Abstract
Objective
Controversy remains over the effect of stimulant treatment on later substance use disorders (SUD). To this end, we examined the risk imparted by stimulant treatment for attention-deficit/hyperactivity disorder (ADHD) on SUD and nicotine dependence in a study of girls with ADHD.
Design
case-controlled, prospective, five-year follow up study.
Participants
adolescent girls with and without ADHD from psychiatric and pediatric sources.
Setting
Massachusetts General Hospital. Blinded interviewers determined all diagnoses with structure interviews.
Main exposure
naturalistic treatment exposure with psychostimulants for ADHD.
Outcomes
We modeled time to onset of SUD and smoking as a function of stimulant treatment.
Results
We ascertained 114 subjects with ADHD (mean age at follow-up of 16.2 yrs) with complete medication and SUD data, of which 94 (82%) subjects were treated with stimulants. There were no differences in SUD risk factors between naturalistically treated and untreated groups other than family history of ADHD. We found no increased risks for cigarette smoking or SUD associated with stimulant therapy. We found significant protective effects of stimulant treatment on the development of any SUD (N = 113; HR = 0.27 (0.125–0.60), χ2=10.57, p=0.001) and cigarette smoking (N = 111; HR = 0.28 (0.14–0.60), χ2=10.05, p=0.001) that were maintained when controlling for conduct disorder. We found no effects of time of onset or duration of stimulant therapy on subsequent SUD or cigarette smoking in ADHD subjects.
Conclusions
Stimulant therapy does not increase, rather reduces the risk for cigarette smoking and SUD during adolescent years in girls with ADHD.
Keywords: ADHD, smoking, nicotine, stimulants, substance use disorders
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a prevalent neurobehavioral disorder occurring in 6–8% of children and 4–5% of adults worldwide [1–3]. Although more prevalent in boys than in girls [3] and the overwhelming majority of the extant literature on ADHD is based on boys, it is very clear that ADHD afflicts a sizeable number of girls and it is as much a source of morbidity and disability for girls as has been documented for boys [4, 5]. ADHD is now considered to be more chronic with from 40–60% of children continuing to manifest prominent ADHD symptoms and impairment through adolescence into adulthood [6–8][9]. Across the lifespan, ADHD has been shown to be associated with high risk for comorbid disruptive, mood, and anxiety disorders [4, 10]. Likewise, a high risk for cigarette smoking and substance use disorders (SUD; including drug and alcohol abuse and dependence) has also been shown in ADHD individuals growing up [11–15].
Among treatments for ADHD, stimulants remain among first line treatments for the disorder [16, 17]. Because ADHD is a well known risk for SUD [12, 13, 18] and because the stimulants are potential drugs of abuse [19], concerns remain as to the possibility for stimulants to increase the subsequent risk for cigarette smoking and SUD in individuals treated for their ADHD [20]. Along those lines, while one group found that cigarette smoking and cocaine abuse was associated with previous stimulant treatment [21], others have reported that stimulant treatment in youth with ADHD does not increase subsequent cigarette or SUD [22] and yet other studies [23–25] including a meta-analysis [26] have shown that it may exert a protective effect against the subsequent cigarette smoking and/or SUD.
Despite the implications of the effects of early stimulant treatment on later SUD, important limitations in the literature exist. For example, previous studies have not generally examined the length of stimulant exposure and later SUD, severity of SUD outcomes, or comorbidity with conduct disorder (CD) [21]. In addition, there is a limited literature that specifically examines the association between stimulant treatment and SUD attending to sex. Yet data suggest that girls with ADHD compared to boys with ADHD may have a substantially higher age matched risk for cigarette and substance use and SUD in early adolescence [27–29]. Moreover, data suggest differences may exist between boys and girls with ADHD in terms of SUD risk associated with prior stimulant treatment. For instance, (Katusic et al [24] reported a difference in the SUD risk reduction associated with stimulant treatment in boys but not girls with ADHD.
The main objective of the present study was to examine the effects of early stimulant treatment on the subsequent risk for cigarette smoking and SUD in adolescent girls with ADHD. Based on our previous work in a similarly aged sample of boys with ADHD [23] we hypothesized that stimulants would be associated with a reduction in the risk for SUD and cigarette smoking. Secondarily, we hypothesized that the duration of treatment would be directly related to the reduction in the risk for SUD [25, 30].
Methods
Subjects
Subjects were derived from a longitudinal case-control family study of girls with and without ADHD as described previously in detail [5]. Briefly at baseline, we studied female subjects aged 6–18 years with ADHD (N=140) and without ADHD (N=122), ascertained from pediatric and psychiatric sources. We excluded potential subjects if they had been adopted, their nuclear family was not available for study, if they had major sensorimotor handicaps (paralysis, deafness, blindness), psychosis, autism, inadequate command of the English language, or a Full Scale IQ less than 80. All of the ADHD subjects met full DSM-III-R diagnostic criteria for ADHD at the time of the clinical referral, and at recruitment they all had active symptoms of the disorder. The present study reports on the 5-year follow-up of the ADHD subjects [5]. Parents and adult offspring provided written informed consent to participate, and parents also provided consent for offspring under the age of 18. Children and adolescents provided written assent to participate. The human research committee at Massachusetts General Hospital approved this study protocol.
A three-stage ascertainment procedure was used to select subjects: in the first stage psychiatric or pediatric clinics referred conducted screening and referred subjects; in the second stage, we confirmed screening by administering a telephone questionnaire a subject’s mother; in the thrid stage, we assessed subjects with diagnostic structured interviews. Because this study had begun prior to the finalization of DSM-IV, our baseline assessment used DSM-III-R based structured interviews but we supplemented these with questions that would allow us to make DSM-IV diagnoses. Psychiatric assessments at the 5 year follow-up relied on the Schedule for Affective Disorders and Schizophrenia for School-Aged Children—Epidemiologic Version (K-SADS-E) for subjects younger than 18 years of age and the Structured Clinical Interview for DSM-IV (SCID) (supplemented with modules from the K-SADS-E to assess childhood diagnoses) for subjects 18 years of age and older. We considered a disorder positive if DSM-IV diagnostic criteria were unequivocally met in either interview. For diagnostic modules, including those assessing all substance use disorders and ADHD, the interviewer asked the subject to characterize the degree of impairment caused by the symptoms in question on their daily functioning on an ordinal scale -- minimal, moderate or severe.
A committee of nine board-certified child and adult psychiatrists who were blind to the subject’s ADHD status, referral source and all other data resolved diagnostic uncertainties. Diagnoses presented for review were considered positive only if a consensus was achieved that criteria were met to a degree that would be considered clinically meaningful. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was .98. Kappa coefficients for individual diagnoses included: ADHD (0.88), conduct disorder (1.0), major depression (1.0), mania (0.95), separation anxiety (1.0), agoraphobia (1.0), panic (.95), substance use disorder (1.0), and tics/Tourette’s (0.89).
Substance Use Measures
Our diagnostic interviews collected data on the lifetime use of nicotine, alcohol, marijuana, and other drugs - all substances with the exception of alcohol and nicotine will be referred to as “drugs.” For every substance used by a given subject, we derived the age of first use, lifetime diagnosis of DSM-IV abuse or dependence, and age of onset from structured interview data. Cigarette smoking refers to age-appropriate diagnosis of DSM-IV smoking dependence.
Statistical Analysis
We compared ADHD subjects at follow-up with and without lifetime history of stimulant medication on follow-up demographic factors. We used t-tests for age and Wilcoxon rank-sum tests for SES, and used Pearsonχ2 tests for binary outcomes. We controlled for demographic confounders if an outcome was significantly predicted by group membership at the α=0.1 level.
We used Cox proportional hazard survival models to estimate the lifetime risk for SUD associated with stimulant therapy. For each outcome, rates are defined as a positive response at any assessment (baseline or follow-up) versus a negative response at both assessments. These models utilize all available data for each subject, including those not assessed at the follow-up; thus, all 140 subjects are included, using as many waves of follow-up data as are available. We used the earliest age at onset as the survival time for cases and the age at most recent interview as the time of censoring for non-cases.
We created an indicator variable for each SUD outcome; this indicator is positive for a subject if: 1) a subject reported a lifetime history of treatment with any stimulant; and 2) the subject did not meet criteria for the substance use outcome before the onset of treatment. Untreated subjects and subjects who began stimulant treatment after the onset of the substance use were scored as negative on this binary variable. Subjects whose treatment and substance outcome began at the same age were impossible to categorize and were dropped from the analysis of that outcome. The statistical significance of each covariate in these regression models was determined by a linear Wald test, and our α-level was set at 0.05. All tests were two-tailed.
Results
We ascertained 114 subjects with complete medication and substance abuse data. These subjects ranged in age from 10 to 24 years of age at the 5-year followup; one-hundred and eight (95%) of subjects identified themselves as Caucasian, while 5 (4%) identified themselves as African-American and one (1%) subject was of unknown ethnicity. We found no differences in age, SES, frequency of family intactness, rates of CD, severity of ADHD impairment, parental history of SUD, or source of ascertainment between exposed and unexposed subjects (Table 1). We did find that subjects with ADHD receiving stimulant treatment were significantly more likely to have parents with a lifetime history of ADHD; all further analyses controlled for parental history of ADHD.
Table 1.
Demographic and stimulant treatment characteristics of girls with ADHD at 5-year follow-up
| No Stimulant Therapy | Stimulant Therapy | |||
|---|---|---|---|---|
| N=20 | N=94 | |||
| Mean | Mean | t1 | p | |
| Subject’s Age | 16.55±4.15 | 16.12±3.55 | 0.48 | 0.6 |
| Mean | Mean | z2 | p | |
| SES3 | 1.89±0.83 | 1.97±1.03 | −0.03 | 1.0 |
| N(%) | N(%) | χ24 | p | |
| Intact | 15(75) | 62(66) | 0.62 | 0.4 |
| Conduct Disorder (Full) | 7(35) | 38(40) | 0.20 | 0.7 |
| Level of ADHD5 | 1.26 | 0.5 | ||
| Impairment6 | ||||
| Mild | 1 (6) | 8 (9) | ||
| Moderate | 14(78) | 59(64) | ||
| Severe | 3(15) | 25(27) | ||
| Parental History of ADHD | 3(15) | 35(37) | 3.67 | 0.06 |
| Parental History of SUD7 | 11(55) | 65(69) | 1.49 | 0.2 |
| Source of Ascertainment | 5(25) | 41(44) | 2.37 | 0.1 |
t-test
Wilcoxon rank-sum test
Socioeconomic status
Pearson χ2-test
Attention-deficit/hyperactivity disorder
As assessed on an ordinal scale by subject reflecting impact on daily functioning
Substance use disorder
Exposure to stimulants
Risk for SUD
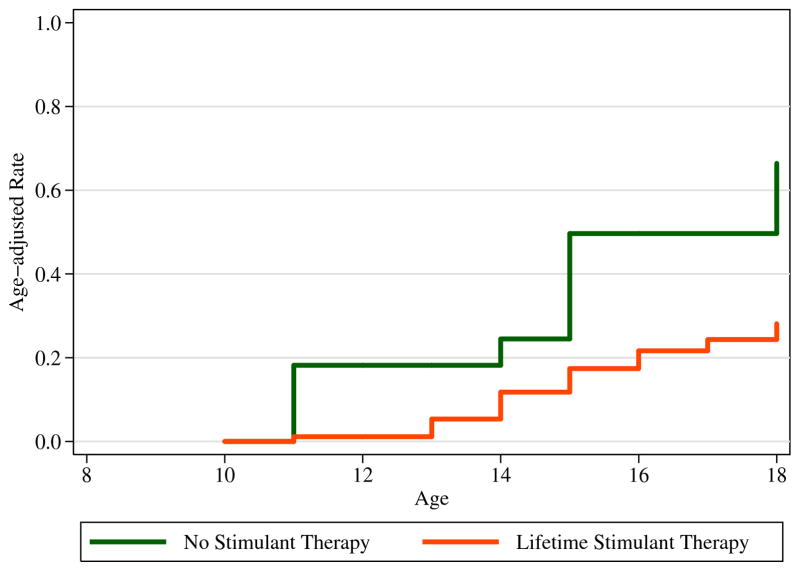
We compared subjects with ADHD with and without exposure to stimulants on age-adjusted rates of developing SUD. We failed to find any evidence of a significantly higher risk for any SUD among subjects exposed to stimulant medication. Instead, we found evidence for a significant protective effect of stimulant exposure on the subsequent development of any SUD (Figure 1). Stimulant exposed girls with ADHD were 73% less likely to manifest a SUD compared to girls who were not exposed to stimulants (see Table 2).
Figure 1.
The effects of prior stimulant exposure on risk for a subsequent substance use disorder (SUD; curves truncated at 18 years).
Table 2.
Rates of substance use disorders in girls at 5 year follow up. Sample sizes in each category depend on stimulant exposure prior to development of the specified substance use disorder, and therefore vary from 91 to 94 for stimulant-treated girls
| No Stimulant Therapy | Stimulant Therapy | ||||
|---|---|---|---|---|---|
| N=20 | N=94 | ||||
| N(%) | N(%) | HR | 95% | CI | |
| SUD8 | 11(48) | 19(21) | 0.306 | 0.138 | 0.679 |
| Alcohol abuse | 5(23) | 11(12) | 0.612 | 0.198 | 1.893 |
| Alcohol dependence | 2(10) | 3( 3) | 0.288 | 0.046 | 1.797 |
| Substance abuse | 8(36) | 11(12) | 0.297 | 0.117 | 0.754 |
| Substance dependenc | 4(19) | 9(10) | 0.565 | 0.163 | 1.958 |
Substance use disorder
We also failed to find any evidence for increases in the risks for class or severity of dependence associated with stimulant treatment. In contrast, we found evidence of specific SUD risk reduction associated with prior stimulant treatment. More specifically, we found a significant protective effect of stimulant exposure on age-adjusted rate of development of drug abuse (N = 112) and although not statistically significant, a lower effect of stimulant exposure on drug dependence as well (N = 112). Likewise, we found no significant effect of stimulant exposure on alcohol abuse (N = 114) or alcohol dependence (N = 114).
Risk for Cigarette Smoking
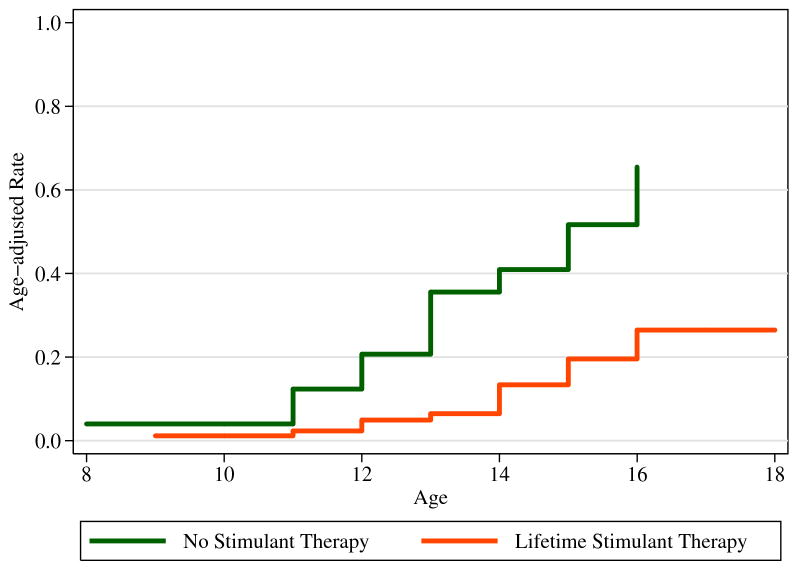
We also evaluated stimulant exposure in relation to the development of cigarette smoking (dependence). We failed to find a significantly higher risk for cigarette smoking (dependence) and prior exposure to stimulant medication. In contrast, we found a significant protective effect of stimulant exposure on age-adjusted rate of development of smoking in our sample (Figure 2). Subjects with ADHD who were previously treated with stimulant had a 72% lower risk and later onset of cigarette smoking relative to ADHD subjects without stimulant treatment (see Table 2).
Figure 2.
The effects of prior stimulant exposure on risk for a subsequent cigarette smoking (dependence; curves truncated at 18 years).
Because the comorbidity with CD is a potent predictor of subsequent risk for SUD and cigarette smoking in subjects with ADHD ([31] we repeated each analysis controlling for the effect of CD. Results showed that controlling for the effect of CD did not change any of the above results. As expected, the effect of CD was significant for overall SUD, drug abuse (N=112, HR=3.61 (1.13–11.5), p=0.03), and drug dependence (N=112, HR=5.0 (1.41–17.5), p=0.01).
Onset and Duration
We found no effect of age of onset of stimulant therapy on the development of any SUD or smoking. Likewise, there was no effect of stimulant duration on the development of cigarette smoking (N=86, HR=1.02 (.87–1.18), χ2=0.04, p=0.8) or SUD (N=90, HR=1.01 (0.90–1.14), χ2=0.06, p=0.8) in subjects with ADHD. We then tested if stimulant therapy affected the duration of alcohol abuse and dependence, drug abuse and dependence and smoking and found no effect of stimulant exposure on duration of SUD (linear regression; N=30, t=0.81, p=0.425) or smoking (linear regression; N=28, t=0.36, p=0.7) in subjects that developed SUD or smoking.
Discussion
In a longitudinal sample of girls with ADHD followed for five years into adolescent years, we found strong evidence that prior treatment with stimulants was associated with a subsequent decreased risk for SUD and cigarette smoking. We did not detect any significant association between age of onset or duration of stimulant treatment and subsequent risk of SUD or cigarette smoking. Similarly, in those who did develop SUD, there was no relationship between stimulant treatment and the severity or duration of SUD. While limited by a relatively small sample of girls who were unmedicated for their ADHD, these findings extend to girls with ADHD previously reported findings in boys with ADHD [22–25] suggesting that prior stimulant treatment does not increase the risk for subsequent SUD and cigarettes smoking; and may instead have a protective effective of SUD and cigarette smoking.
The present results replicate our previous findings in a boys with ADHD suggesting a protective effect of stimulant treatment against subsequent alcohol and drug use disorders [23]. The current work adds to a growing body of literature showing general reductions in SUD stimulant-treated children with ADHD in their adolescent years [26]. Our results documenting protective effects of stimulants in girls with ADHD are not entirely consistent with those of (Katusic et al [24] that showed that the protective effect of stimulants against SUD was limited to boys with ADHD. The reasons for discrepancy are probably due to the relatively small sample size of girls in the Katusic sample that limited their Power to detect meaningful differences.
The protective effects of stimulants against the development of SUD is particularly noteworthy considering that a greater proportion of our adolescent girls with ADHD were more fully into the age of risk for SUD compared to our boys with ADHD when they were assessed [27]. Furthermore, our data have shown that girls with ADHD have an almost two year earlier onset of SUD relative to boys with ADHD (17 years vs. 19 years, respectively) [12, 32, 33].
Studies examining the effects of stimulant therapy on subsequent SUD have generally shown more of a protective effect in adolescents and a rather neutral effect in adults [26] leading to the notion that the stimulants may delay rather than protect against subsequent SUD. More research is needed to understand this developmental effect of stimulants (e.g. persistence of treatment vs. underlying biological effect) on subsequent substance use and to further clarify their protective mechanisms.
Our results are among the first to demonstrate a clinically and statistically significant reduction in the risk and delayed onset of cigarette smoking associated with stimulant treatment in girls with ADHD. Our current results are consistent with epidemiological evidence from Germany [25] indicating delays in the onset of smoking and lower rates of smoking associated with stimulant treatment in ADHD subjects. Our data are also consistent with a recently reported prospective study that found an association between stimulant therapy and diminished risk for cigarette smoking [34]. However, our findings are in contradistinction with an older naturalistic study by Lambert and colleagues [21] that showed higher risk for tobacco dependence in treated ADHD subjects; however, the stimulant treated group had an overrepresentation of CD, a strong predictor of SUD and cigarette smoking [31, 35]. Our data may be of further importance given prior work in boys with ADHD showing that early cigarette smoking in ADHD is related to a very high risk for subsequent SUD [36].
While the mechanism of risk reduction for SUDs and cigarette smoking remain unclear, some recent preclinical data may shed light on this important area. For instance, Augustyniak et al [37] showed prepubertal exposure of methylphenidate in an animal model of ADHD (spontaneous hypertensive rat) resulted in diminished sensitivity to the incentive properties of cocaine in adulthood without altering the responses of mesolimbic dopamine system. Similarly, enduring effects of early exposure of methylphenidate to rat pups resulted in diminished subsequent behaviors in these animals that was synonymous with SUD [38]. Psychosocial considerations explaining the reduced risk of SUD associated with stimulant treatment also need be considered. For instance, decreased risk for SUD may be related to those families who seek out appropriate treatment for their children. Alternatively, it may be that the necessary supervision and heightened monitoring and of youth receiving stimulants is associated with the reduced SUD. Clearly, more work is necessary in understanding if the risk reduction for SUD and cigarette smoking in adolescents with ADHD treated with stimulants is related to a biological, psychosocial, or a combined mechanism of action.
These results must be considered in the light of the methodological limitations. Our naturalistic study design cannot provide evidence as compelling as that produced by a randomized, controlled study of stimulant treatment. Because participating subjects were referred and largely Caucasian we do not know if our results will generalize to ADHD children in the general population, or to other racial or ethnic backgrounds. Furthermore, because the girls with ADHD in our sample were mostly adolescents, they had not yet not fully transitioned through the age of risk for SUD and cigarette smoking. The small size of our sample of untreated girls with ADHD also limits our statistical power. Although our study was prospective, we still depended on retrospectively (i.e., within the intervals between assessments) reported ages of treatment and cigarette and substance use disorder onset to establish the temporal sequence. We relied on structured interview data and not objective measures (e.g. urine toxicology) to determine dependence on cigarettes or SUD and may have underestimated lifetime rates of these disorders. However, recent data suggest that structured interview-derived substance use data may be more sensitive to determine past SUD compared to objective measures [39]. We also did not examine the role of other treatment modalities and SUD. It is notable, however, that previous work failed to find any relationship between psychotherapy and later SUD outside of stimulant treatment.
Despite these limitations, this study provides evidence for the first time that prior stimulant treatment does not increase the subsequent risk for and may have protective effects against the development of cigarette smoking and SUD in adolescent girls with ADHD. These data add to a growing literature documenting that stimulant treatment of ADHD may diminish the risk for cigarette smoking and SUD in adolescence. These results should allay lingering concerns among clinicians and families about future substance problems when prescribing stimulants to a child with ADHD. Future research should focus on more salient predictors and moderators of SUD risk in girls with ADHD as well as following this group fully through the ages of SUD risk.
Acknowledgments
This project was supported by grants DA R01 DA14419 & K24 DA016264 from the National Institutes of Health, Bethesda, M (Dr. Wilens) and the Lilly Foundation.
Footnotes
Author Contributions: Dr. Wilens had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Biederman, Faraone.
Acquisition of data: Schillinger, Westerberg, Wilens, Biederman.
Analysis and interpretation of data: Adamson, Monuteaux, Faraone, Wilens, Biederman.
Drafting of the manuscript: Adamson, Faraone, Biederman.
Critical revision of the manuscript for important intellectual content: Schillinger, Westerberg, Monuteaux, Faraone, Wilens, Biederman.
Statistical analysis: Adamson, Monuteaux, Faraone.
Obtained funding: Faraone, Biederman.
Administrative, technical, or material support: Schillinger, Westerberg, Wilens, Biederman.
Study supervision: Monuteaux, Wilens, Biederman.
References
- 1.Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: Results from the national comorbidity survey replication. American Journal of Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faraone SV, et al. The Worldwide Prevalence of ADHD: Is it an American Condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- 3.Polanczyk G, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 4.Hinshaw SP. Preadolescent girls with attention-deficit/hyperactivity disorder: I. Background characteristics, comorbidity, cognitive, and social functioning, and parenting practices. J Consult Clin Psych. 2002;70(5):1086–1098. doi: 10.1037//0022-006x.70.5.1086. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, et al. Psychopathology in females with attention-deficit/hyperactivity disorder: A controlled, five-year prospective study. Biological Psychiatry. 2006;60(10):1098–105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Weiss G, editor. Child and Adolescent Psychiatric Clinics of North America. W.B. Saunders Company; Philadelphia: 1992. Attention-Deficit Disorder. [Google Scholar]
- 7.Biederman J, Faraone S, Mick E. Age dependent decline of ADHD symptoms revisited: Impact of remission definition and symptom subtype. American Journal of Psychiatry. 2000;157:816–817. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 8.Wolraich ML, et al. Attention-deficit/hyperactivity disorder among adolescents: a review of the diagnosis, treatment, and clinical implications. Pediatrics. 2005;115(6):1734–46. doi: 10.1542/peds.2004-1959. [DOI] [PubMed] [Google Scholar]
- 9.Pliszka SR, et al. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642–57. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- 10.Biederman J, et al. Young Adult Outcome of Attention Deficit Hyperactivity Disorder: A Controlled 10 year Follow-Up Study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 11.Milberger S, et al. ADHD is associated with early initiation of cigarette smoking in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Wilens TE, et al. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. Journal of Nervous and Mental Disease. 1997;185(8):475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Molina B, Pelham W. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 14.Molina BS, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1028–40. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- 15.McGough JJ, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162(9):1621–7. doi: 10.1176/appi.ajp.162.9.1621. [DOI] [PubMed] [Google Scholar]
- 16.Greenhill LL, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 Suppl):26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 17.Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):1033–44. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 18.Biederman J, et al. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. American Journal of Psychiatry. 1995;152(11):1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- 19.Kollins SH, MacDonald EK, Cush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: A review. Pharmacology Biochemistry and Behavior. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 20.Vitiello B. Long-term effects of stimulant medications on the brain: possible relevance to the treatment of attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2001;11(1):25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- 21.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disablities. 1999;31(6):533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 22.Barkley RA, et al. Does the treatment of ADHD with stimulant medication contribute to illicit drug use/abuse? A 13 year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- 23.Biederman J, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 24.Katusic SK, et al. Psychostimulant Treatment and Risk for Substance Abuse Among Young Adults with a History of Attention-Deficit/Hyperactivity Disorder: A Population-Based, Birth Cohort Study. J Child Adolesc Psychopharmacol. 2005;15(5):764–76. doi: 10.1089/cap.2005.15.764. [DOI] [PubMed] [Google Scholar]
- 25.Huss M. ADHD and Substance abuse. IX Annual European Congress of Psychiatry; Hamburg. 1999. [Google Scholar]
- 26.Wilens T, et al. Does stimulant therapy of ADHD beget later substance abuse: A metanalytic review of the literature. Pediatrics. 2003;11(1):179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 27.Milberger S, et al. Associations between ADHD and psychoactive substance use disorders: Findings from a longitudinal study of high-risk siblings of ADHD children. American Journal on Addictions. 1997;6:318–329. [PubMed] [Google Scholar]
- 28.Biederman J, et al. Clinical correlates of ADHD in females: Findings from a large group of girls ascertained from pediatric and psychiatric referral sources. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(8):966–975. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Disney ER, et al. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry. 1999;156(10):1515–21. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- 30.Huss M, Lehmkuhl U. Methylphenidate and substance abuse: a review of pharmacology, animal, and clinical studies. J Atten Disord. 2002;6(Suppl 1):S65–71. doi: 10.1177/070674370200601s09. [DOI] [PubMed] [Google Scholar]
- 31.Mannuzza S, et al. Adult outcome of hyperactive boys: Educational achievement, occupational rank, and psychiatric status. Archives of General Psychiatry. 1993;50:565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 32.Milberger S, et al. Familial risk analysis of the association between attention-deficit/hyperactivity disorder and psychoactive substance use disorders. Arch Pediatr Adolesc Med. 1998;152:945–951. doi: 10.1001/archpedi.152.10.945. [DOI] [PubMed] [Google Scholar]
- 33.Wilens T. Attention-deficit/hyperactivity disorder and the substance use disorders: The nature of the relationship, subtypes at risk and treatment issues. In: Spencer T, editor. Psychiatric Clinics of North America. Saunders Press; Philadelphia: 2004. pp. 283–301. [DOI] [PubMed] [Google Scholar]
- 34.Monuteaux MC, et al. A Randomized Clinical Trial of Bupropion for the Prevention of Smoking in Youth with Attention-DeficitHyperactivity Disorder. Journal of Clinical Psychiatry. doi: 10.4088/jcp.v68n0718. in press. [DOI] [PubMed] [Google Scholar]
- 35.Robins LN. Deviant children grown up. Baltimore: Williams and Wilkins; 1966. [Google Scholar]
- 36.Biederman J, et al. Is cigarette smoking a gateway drug to subseqeunt alcohol and illicit drug use disorders? A controlled study of youths with and without ADHD. Biological Psychiatry. 2006;59:258–64. doi: 10.1016/j.biopsych.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Augustyniak PN, et al. Differential behavioral and neurochemical effects of cocaine after early exposure to methylphenidate in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2006;167(2):379–82. doi: 10.1016/j.bbr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Carlezon WA, Mague SD, Anderson SL. Enduring behavioral effects of early exposure to methylphenidate in Rats. Biological Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Gignac M, et al. Assessing cannabis use in adolescents and young adults: what do urine screen and parental report tell you? J Child Adolesc Psychopharmacol. 2005;15(5):742–50. doi: 10.1089/cap.2005.15.742. [DOI] [PubMed] [Google Scholar]