We present a facile method for fine tuning the optical properties of Au–Ag nanocages with oxygen (from air) and a water-soluble thiol. When nanocages were dispersed in an aqueous solution containing the thiol, the pure Ag inside the cages was selectively etched away. As a result, the wall thickness of the nanocages decreased and the localized surface plasmon resonance (LSPR) peak was red shifted. We also found that the extent of etching and LSPR peak position could be both controlled by varying the concentration of the thiol solution, allowing us to tailor and tune the optical properties of nanocages without involving galvanic replacement and dealloying reactions.
Successful synthesis of noble-metal nanostructures with specific sizes, shapes, and morphologies has made it possible to tailor and tune their properties for various applications including catalysis,1 optical sensing,2 drug delivery,3 and biomedical imaging.4 The optical properties of Au and Ag nanostructures have received much attention because their LSPR peaks are tunable throughout the visible and near-infrared (NIR) regions.5 For Au-based nanoboxes, nanocages, and nanoshells, in particular, their LSPR peaks can be conveniently controlled by varying the ratio of wall thickness to outer dimension. For Au-based nanostructures with LSPR peaks tuned into the NIR transparent window (800–900 nm) of soft tissue, they have been explored for a broad range of in vivo applications.
It is well-known that both galvanic replacement and selective etching are useful tools for controlling the shape and morphology of metal nanostructres.6,7 For example, Au–Ag nanoboxes and nanocages can be made with controllable porosities and various morphologies by reacting Ag nanocubes with HAuCl4 in an aqueous solution.6 It has also been shown that the remaining Ag can be selectively removed from Au–Ag alloy nanocages with Fe(NO3)3 or NH4OH as a wet etchant.7 With the use of these two techniques, a controllable red shift was observed for the LSPR peak as the reaction proceeded. In this Communication, we present another facile method for controlling the wall thickness of Au–Ag nanocages and thus their optical properties. The key is to use a water-soluble thiol as an etchant (when combined with oxygen from air) to selectively dissolve the Ag remaining inside the Au–Ag nanocages. This new protocol is advantageous over previous methods when we want to fine tune the LSPR spectra of Au–Ag nanocages without significantly modifying the elemental composition and structure of the alloyed walls. We found that the un-reacted, pure Ag trapped inside the Au–Ag nanocages could be removed in the presence of a water-soluble thiol such as methoxy-terminated poly(ethylene glycol)-SH (mPEG-SH) or mercaptoethanol [HO(CH2)2SH]. The overall thickness and LSPR peak position of the resultant nanocages could be precisely tuned by varying the reaction time and/or the concentration of the thiol.
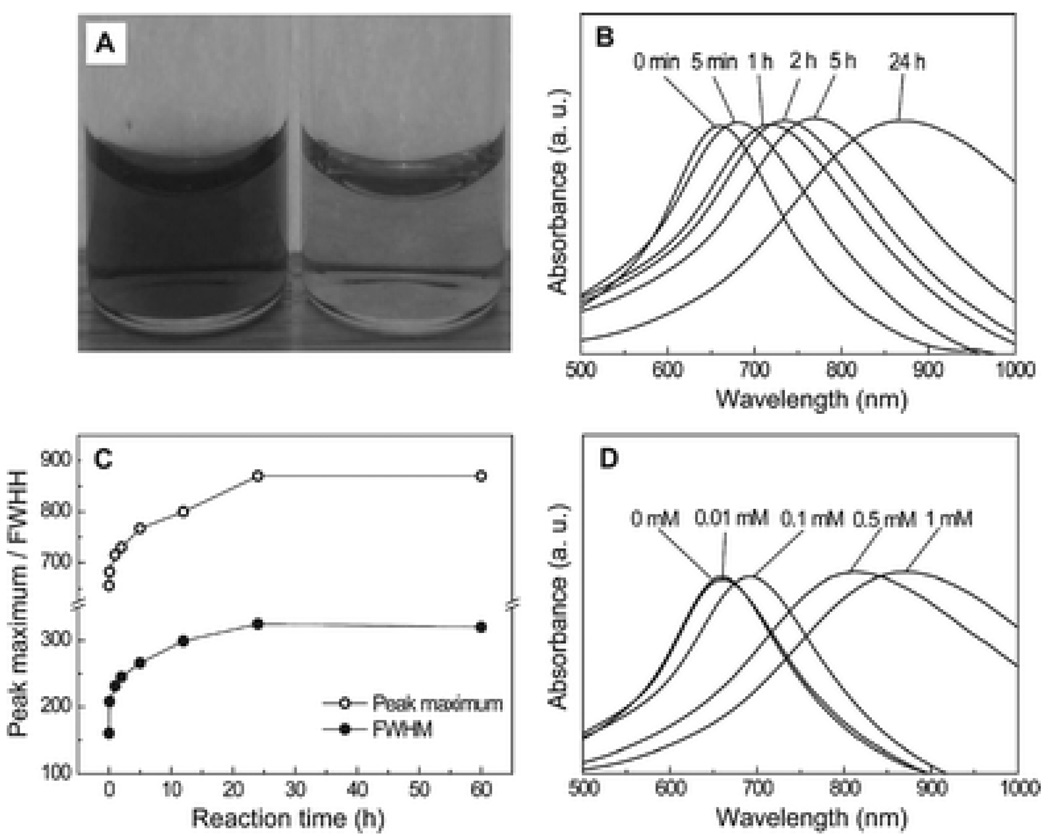
Fig. 1A shows the depth of color change for the Au–Ag nanocages before (left) and after (right) reacting with 1 mM mPEG-SH for 24 h, with the concentration of nanocages in the thiol solution being 1 nM. The Au–Ag nanocages were prepared by titrating 40 nm Ag nanocubes with HAuCl4 solution. The detailed experimental procedures for the synthesis of Ag nanocubes and the Au–Ag nanocages can be found in ref. 6b. Briefly, AgNO3 was reduced by ethylene glycol in the presence of poly(vinyl pyrrolidone) (PVP) and a trace amount of NaHS to generate uniform Ag nanocubes. We then transformed the Ag nanocubes into Au–Ag nanocages by adding 0.2 mM HAuCl4 aqueous solution to the Ag nanocubes dispersed in a PVP aqueous solution at 100 °C. Once the LSPR peak had reached 650 nm, the addition of HAuCl4 was immediately stopped. After washing once with NaCl to remove AgCl and several times with deionized (DI) water, the suspension of nanocages displayed a dark blue color. When the nanocages were re-suspended in a 1 mM mPEG-SH (Mn≈5000, Laysan Bio Inc.) aqueous solution and left for 24 h at room temperature, the suspension of nanocages changed to a lighter blue, indicating that the LSPR peak had been modified. As shown in Fig. 1B, the LSPR peak shifted continuously from 650 to 860 nm over the course of the reaction. Analysis of the spectra (Fig. 1C) indicated that both the peak maximum and the full width at half maximum (FWHM) of the peak increased with the elongation of reaction time. After 24 h, there was no further change for the peak position and FWHM. The LSPR peak could also be tuned in a controllable way by varying the concentration of the thiol in the solution. Fig. 1D shows the extinction spectra taken from the same batch of Au–Ag nanocages after they had reacted with mPEG-SH of different concentrations for 24 h. As the concentration of mPEG-SH increased, the peak position also shifted to longer wavelengths.
Fig. 1.
(A) Photograph showing the depth of colour change of 1 nM Au–Ag nanocages before (left) and after (right) reacting with 1 mM mPEG-SH aqueous solution for 24 h. (B) UV-Vis spectra of 1 nM Au–Ag nanocages as a function of reaction time with 1 mM mPEG-SH aqueous solution. (C) Change in the full width at half maximum (FWHM) from UV-Vis spectra as a function of reaction time with 1 mM mPEG-SH aqueous solution. (D) UV-Vis spectra of 1 nM Au–Ag nanocages plotted as a function of the concentration of mPEG-SH aqueous solution (reaction time: 24 h).
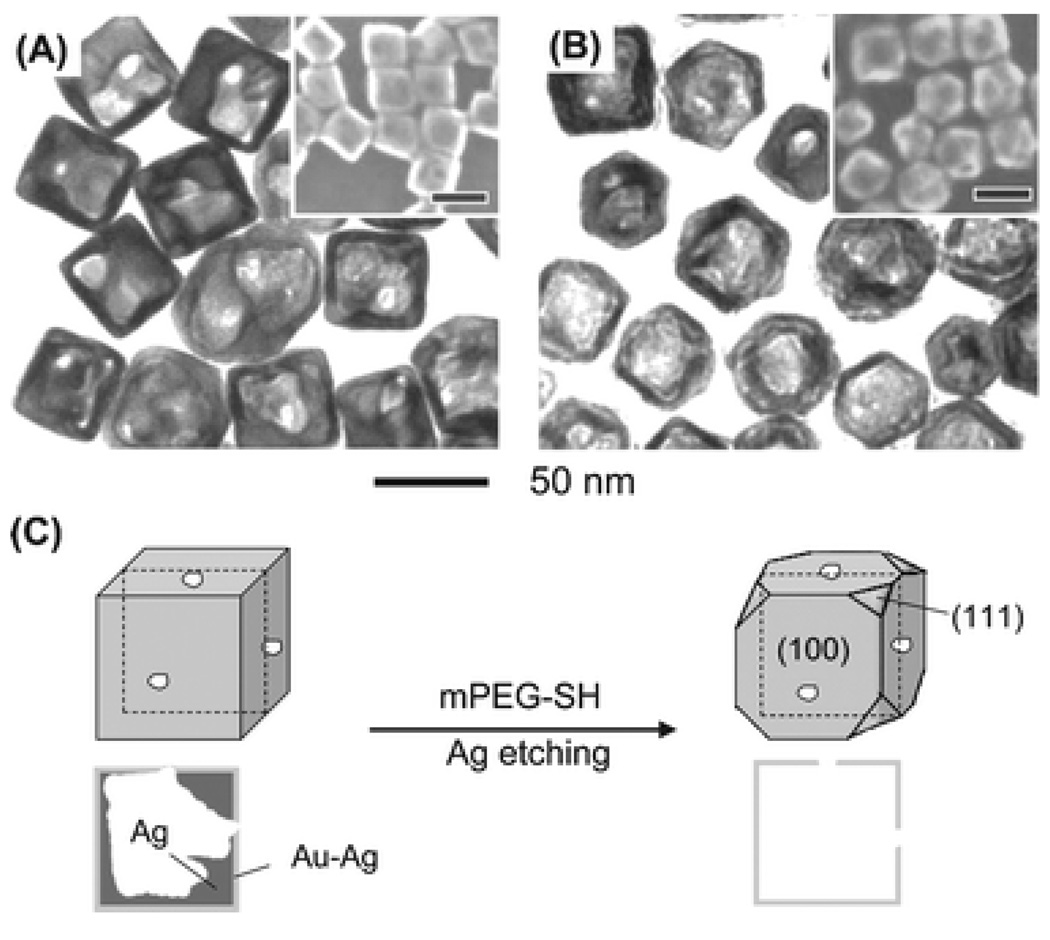
We next investigated the effect of mPEG-SH on the morphology and geometry of the Au–Ag nanocages by TEM and SEM (Fig. 2, A and B). Before reaction with mPEG-SH (Fig. 2A), most of the nanocages had a cubic shape with a partially hollow structure in the interior because there was still a significant amount of un-reacted, pure Ag. The nanocages were transformed into completely hollow structures (Fig. 2B), and some of their corners became slightly truncated (also see the inset) during the reaction. We also measured the average wall thicknesses of the nanocages from the TEM images, and the results indicate that the thickness decreased from 5.3 ± 1.1 nm to 4.0 ± 1.2 nm due to the removal of Ag from the interior. Meanwhile, the average size of the nanocages was not very different: it was 41.2 ± 4.4 nm before reaction and 39.8 ± 4.9 nm after reaction. These morphological (and dimensional) changes are responsible for the spectral changes observed during the reaction with 1 mM mPEG-SH. In particular, we could attribute the continuous red shift in LSPR peak position to the gradual decrease in wall thickness for the nanocages. It has been reported that the LSPR peaks of Au-based nanocages were shifted to longer wavelength as the wall thickness was reduced.4b,7,8 As the concentration of thiol solution increased, the wall thickness of the nanocages was also found to decrease, causing a red shift for the LSPR peak position.
Fig. 2.
TEM micrographs (with SEM insets) showing the morphology of Au–Ag nanocages before (A) and after (B) reacting with 1 mM mPEG-SH aqueous solution for 24 h. The scale bars in the SEM images are 50 nm. (C) A schematic of the mechanism for selective etching of pure Ag from the interior of Au–Ag nanocages by reacting with mPEG-SH. The cross-sectional views (dashed line) are shown below for a better view of the interior structure of the nanocages.
Theoretical calculations indicate that the FWHM of an LSPR peak is determined by surface scattering and radiation damping, in other words, the wall thickness and the volume of the particle.9 Since the volumes of the Au–Ag nanocages were more or less similar at different reaction times, we believe that the increase in FWHM was largely caused by the enhanced surface scattering of Au–Ag nanocages due to the reduction in wall thickness after reacting with mPEG-SH.
The reaction with mPEG-SH also caused a change in the chemical composition of the Au–Ag nanocages. We performed elemental analysis of the Au–Ag nanocages by energy-dispersive spectroscopy (EDS) and the results are shown in Table 1. The Au–Ag nanocages originally consisted of Ag and Au with a molar ratio of 4:1, but this ratio dropped to 3:1 after the nanocages had reacted with 1 mM mPEG-SH. In addition, a considerable amount of sulfur was detected from the nanocages after reaction. These results indicate that some of the Ag was selectively etched from the nanocages by mPEG-SH and the thiol was immobilized on the surface of the nanocages. To clarify the function of mPEG-SH, we added 1 mM PEG (Mn≈5000, Aldrich) to an aqueous dispersion of the same batch of Au–Ag nanocages. Even after 24 h, we did not observe any shift or broadening for the LSPR peak. This result suggests that the etching of Ag critically depends on the thiol group of mPEG-SH.
Table 1.
Relative atomic concentrations of Au, Ag, and S in Au–Ag nanocages before and after reacting with 1 mM mPEG-SH at different concentrations. The reaction time was 24 h, and the analysis was carried out using EDS
| Atomic concentration (%) | |||
|---|---|---|---|
| Au | Ag | S | |
| Before etching | 19.2 ± 0.1 | 80.8 ± 0.1 | 0 |
| After etching | 21.6 ± 1.0 | 58.3 ± 1.0 | 20.1 ± 1.1 |
The thiol mainly removed the pure Ag from the interior of nanocages via oxidative etching, in which the thiol serves as a coordination ligand, and O2 from air serves as the oxidant. In an aqueous solution containing O2 and H+, it is possible for O2 to be reduced to water by selectively oxidizing Ag to Ag+. This is due to the fact that the reduction potential of O2 (E0 = 1.23 V) is higher than that of Ag (E0 = 0.80 V) but lower than that of Au (E0 = 1.50 V). We suggest that the thiol compound can help to reduce O2 by supplying the H+ after reacting with Ag+ ions to form mPEG-S-Ag. In the present work, we used the Au–Ag nanocages consisting of a Au–Ag alloy for the wall and pure Ag in the interior (Fig. 2C). By selectively dissolving the pure Ag from the interior of the nanocage with mPEG-SH, we obtained nanocages with essentially no pure Ag inside the cage. A small amount of Ag at the corner of the nanocages was also removed from the Au–Ag alloy walls, causing slight truncation at corners when compared with the starting nanocages. However, there was no significant removal of Ag from the Au–Ag alloy walls by mPEG-SH. The shift in LSPR peak and the transformation of the morphology did not proceed any further even after a longer reaction time (ca. one week) or with the use of a higher concentration of mPEG-SH (4 mM). In addition, we did not observe any shift in the LSPR peak for the Au–Ag nanocages that did not contain any pure Ag inside the nanocage after reaction with 1 mM mPEG-SH (data not shown). This observation implies that the etching ability of water-soluble thiol compounds is not as strong as those of NH4OH or Fe(NO3)3 which could also dissolve most of the Ag from the Au–Ag alloy walls through a dealloying process.7 In these cases, the dealloying process transformed cubic nanocages into porous nanoframes, and further shifted the LSPR peak to 1200 nm. By selectively etching the Ag inside nanocages only, the use of a water-soluble thiol is advantageous when we just want to fine tune the LSPR spectra of Au–Ag nanocages without severe transformation of the Au–Ag alloy composition and wall structure.
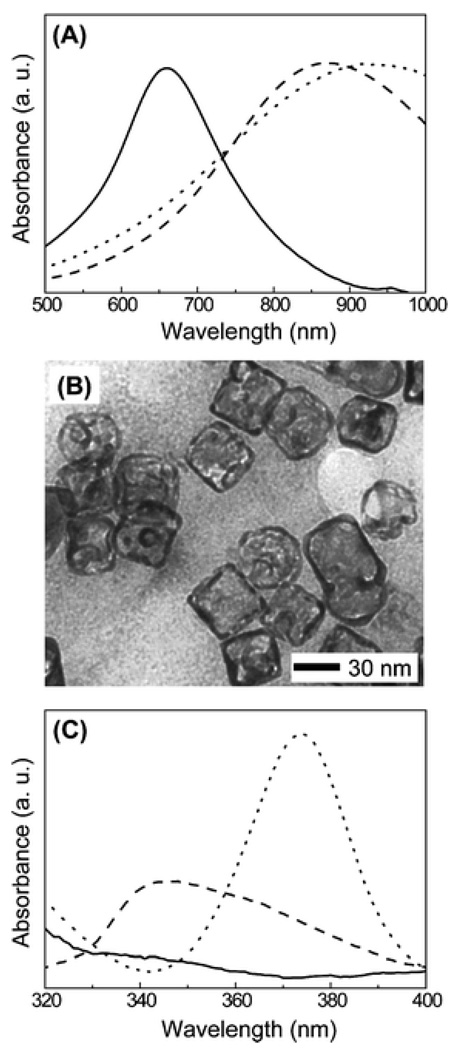
We also investigated the role of the thiol in Ag etching by using a different thiol, 2-mercaptoethanol or HS(CH2)2OH. We also observed a similar shift for the LSPR peak when the Au–Ag nanocages were reacted with 1 mM HS(CH2)2OH, but the spectra became much broader (data not shown). We found that the Au–Ag nanocages slowly precipitated out in 2 h after adding HS(CH2)2OH, implying that the etching process might have been stopped due to aggregation of thiol-capped nanocages. To prevent the nanocages from aggregating, the Au–Ag nanocages were reacted with 0.1 mM mPEG-SH for 24 h, followed by the addition of 1 mM HS(CH2)2OH. As shown in Fig. 3A, the absorption spectra were similar to those obtained for the reaction with 1 mM mPEG-SH. Since 0.1 mM mPEG-SH only caused a shift of ~50 nm (Fig. 1D), the additional shift in peak position could be attributed to the etching by HS(CH2)2OH. We also observed the morphology of nanocages by TEM (Fig. 3B), and found that the Ag in the interior of the nanocages was etched away due to the reactions with both 0.1 mM mPEG-SH and 1 mM HS(CH2)2OH. These results further confirm that the etching of Ag from the Au–Ag nanocages requires a thiol group.
Fig. 3.
(A) UV-Vis spectra of 1 nM Au–Ag nanocages (solid line), after reacting with 1 mM mPEG-SH aqueous solution (dashed line), and after reacting with 0.1 mM mPEG-SH and then 1 mM HS(CH2)2OH (dotted line). (B) TEM micrographs after reacting with 0.1 mM mPEG-SH and then 1 mM HS (CH2)2OH. (C) Absorption spectra in the UV for 1 nM Au–Ag nanocages (solid line) after reacting with 1 mM mPEG-SH aqueous solution (dashed line), and after reacting with 0.1 mM mPEG-SH and then 1 mM HS (CH2)2OH (dotted line). All the reaction times were 24 h.
Although water-soluble thiol compounds can etch Ag from the interior of Au–Ag nanocages, it is unclear whether the silver–thiol compounds formed were in a completely water-soluble form or in the polymeric form, [Ag-S-R]n.10 We recorded the absorption spectra from 320 to 400 nm for the aqueous dispersions of Au–Ag nanocages after reaction with the two thiol compounds (Fig. 3C). When reacting with 1 mM mPEG-SH, there was a small peak around 340 nm, which is different from the nanocages reacted with 1 mM HS (CH2)2OH or 0.1 mM mPEG-SH followed by 1 mM HS(CH2)2OH. For the latter case, a peaks was detected around 370 nm and the intensity was much stronger than the case when the nanocages reacted with 1 mM mPEG-SH only. It has been reported that the existence of the peak in this region could be attributed to the polymeric form of Ag-S-R.11 Therefore, for the reaction between HS(CH2)2OH and Au–Ag nanocages, it seems to be that Ag-S(CH2)2OH existed in a polymeric form due to its decreased solubility in the aqueous solution.11,12 On the other hand, there is only a small peak in this region when the nanocages were reacted with mPEG-SH, implying that mPEG-S-Ag existed mostly in a water-soluble form.
In summary, we have demonstrated the use of oxygen (from air) and a water-soluble thiol compound for tailoring and fine-tuning the optical properties of Au–Ag nanocages through selective etching of pure Ag from the interior. This method is advantageous because it does not require additional metal precursors (HAuCl4) and there is no substantial modification to the wall composition and structure of the product as typically seen with other etchants. In addition, this unique approach for tuning the LSPR properties can be done entirely in an aqueous system.
Acknowledgements
This work was supported by a NIH Director's Pioneer Award (5DP1OD000798). E.C.C. was also partially supported by a fellowship from the Korea Research Foundation (KRF-2007-357-D00070).
Footnotes
This paper is part of a Journal of Materials Chemistry theme issue on inorganic nanoparticles for biological sensing, imaging, and therapeutics. Guest editor: Jinwoo Cheon.
References
- 1.Kim SW, Kim M, Lee WY, Hyeon T. J. Am. Chem. Soc. 2002;124:7642. doi: 10.1021/ja026032z. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Xia Y. Anal. Chem. 2002;74:5297. doi: 10.1021/ac0258352. [DOI] [PubMed] [Google Scholar]
- 3.Portney NG, Ozkan M. Anal. Bioanal. Chem. 2006;384:620. doi: 10.1007/s00216-005-0247-7. [DOI] [PubMed] [Google Scholar]
- 4.(a) Chen J, Saeju F, Wiley BJ, Cang H, Cobb MJ, Li Z-Y, Au L, Zhang H, Kimmey MB, Li X, Xia Y. Nano Lett. 2005;5:473. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]; (b) Chen J, Wiley BJ, Li Z-Y, Campbell D, Saeki F, Cang H, Au L, Lee J, Li X, Xia Y. Adv. Mater. 2005;17:2255. [Google Scholar]; (c) Cang H, Sun T, Li Z-Y, Chen J, Wiley BJ, Xia Y, Li X. Opt. Lett. 2005;30:3048. doi: 10.1364/ol.30.003048. [DOI] [PubMed] [Google Scholar]; (d) Loo C, Lin A, Hirsch L, Lee MH, Barton J, Halas N, West J, Drezek R. Technol. Cancer Res. Treat. 2004;3:33. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]; (e) Chen J, Wang D, Xi J, Au L, Siekkinen A, Warsen A, Li Z-Y, Zhang H, Xia Y, Li X. Nano Lett. 2007;7:1318. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hirsch LR, Gobin AM, Lowery AR, Tam F, Drezek RA, Halas NJ, West JL. Ann. Biomed. Eng. 2006;34:15. doi: 10.1007/s10439-005-9001-8. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Halas NJ. MRS Bull. 2005;30:338. [Google Scholar]
- 6.(a) Sun Y, Xia Y. J. Am. Chem. Soc. 2004;126:3892. doi: 10.1021/ja039734c. [DOI] [PubMed] [Google Scholar]; (b) Skrabalak SE, Au L, Li X, Xia Y. Nature Protocols. 2007;2:2183. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Au L, McLellan J, Li Z-Y, Marquez M, Xia Y. Nano Lett. 2007;7:1764. doi: 10.1021/nl070838l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiley B, Sun YG, Chen J, Cang H, Li Z-Y, Li XD, Xia Y. MRS Bull. 2005;30:356. [Google Scholar]
- 9.Hu M, Petrova H, Sekkinen AR, Chen J, McLellan JM, Li Z-Y, Marquez M, Li X, Xia Y, Hartland GV. J. Phys. Chem. B. 2006;110:19923. doi: 10.1021/jp0621068. [DOI] [PubMed] [Google Scholar]
- 10.Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 5th Ed. Canada: John Wiley & Sons, Inc; 1988. [Google Scholar]
- 11.Fijolek HG, Gonzalez-Duarte P, Park SH, Suib SL, Natan MJ. Inorg. Chem. 1997;36:5299. [Google Scholar]
- 12.Baena MJ, Espinet P, Lequerica MC, Levelut AM. J. Am. Chem. Soc. 1992;114:4182. [Google Scholar]