI] [PubMed] [Google Scholar]
- 2.Corley PA. Acquired Immune Deficiency Syndrome: the glucocorticoid solution. Med Hypotheses. 1996;47:49–54. doi: 10.1016/s0306-9877(96)90043-2. [DOI] [PubMed] [Google Scholar]
- 3.Antoni MH, Cruess S, Cruess DG, et al. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Ann Behav Med. 2000;22(1):29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- 4.Antoni MH, Cruess DG, Kimas N, et al. Stress management and immune system reconstitution in symptomatic HIV-infected gay men over time: Effects on transitional naive T cells (Cd4+Cd45RA+DC29+) Am J Psychiatry. 2002;159(1):143–145. doi: 10.1176/appi.ajp.159.1.143. [DOI] [PubMed] [Google Scholar]
- 5.Balbin EG, Ironson GH, Solomon GF. Stress and coping: The psychoneuroimmunology of HIV/AIDS. Bailliere's Clinical Endocrinology and Metabolism. 1999;13(4):615–633. doi: 10.1053/beem.1999.0047. [DOI] [PubMed] [Google Scholar]
- 6.Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: Mediation by the autonomic nervous system. Biol Psychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- 7.Cruess DG, Antoni MH, Kumar M, Schneiderman N. Reductions in salivary cortisol are associated with mood improvement during relaxation training among HIV-seropositive men. J Behav Med. 2000;23(2):107–121. doi: 10.1023/a:1005419917023. [DOI] [PubMed] [Google Scholar]
- 8.CDC CfDCaP . HIV/AIDS Surveillance Report. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2003. pp. 1–64. 2004. [Google Scholar]
- 9.Mannheimer SB, Matts J, Telzak E, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- 10.Grassi L, Righi R, Sighinolfi L, Makoui S, Ghinelli F. Coping styles and psychosocial- related variables in HIV-infected patients. Psychosomatics. 1998;39:350–359. doi: 10.1016/S0033-3182(98)71323-4. [DOI] [PubMed] [Google Scholar]
- 11.Vosvick M, Gore-Felton C, Koopman C, Thoresen C, Krumboltz J, Spiegel D. Maladaptive coping strategies in relation to quality of life among HIV+ adults. AIDS & Behavior. 2002;6(1):97–106. [Google Scholar]
- 12.Penendo FJ, Antoni MH, Schneiderman N, et al. Dysfunctional attitudes, coping, and depression among HIV-seropositive men who have sex with men. Cognitive Therapy & Research. 2001;25(5):591–606. [Google Scholar]
- 13.Weaver KE, Antoni MH, Lechner SC, et al. Perceived stress mediates the effects of coping on the quality of life of HIV-positive women on Highly Active Antiretroviral Therapy. AIDS and Behavior. 2004;8(2):175–183. doi: 10.1023/B:AIBE.0000030248.52063.11. [DOI] [PubMed] [Google Scholar]
- 14.Weaver KE, Llabre MM, Duran RE, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health Psychol. 2005;24(4):385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- 15.Antoni MH, Cruess DG, Klimas N, et al. Increases in a marker of immune system reconstitution are predated by decreases in 24-h urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic HIV-infected men. J Psychomatic Research. 2005;58:3–13. doi: 10.1016/j.jpsychores.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Ano GG, Vasconcelles EB. Religious coping and psychological adjustment to stress: A meta- analysis. J Clin Psychol. 2004;61(4):461–480. doi: 10.1002/jclp.20049. [DOI] [PubMed] [Google Scholar]
- 17.Tuck I, Alleyne R, Thinganjana W. Spirituality and stress management in healthy adults. Journal of Holistic Nursing. 2006;24(4):245–253. doi: 10.1177/0898010106289842. [DOI] [PubMed] [Google Scholar]
- 18.Smith TB, McCullough ME, Poll J. Religiousness and depression: Evidence for a main effect and the moderating influence of stressful life events. Psychol Bull. 2003;129:614–636. doi: 10.1037/0033-2909.129.4.614. [DOI] [PubMed] [Google Scholar]
- 19.Ironson G, Stuetzle R, Fletcher MA. An increase in religiousness/spirituality occurs after HIV diagnosis and predicts slower disease progression over 4 years in people with HIV. J Gen Intern Med. 2006;21(S5):S62–S68. doi: 10.1111/j.1525-1497.2006.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. 2003a;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 21.Koenig HG, McCullough ME, Larson DB. Handbook of religion and health. Oxford University Press; New York: 2001. [Google Scholar]
- 22.Koenig HG, Cohen HJ, George LK, Hays JC, Larson DB, Blazer DG. Attendance at religious services, interleukin-6, and other biological parameters of immune function in older adults. Int J Psychiatry in Med. 1997;27:233–250. doi: 10.2190/40NF-Q9Y2-0GG7-4WH6. [DOI] [PubMed] [Google Scholar]
- 23.Pargament KI, Smith B, Koenig HG. Patterns of positive and negative religious coping with major life stressors. J Sci Study Religion. 1998;1998(37):710–724. [Google Scholar]
- 24.Zinnbauer BJ, Pargament KI. Measurement in the psychology of religion and spirituality: Current status and evaluation. In: Paloutzian RF, Park CL, editors. Handbook of the psychology of religion and spirituality. Guilford Press; New York: 2005. pp. 43–61. [Google Scholar]
- 25.Hill PC, Pargament KI. Advances in the conceptualization and measurement of religion and spirituality: Implications for physical and mental health research. Am Psychol. 2003;58(1):64–74. doi: 10.1037/0003-066x.58.1.64. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Seidlitz L. Spirituality moderates the effect of stress on emotional and physical adjustment. Personality and Individual Differences. 2002;32:1377–1390. [Google Scholar]
- 27.Woods TE, Ironson GH. Religion and spirituality in the face of illness: How cancer, cardiac and HIV patients describe their spirituality/religiosity. J Health Psychology. 1999;4:393–412. doi: 10.1177/135910539900400308. [DOI] [PubMed] [Google Scholar]
- 28.Cotton S, Puchalski CM, Sherman SN, et al. Spirituality and religion in patients with HIV/AIDS. J Gen Intern Med. 2006;21(S5):S5–S13. doi: 10.1111/j.1525-1497.2006.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall BA. Patterns of spirituality in persons with advanced HIV disease. Res Nurs Health. 1998;21:143–153. doi: 10.1002/(sici)1098-240x(199804)21:2<143::aid-nur5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Ironson G, Solomon GF, Balbin EG, et al. The Ironson-Woods spirituality/religiousness index is associated with long survival, health behaviors, less distress, and low cortisol in people with HIV/AIDS. Ann Behav Med. 2002;24:34–48. doi: 10.1207/S15324796ABM2401_05. [DOI] [PubMed] [Google Scholar]
- 31.Koenig HG. Psychoneuroimmunology and the faith factor. J Gender-Specific Med. 2000;3(5):37–44. [PubMed] [Google Scholar]
- 32.Lorenz KA, Wenger N, Hays RD, et al. In: Description and predictors of religiosity and spirituality in a nationally representative sample of patients with HIV infection from the HIV cost and services utilization study (HCSUS) Research AfHS, editor. vol 16. 1999. pp. 396–7. [Google Scholar]
- 33.Pargament KI, McCarthy S, Shah P, et al. Religion and HIV: A review of the literature and clinical implications. South Med J. 2004;97(12):1201–1209. doi: 10.1097/01.SMJ.0000146508.14898.E2. [DOI] [PubMed] [Google Scholar]
- 34.Simoni JM, Martone MG, Kerwin J. Spirituality and psychological adaptation among women with HIV/AIDS: Implications for counseling. J Counseling Psychology. 2002;49:139–147. [Google Scholar]
- 35.Somlai AM, Heckman TG. Correlates of spirituality and well-being in a community sample of people living with HIV disease. Mental Health, Religion & Culture. 2000;3(1) [Google Scholar]
- 36.Sowell R, Moneyham L, Hennessy M, Guillory J, Demi A, Seals B. Spiritual activities as a resistance resource for women with human immunodeficiency virus. Nurs Res. 2000;49(2):73–82. doi: 10.1097/00006199-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Szaflarski M, Richey PN, Leonard AC, et al. Modeling the effects of spirituality/religion on patients' perceptions of living with HIV/AIDS. J Gen Intern Med. 2006;21(S5):S28–S38. doi: 10.1111/j.1525-1497.2006.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarakeshwar N, Khan N, Sikkema KJ. A relationship-based framework of spirituality for individuals with HIV. AIDS & Behavior. 2006;10(1):59–70. doi: 10.1007/s10461-005-9052-8. [DOI] [PubMed] [Google Scholar]
- 39.Tuck I, McCain NL, Elswick RKJ. Spirituality and psychosocial factors in persons living with HIV. J Adv Nurs. 2001;33(6):776–783. doi: 10.1046/j.1365-2648.2001.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bormann JE, Gifford AL, Shively M, et al. Effects of spiritual mantram repetition on HIV outcomes: A randomized controlled trial. J Behav Med. 2006;29(4):359–376. doi: 10.1007/s10865-006-9063-6. [DOI] [PubMed] [Google Scholar]
- 41.Margolin A, Beitel M, Schuman-Oliver Z, Avants SK. A controlled study of a spiritually- focused intervention for increasing motivation for HIV prevention among drug users. AIDS Educ Prev. 2006;18(4):311–322. doi: 10.1521/aeap.2006.18.4.311. [DOI] [PubMed] [Google Scholar]
- 42.Tarakeshwar N, Pearce MJ, Sikkema KJ. Development and implementation of a spiritual coping group intervention for adults living with HIV/AIDS: A pilot study. Mental Health, Religion & Culture. 2005;8(3):179–190. [Google Scholar]
- 43.Bormann JE, Oman D. Mantram or holy name repetition: Health benefits from a portable spiritual practice. In: Plante TG, Thoresen C, editors. Spirit, science and health: How the spiritual mind fuels physical wellness. Praeger; Westport, CT: 2007. pp. 94–112. [Google Scholar]
- 44.Oman D, Driskill JD. Holy name repetition as a spiritual exercise and therapeutic technique. J Psychol Christianity. 2003;22(1):5–19. [Google Scholar]
- 45.Bormann JE. Frequent, silent mantram repetition: A Jacuzzi for the mind. Topics in Emergency Medicine. 2005;27(2):163–166. [Google Scholar]
- 46.Easwaran E. The mantram handbook. 4th ed. Nilgiri Press; Tomales, CA: 2001. [Google Scholar]
- 47.Easwaran E. Strength in the storm: Creating calm in difficult times. Nilgiri Press; Tomales, CA: 2005. [Google Scholar]
- 48.Bormann JE, Becker S, Gershwin M, et al. Relationship of frequent mantram repetition to emotional and spiritual well-being in healthcare workers. Journal of Continuing Education in Nursing. 2006;37(5):218–224. doi: 10.3928/00220124-20060901-02. [DOI] [PubMed] [Google Scholar]
- 49.Bormann JE, Smith TL, Becker S, et al. Efficacy of frequent mantram repetition on stress, quality of life, and spiritual well-being in veterans: A pilot study. J Holistic Nurs. 2005;23(4):394–413. doi: 10.1177/0898010105278929. [DOI] [PubMed] [Google Scholar]
- 50.Bormann JE, Smith TL, Shively M, Dellefield ME, Gifford AL. Self-monitoring of a stress reduction technique using wrist-worn counters. Journal for Healthcare Quality. 2007;29(1):47–55. doi: 10.1111/j.1945-1474.2007.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 51.Wolf DB, Abell N. Examining the effects of meditation techniques on psychosocial functioning. Research on Social Work Practice. 2003;13(1):27–42. [Google Scholar]
- 52.Ironson G, Stuezle R, Fletcher MA, Ironson D. View of god is associated with disease progression in HIV [abstract] Ann Behav Med. 2006;31(Suppl):S074. [Google Scholar]
- 53.Cockrell JR, Folstein MF. Mini Mental State Examination (MMSE) Psychopharmacology. 1988;24:689–692. [PubMed] [Google Scholar]
- 54.Brady MJ, Peterman AH, Fitchett G, Mo M, Cella D. A case for including spirituality in quality of life measurement in oncology. Psychooncology. 1999;8(5):417–428. doi: 10.1002/(sici)1099-1611(199909/10)8:5<417::aid-pon398>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 55.Mytko J, Knight SJ. Body, mind and spirit: Towards the integration of religiosity and spirituality in cancer quality of life research. Psychooncology. 1999;8:439–450. doi: 10.1002/(sici)1099-1611(199909/10)8:5<439::aid-pon421>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual wellbeing in people with cancer: The Functional Assessment of Chronic Illness Therapy--Spiritual Well-Being Scale (FACIT-Sp) Ann Behav Med. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 57.Leserman J, Petitto JM, Golden RN, et al. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. American Journal of Psychiatry. 2000;151:1221–1228. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 58.Gallagher-Thompson D, Lovett S, Rose J, et al. Impact of psychoeducational interventions on distressed family caregivers. J Clin Geropsychology. 2000;6:91–110. [Google Scholar]
- 59.Bentler PM. Compartive fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 60.Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satorra A, Bentler PM. Scaling corrections for chi-square statistics in covariance structure analysis. Journal of Business and Economic Statistics. 1998;1:301–313. [Google Scholar]
- 62.Bentler PM, Yuan KH. Structural equation modeling with small sample: Test statistics. Multivariate Behavorial Research. 1999;34:181–197. doi: 10.1207/S15327906Mb340203. [DOI] [PubMed] [Google Scholar]
- 63.Collazos J, Mayo J, Martinez E, Ibarra S. Serum cortisol in HIV-infected patients with and without highly active antiretroviral therapy. AIDS. 2003;17:123–6. doi: 10.1097/00002030-200301030-00018. [DOI] [PubMed] [Google Scholar]
- 64.Antoni MH, Cruess DG, Cruess S, Lutgendorf S, Kumar M, Ironson G, Klimas N, Fletcher MA. Schneiderman: Cognitive-behavioral stress management intervention effects on anxiety, 24-hr urinary norepinephrine output, and t-cytotoxic/suppressor cells over time among symptomatic HIV-infected gay men. J Consult Clin Psychol. 2000;68(1):31–45. doi: 10.1037//0022-006x.68.1.31. [DOI] [PubMed] [Google Scholar]
- 65.Leserman J. The effects of stressful life events, coping, and cortisol on HIV infection. CNS Spectrums. 2003b;8(1):25–30. doi: 10.1017/s1092852900023439. [DOI] [PubMed] [Google Scholar]
- 66.Chesney MA, Chambers D, Taylor JM, Johnson LM, Folkman S. Coping effectiveness training for men living with HIV: Results from a randomized clinical trial testing a group-based intervention. Psychosomatic Medicine. 2003;65:1038–1046. doi: 10.1097/01.psy.0000097344.78697.ed. [DOI] [PubMed] [Google Scholar]
- 67.Robinson FP, Mathews HL, Witek-Janusek L. Psycho-endocrine-immune response to Mindfulness-Based Stress Reduction in individuals infected with the Human Immunodeficiency Virus: A quasiexperimental study. The Journal of Alternative and Complementary Medicine. 2003;9(5):683–694. doi: 10.1089/107555303322524535. [DOI] [PubMed] [Google Scholar]
- 68.Cole S, Kemeny ME, Taylor SE. Social identity and physical health: Accelerated HIV progression in rejection-sensitive gay men. J Pers Soc Psychol. 1997;72(2):320–335. doi: 10.1037//0022-3514.72.2.320. [DOI] [PubMed] [Google Scholar]
- 69.Thomson JE. The place of spiritual well-being in hospice patients' overall quality of life. Hospice Journal. 2000;15(2):13–27. [PubMed] [Google Scholar]
- 70.Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. Lawrence Erlbaum Associates. 2004:561–563. [Google Scholar]
- 71.Kessler RC. The use of change scores as criteria in longitudinal survey research. Qual and Quant. 1977;(11):43–66. [Google Scholar]
- 72.Kreft IGG, de Leeuw J. Introducing multilevel modeling. Sage; Thousand Oaks, CA: 1998. [Google Scholar]
- 73.Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime protocols in noncompliant subjects. Psychosom Med. 2003;(65):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- 74.Christeff N, Melchior JC, de Truchis P, Perronne C, Nunez EA, Gougeon ML. Lipodystrophy defined by a clinical score in HIV-infected men on highly active antiretroviral therapy: correlation between dyslipidaemia and steroid hormone alterations. AIDS. 1999;13:2251–2260. doi: 10.1097/00002030-199911120-00007. [DOI] [PubMed] [Google Scholar]
- 75.Yanovski JA, Miller KD, Kino T, et al. Endocrine and metabolic evaluation of human immunodeficiency virus-infected patients with evidence of protease inhibitor-associated lipodystrophy. J Clin Endocrinol Metab. 1999;84(6):1925–31. doi: 10.1210/jcem.84.6.5740. [DOI] [PubMed] [Google Scholar]