Abstract
Merlin loss causes benign tumours of the nervous system, mainly schwannomas and meningiomas. Schwannomas show enhanced Rac1 and Cdc42 activity, The p21-activated kinase 2 (PAK2) activation and increased ruffling and cell adhesion. PAK regulates activation of merlin. PAK has been proposed as a potential therapeutic target in schwannomas. However where PAK stands in the Rac pathway is insufficiently characterised. We used a novel small molecule PAK inhibitor, IPA-3, to investigate the role of PAK activation on Rac1/Cdc42 activity, cell spreading and adhesion in human primary schwannoma and Schwann cells. We show that IPA-3 blocks activation of PAK2 at Ser192/197 that antagonises PAKs interaction with Pix. Accordingly, Pix-mediated Rac1 activation is decreased in IPA-3 treated schwannoma cells, indicating that PAK acts upstream of Rac. We show that this Rac activation at the level of focal adhesions in schwannoma cells is essential for cell spreading and adhesion in Schwann and schwannoma cells.
Keywords: schwannoma, PAK, merlin, small GTPases
Introduction
Merlin is a tumour suppressor whose loss leads to the formation of benign tumours of the nervous system, mainly schwannomas and meningiomas that are also hallmarks of the inherited disease neurofibromatosis type 2 (NF2). P21-activated kinase (PAK) phosphorylates merlin and is inhibited by merlin in a negative feedback-loop. PAK is thus being regarded as potential therapeutic target in NF2 (Xiao et al. 2002;Kissil et al. 2003;Hirokawa et al. 2004). PAK can crosstalk to the MEK/ERK pathway (Beeser et al. 2005) which could affect Schwann cell proliferation, but foremost PAK is known to act as an effector of the RhoGTPases Rac1 and Cdc42 and is thereby supposed to regulate cellular processes like cell spreading, formation of ruffles and adhesion to the extracellular matrix (Bokoch 2003;Zhao and Manser 2005). Merlin-deficient human primary schwannoma cells do show elevated levels of activated Rac1 that colocalises with phospho-PAK2 at the membrane (Kaempchen et al. 2003;Flaiz et al. 2007). Ongoing ruffling through Rac1 activation leads to decreased intercellular adhesion in schwannoma cells (Flaiz et al. 2008) and subsequent loss of contact inhibition of growth in fibroblasts and keratinocytes (Lallemand et al. 2003). In addition, merlin-deficient human schwannoma cells show elevated levels of Cdc42, overexpression of integrin beta1 and numerous stable paxillin-containing focal adhesions and increased adhesion to ECM (Utermark et al. 2003;Flaiz et al. 2009). Activation of Rac in merlin deficient cells (Shaw et al. 2001;Kaempchen et al. 2003) has also been linked to an inability to ensheath axons (Nakai et al. 2006). GTPase activation at the membrane is seen as one of the major properties linked to merlin deficiency and accounts for many of the pathological processes in merlin-deficient tumours. Despite considering PAK as a target, PAKs position in the Rac pathway and PAKs role in cell spreading, ruffling and adhesion in merlin-deficient tumours is yet insufficiently characterised. Recently we found evidence for Rac activation through the Rac/Cdc42 exchange factor Pix at the levels of focal adhesions, thereby providing a link between the pathological adhesion and increased Rac1 activation in schwannoma. (Flaiz et al. 2009). PAK interacting exchange factor (Pix) (Rosenberger and Kutsche 2006) localises to paxillin via G-protein coupled receptor kinase-interacting protein (GIT) in a complex with PAK (Manser et al. 1998). Using different cell lines ten Klooster et al. 2006 provided an interesting model in which PAK dissociates from Pix in focal adhesions after phosphorylation through Cdc42 that itself has been activated through integrin engagement after adhesion to the extracellular matrix (ten Klooster et al. 2006). After phosphorylated PAK has dissociated from Pix, Rac can be activated and translocates to the membrane. According to this model, PAK is not only a Rac effector, but also acts upstream of Rac, a phenomenon that was suggested before in cell lines after PAK overexpression (Obermeier et al. 1998). Thus we hypothesise that in merlin deficient schwannoma cells activated PAK is leading to Rac activation and altered adhesion
Using the novel isoform-selective, small-molecule PAK inhibitor IPA-3 (“inhibitor targeting PAK1 activation”) targeting the autoregulatory mechanism of of PAK 1, 2 and 3 (Deacon et al. 2008), we show that PAK acts downstream of Cdc42 but upstream of Rac1 in merlin-deficient human primary schwannoma cells. PAK and the Pix-PAK-mediated Rac activation are involved in cell spreading and adhesion of human primary schwannoma cells. We show that PAK activity is also needed for normal Schwann cell spreading as suggested by Thaxton et al. 2007 as well as for adhesion to the extracellular matrix and development of polarity. Taken together our findings contribute to the understanding of the mechanism of GTPase activation in schwannoma and the role of PAK in cell spreading and adhesion in Schwann and schwannoma cells. They demonstrate that IPA-3 can be used to elucidate PAK functions in human primary cells, that PAK acts upstream of Rac and is a relevant target in schwannomas.
Material and Methods
Preparation of human primary Schwann and schwannoma cells
Schwann cells were obtained from peripheral nerves from tissue donors not carrying any predisposition to a peripheral neuropathy. Schwannomas were kindly provided by NF2 patients after informed consent. Diagnosis of NF2 was based on clinical criteria defined by the NIH Consensus Conference on Neurofibromatosis. Isolation and culturing were carried out as previously described (Rosenbaum et al. 1998). Briefly, cells were cultured in proliferation medium and grown on poly-L-lysine/laminin coated 6-well plates (Greiner bio-one, Stonehouse, UK), 8-well permanox chamber slides (Nunc, Wiesbaden, Germany) or glass-bottom-dishes (Mattek, Ashland, MA, USA). At least three different schwannoma from different patients or Schwann cells from three different donors were used in each experiment. Every experiment was carried out between the second and the fourth passage, when proliferation rates are best and the number of fibroblasts is neglible (less than 2 %, routinely checked by S-100 staining).
Cell viability assay
The effect of IPA-3 and PIR-3.5 on schwannoma cell’s viability were assessed with a cell titer 96© aqueous non-radioactive cell proliferation assay (MTS test from Promega).
Human primary schwannoma cells were grown on 96 well plates for 2 days. Cells were left untreated or treated with 5 µM IPA-3, 20 µM IPA-3 or 20 µM PIR-3.5 for 24 hours. The MTS-solution was left on the cells for 3 hours, before the absorbance at 490 nm was measured. The experiments were conducted three times and mean and standard error of the mean was calculated with Excel.
Western Blot analysis
Schwannoma cells were grown until subconfluent and serum starved overnight. Cells were left untreated or preincubated with 2 µM, 5 µM or 20 µM IPA-3 (“inhibitor targeting PAK1 activation” 2,2’-dihydroxy-1.1’-dinaphthylsulfide) or 20 µM of the control substance PIR-3.5 (“PAK1 inhibitor relative 3.5” 2-naphthalenol-6,6’-dithiobis) for 10minutes, before stimulation with 10 % FCS (PAA, Pasching, Austria), 0.5 µM forskolin (Sigma-Aldrich, St.Louis, USA), 10 nM β1-heregulin144–244, (Mark Sliwkowski, Genentech, San Francisco, USA), 0.5 mM 3-isobutyl-1-methylxanthin and 2.5 µg/ml insulin (both from Sigma-Aldrich) for 5 minutes. Cells were lysed as previously described (Utermark et al. 2005a). Equal amounts of total cell lysate were separated by SDS-PAGE on a 12 % polyacrylamide gel. Blocking was done in TBS-T containing 5 % milk, 2 % BSA. Membranes were then incubated with anti-phospho-PAK1/2 (PAK1 serines, 198/203; PAK2 serines, 192/197, 1:500, Cell Signaling Technology, Davis MA, USA) followed by incubation with appropriate HRP-conjugated secondary antibodies (Biorad Laboratories, Hercules, USA). As actin and tubulin are differentially regulated in our system (cDNA array, data not shown), we use RhoGDI (anti-RhoGDI, 1:500, Santa Cruz, CA, USA) that is not regulated in our system (Hanemann et al. 2006) as a loading control. ECL (Amersham, Buckinghamshire, UK) was used for detection. Optical density measurements were performed, after correction of background levels, with a Biorad FluorS Multi Imager, using the Quantity One software (Bio-Rad Laboratories). Experiments were carried out at least three times, using different patient material. Protein levels of IPA-3 or PIR-3.5 treated cells were normalised to levels of untreated schwannoma cells to allow comparison of blots. Mean and standard error of the mean were calculated with Excel. Two-tailed student’s t-test was performed to find out whether the detected difference is significant.
Rac G-protein linked immunosorbent assay (GLISA)
To measure the effect of IPA-3 or PIR-3.5 on Rac activation, human primary schwannoma and for comparison untreated Schwann cells were cultured in Poly-L-lysin/laminin coated 6 well plates until 70 % confluent. Cells were serum starved over night. Schwannoma cells were either left untreated or pre-incubated with 20 µM PIR-3.5 or 2 µM, 5 µM or 20 µM IPA-3 for 10 minutes. Cells were then stimulated with 10 % FCS (PAA), 0.5 µM forskolin (Sigma-Aldrich), 10 nM β1-heregulin144–244 (Genentech), 0.5 mM 3-isobutyl-1-methylxanthin (IBMX, Sigma-Aldrich) and 2.5 µg/ml insulin (Sigma-Aldrich) for 5 minutes. Rac1-GTP was detected using the GLISA Rac1 activation assay biochem Kit™ (absorbance based) from cytoskeleton (cytoskeleton, Denver, USA) using the aforementioned procedure. Briefly, cells were lysed according to the manufacturer’s protocol and lysates incubated on 96-well plates that contain a Rac-GTP-binding protein. Bound Rac-GTP is detected with a Rac specific primary antibody and a HRP-conjugated secondary antibody. Appropriate controls were carried out (positive control: Rac control protein, negative control: lysis buffer alone). Levels of Rac1-GTP in IPA-3 or PIR-3.5 treated human primary schwannoma cells from 3 different patients were normalised to the corresponding untreated cells. Mean and standard error of the mean were calculated with Excel and the two-tailed student’s t-test was performed to find out whether the detected differences was significant.
Cdc42 activation assay
Serum starved schwannoma cells were left untreated or pre-treated with 20 µM IPA-3 for 30 minutes, before stimulating as described above. Cdc42 assay was performed as described (Flaiz et al. 2007). Briefly Cells were lysed and probes were placed on Swell Gel Immobilized Glutathione Disc and 20 µg of GST-PAK1-PBD were added. As controls cell lysates, obtained from a mesothelioma cell line, were incubated with 0.1 mM GTPγS (positive control) or 1 mM GDP (negative control). A small portion of Schwann and schwannoma cell lysate was removed prior to the pull down procedure to analyse total Cdc42 levels. Samples, controls and untreated cell lysates were then separated by 12 % SDS-PAGE, blotted and blocked with TBS-T containing 2 % BSA, 5 %milk, incubated with anti-Cdc42 as described (Flaiz et al. 2007). Optical density measurements were performed, as described above. All experiments were carried out at least three times, using different patient material. Protein levels of IPA-3 treated cells were normalised to the levels of untreated schwannoma cells to allow comparison of blots. Mean and standard error of the mean were calculated with Excel and the two-tailed student’s t-test was performed to find out whether the detected differences are significant.
Ruffling/cell spreading assay, immunocytochemistry
Schwann and schwannoma cells were serum starved over night prior to trypsination. Trypsination was stopped in 10 ml DMEM (Gibco), containing 2.5 mg soy bean trypsine inhibitor (Sigma-Aldrich) and 1 % BSA (PAA). Cells were centrifuged, the pellet resuspended in DMEM and left untreated or treated with IPA-3 or PIR-3.5 as above. After stimulation with 10 % FCS (PAA) 0.5 µM forskolin (Sigma-Aldrich), 10 nM β1-heregulin144–244 (Genentech), 0.5 mM 3-isobutyl-1-methylxanthin and 2.5 µg/ml insulin (both from Sigma-Aldrich), equal cell numbers were seeded into poly-L-lysine/laminin coated, flexiperm containing lumox dishes (Greiner). Cells were allowed to spread for 30 minutes. For washout experiments, inhibitor-containing medium was changed to inhibitor-free one and cells were allowed to spread for another 30 minutes. Cells were fixed in 4 % paraformaldehyde, permeablised with 1 % TritonX-100 and blocked with 10 % normal goat serum and incubated anti-p34-Arc (1:100 upstate Biotechnology). Appropriate Cy3 or Alexa Fluor 633-labelled secondary antibodies were used. Alexa Fluor 488-labelled phalloidin was used to visualise filamentous actin (1:100 Molecular Probes). To investigate cell’s ability to spread cells were imaged with a 20X objective under a Zeiss510meta confocal microscope. Spread and unspread cells were counted manually. In addition cell spreading areas of 50 cells per condition were measured using the volocity software. Cell spreading areas of IPA-3 and PIR-3.5 treated cells were normalised to the cell spreading areas of corresponding untreated schwannoma cells. Representative images of each condition were taken with a 63X objective and are shown in galleries of three different cells. In addition, z-stacks from the confocal laser scanning microscope were exported to a workstation and used to create 3D animations of Schwann and schwannoma cells using the volocity software according to software manual. Schwann and schwannoma cells from 3 different patients/donors were analysed. Mean and standard error of the mean of cell spreading areas were calculated with Excel and the two-tailed student’s t-test was performed to find out whether the detected differences are significant.
Adhesion Assay
Schwann and schwannoma cells were serum starved over night prior to trypsination. Trypsination was stopped as described above. Cells were centrifuged, the pellet resuspended in DMEM and left untreated or treated as above with IPA-3 or PIR-3.5 After stimulation as described, quintuples of equal cell numbers per condition were seeded into poly-L-lysine/laminin treated 24 well plates. After 3 h incubation at 37°C, cells were rinsed twice with PBS to wash away loose cells. Adherent cells were fixed in 4 % paraformaldehyde (Sigma-Aldrich) and subsequently counted under an Olympus phase contrast microscope. Number of adherent schwannoma cells under 2 µM, 5 µM or 20 µM IPA-3 or 20µM PIR-3.5 were normalised to number of corresponding untreated schwannoma cells. Schwann and schwannoma cells from 3 different patients/donors were analysed. Mean and standard error of the mean were calculated with Excel and the two tailed student’s t-test was performed.
Results
IPA-3 reduces PAK2 phosphorylation at Ser192/197
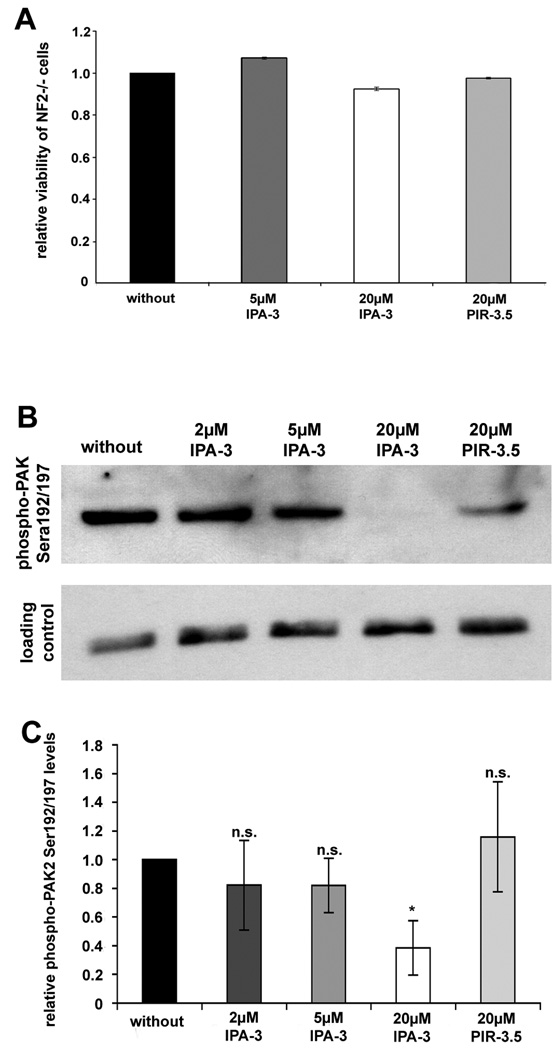
To test if the novel Group I PAK inhibitor IPA-3 is effective in human primary schwannoma cells, we first analysed if it affected cell viability. Neither IPA-3 nor the control substance PIR-3.5 with concentrations up to 20 µM affected cell viability, as a cell viability assay (MTS test) revealed (Fig.1A). IPA-3 has been shown to inhibit PAK autophosphorylation in vitro (Deacon et al. 2008) and Ser199/204 (PAK1) /Ser192/197 (PAK2) phosphorylation antagonises the Pix-PAK-interaction (Zhao and Manser 2005). The release of PAK from Pix is likely crucial for facilitating activation of Rac1 (ten Klooster et al. 2006). 20 µM of IPA-3 clearly reduced PAK2 phosphorylation (the major phosphorylated PAK isoform in human Schwann cells (Flaiz et al. 2007)) at Ser192/197 in human primary schwannoma cells, whereas 20 µM of the structurally related control compound PIR-3.5 (using the same highly diluted carrier) had only a mild effect on PAK phosphorylation (Fig.1B, C ). 2 µM and 5 µM IPA-3 only slightly decreased PAK2 phosphorylation. No band for phospho-PAK1 (Ser199/204) was detected. These findings show that IPA-3 efficiently blocks phosphorylation of PAK2 at Ser192/197 in human schwannoma cells.
Figure 1.
Effect of IPA-3 and PIR-3.5 on cell viability and on PAK phosphorylation at Ser199/204//Ser192/197 in human primary schwannoma cells
(A): Human primary schwannoma cells were left untreated (without) or treated with 5 µM IPA-3, 20 µM or 20 µM PIR-3.5 for 24 hours. MTS-assays revealed that 5 µM IPA-3 and 20 µM PIR-3.5 had no effect on schwannoma cell’s viability. Even a treatment with 20 µM IPA-3 for 24 hours, reduced schwannoma cell’s viability by only 7.6%. Viable schwannoma cells were measured using a non-radioactive cell proliferation assay (MTS-assay). Bars represent mean of the percentage of viable cells normalised to cells without treatment (n=3). Scale bars represent standard error of the mean.
(B): Western Blot analysis revealed that 20µM IPA-3 clearly reduces PAK2 phosphorylation at Ser192/197 in human primary schwannoma cells. The ineffective control substance PIR-3.5 only mildly affects PAK phosphorylation. Rho-GDI, that is not regulated in schwannoma cells, served to control equal loading on each filter.
(C): Optical density measurements, normalised to phospho-PAK2 Ser192/194 levels in untreated cells, are depicted as bars. Scale bars represent standard error of the mean (SEM). 20 µM IPA-3 reduced PAK2 phosphorylation at Ser192/194 to 40 % of phospho-PAK2 levels of untreated schwannoma cells (*: significant; p<0.05 in student’s t-test), whereas 2 µM and 5 µM IPA-3, as well as 20 µM PIR-3.5 did not significantly alter phospho PAK2 Ser192/194 levels. Phospho-PAK2 localises to the membrane of untreated human primary schwannoma cells (NF2−/−)
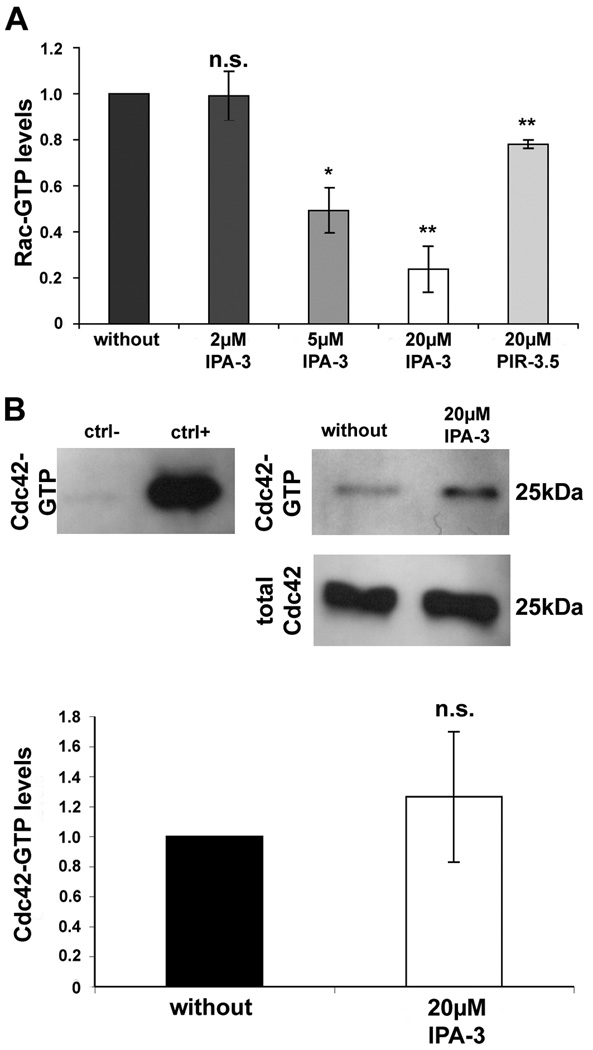
IPA-3 reduces Rac1 but not Cdc42 activation
Previous results have already shown that at least part of the Rac activation in human schwannoma occurs through beta-Pix that localises to focal adhesions (Flaiz et al. 2009). PAK and Rac compete for binding to beta-Pix and PAK activation and subsequent dissociation from focal adhesions after phosphorylation could allow Rac to bind to beta-Pix (ten Klooster et al. 2006). Since IPA-3 reduced PAK2 autophosphorylation at Ser192/197, PAK should stay associated with beta-Pix and therefore prevent beta-Pix induced Rac activation. GLISA analysis showed clearly reduced Rac-GTP levels after incubation with 5µM and 20µM of the PAK inhibitor in a dose-dependent manner in human primary schwannoma cells supporting this hypothesis (Fig.2A). 2 µM IPA-3 does not have a significant effect on Rac1 activation. The control compound PIR-3.5, used at a high concentration (20 µM), had only a mild effect on Rac activation. Whereas PAK activity clearly influences the activation of Rac, Cdc42 is reported to act upstream of PAK by promoting its autophosphorylation and activation (Manser et al. 1994). If this is true in human schwannoma cells, blocking of PAK activation by IPA-3 should not affect levels of Cdc42-GTP. Pull down analysis showed that incubation with 20 µM IPA-3 that clearly reduced Rac-GTP levels, did not significantly alter Cdc42-GTP levels (Fig.2B). Collectively these data suggest that PAK is downstream of Cdc42, but upstream of Rac in human schwannoma cells.
Figure 2.
IPA-3 effect on GTPase activation in human primary schwannoma cells
(A): Blocking of PAK activation with 5 µM and 20 µM IPA-3 reduced levels of Rac-GTP to about 50 % (5µM IPA-3) and 20 % (20µM IPA-3) of Rac-GTP levels of untreated cells (*: significantly for 5 µM IPA-3, p<0.05 and **: highly significantly for 20 µM IPA-3, p<0.01in student’s t-test) in schwannoma cells. 2 µM IPA-3 and 20 µM of the control substance PIR-3.5 did not significantly alter Rac-GTP levels. Rac-GTP levels were determined using GLISA assays. Scale bar represent standard error of the mean (B): Western Blot analysis for Cdc42 showed slightly increased Cdc42-GTP levels (after pull-down assay with the PAK-binding domain PBD) after 20 µM IPA-3 treatment (not significant in student’s t-test, scale bars represent standard error of the mean). Total Cdc42 levels were measured to confirm equal Cdc42 expression. Positive control (ctrl+) and negative control (ctrl-): cell lysate from a mesotheliome cell line treated with GTPγS or GDP, respectively.
IPA-3 reduces cell spreading in human primary Schwann and schwannoma cells
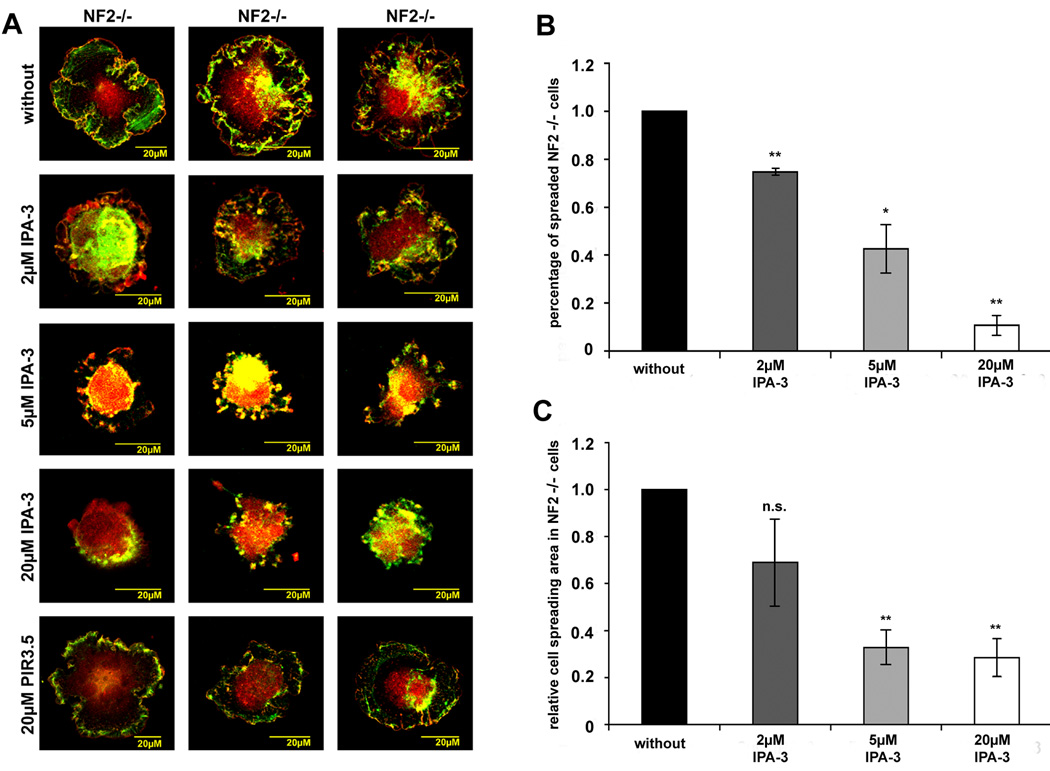
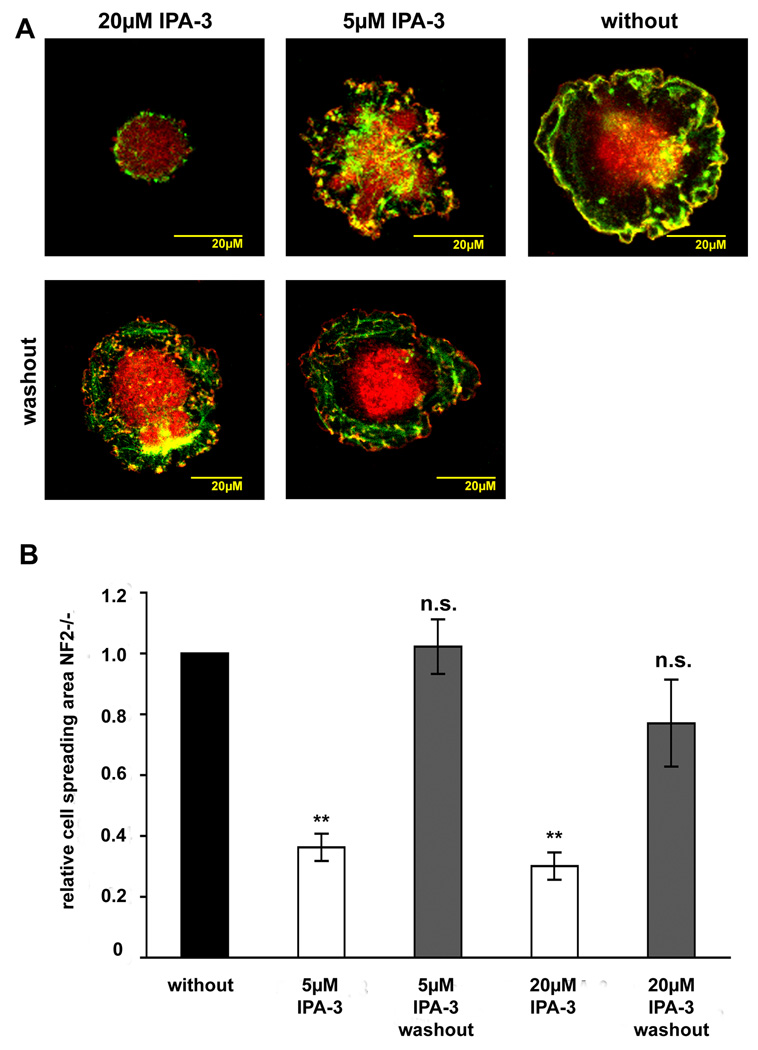
Schwannoma cells characteristically display increased cell spreading and intense ruffling (Pelton et al. 1998;Rosenbaum et al. 1998;Utermark et al. 2005b), that have been linked to Rac1 activation. The commercially available Rac1 inhibitor NSC23766 that blocks Rac1 activation through its guanine exchange factors Tiam and Vav, efficiently blocked ruffling, but did not affect cell spreading in schwannoma cells (Nakai et al. 2006). We therefore determined if inhibiting the PAK-PIX-mediated Rac and PAK activation with IPA-3 would affect cell spreading and ruffling in human primary schwannoma cells. Schwannoma cells were left untreated or treated with 2 µM, 5 µM or 20 µM IPA-3 or with 20 µM PIR-3.5 for 10 minutes prior to seeding on poly-L-lysine/laminin coated dishes where they were allowed to spread for 30 minutes. In order to visualise cell borders, ruffles and the cell body, we stained the cells with p34-Arc, a subunit of the Arp2/3 complex that is enriched in lamellipodia and schwannoma cell periphery (Flaiz et al. 2007) and F-actin. Morphological analysis revealed that untreated schwannoma cells were well spread after 30 minutes (Fig. 3 A). Increasing amounts of IPA-3 reduced numbers of spread cells in a dose-dependent manner (Fig.3 A, B). This was further verified when cell spreading areas were quantitatively measured using the volocity software (Fig.3 C). The control substance PIR-3.5 (20µM) had no effect on cell spreading. If the inhibitor was removed through media changing after 30 minutes (“washout”) and cells were allowed to spread for another 30 minutes, morphology of cells resembled untreated schwannoma cells (Fig.4 A). Washout of 5µM IPA-3 restored cell spreading area completely and washout of 20µM IPA-3 at least partly (Fig.4 B). These findings indicate that the effects of IPA-3 are reversible.
Figure 3.
IPA-3 effect on cell spreading in human primary schwannoma cells (NF2−/−) Schwannoma cells that were allowed to spread for 30 minutes in the absence or presence of the PAK inhibitor IPA-3 or the control substance PIR-3.5 on poly-L-lysine/laminin coated dishes were stained for the Arp2/3 complex (red) a marker for lamellipodia and ruffles and F-actin (green) to visualise cell morphology. The picture gallery shows three representative cells (A). Number of spreaded cells (B) and cell spreading area (C) were determined and normalised to values of untreated schwannoma cells. Cell spreading is reduced in the presence of IPA-3 in a dose-dependent manner. IPA-3 also reduced but not abolished cell ruffling in schwannoma cells. The control substance PIR-3.5 did not significantly alter cell spreading or ruffling (scale bars represent standard error of the mean; *: p<0.05 significant, **: p<0.01 highly significant, ***: p<0.001 very highly significant, n.s. : not significant in student’s t-test).
Figure 4.
Washout of IPA-3 in cell spreading experiments in human primary schwannoma cells (NF2−/−)
Schwannoma cells that were allowed to spread for 30 minutes in the absence or presence of the PAK inhibitor IPA-3 or the control substance PIR-3.5 on poly-L-lysine/laminin coated dishes were stained for the Arp2/3 complex (red) a marker for lamellipodia and ruffles and F-actin (green) to visualise cell morphology. IPA-3 was washed out after 30 minutes by changing inhibitor-containing media to inhibitor-free media. Cells were allowed to spread for another 30 minutes. Washout restored normal schwannoma cell morphology (A). Washout of 5 µM IPA-3 leads to the same cell spreading area compared to untreated cells and washout of 20 µM IPA-3 to an only slightly reduced cell spreading area that was clearly higher than in cells were inhibitor was left on (B).
To visualise cell spreading and ruffling of the whole cell more clearly we created 3D animations of schwannoma cells in the presence or absence of 5µM or 20µM IPA-3. Untreated schwannoma cells show intense ruffling all around the cell periphery (supplementary figure 1). As already analysed by other methods, the 3D animation of schwanoma cells treated with IPA-3 clearly showed disturbed cell spreading in a dose-dependent manner, with more rounded cells at higher inhibitor concentrations (supplementary figure 2 and supplementary figure 3). Ruffling seems to be reduced but not abolished in inhibitor treated cells, compared to untreated cells. In summary we claim that PAK and/or the Pix-PAK-mediated Rac1 activation are involved in cell spreading, but seems not to abolish ruffling in human primary schwannoma cells.
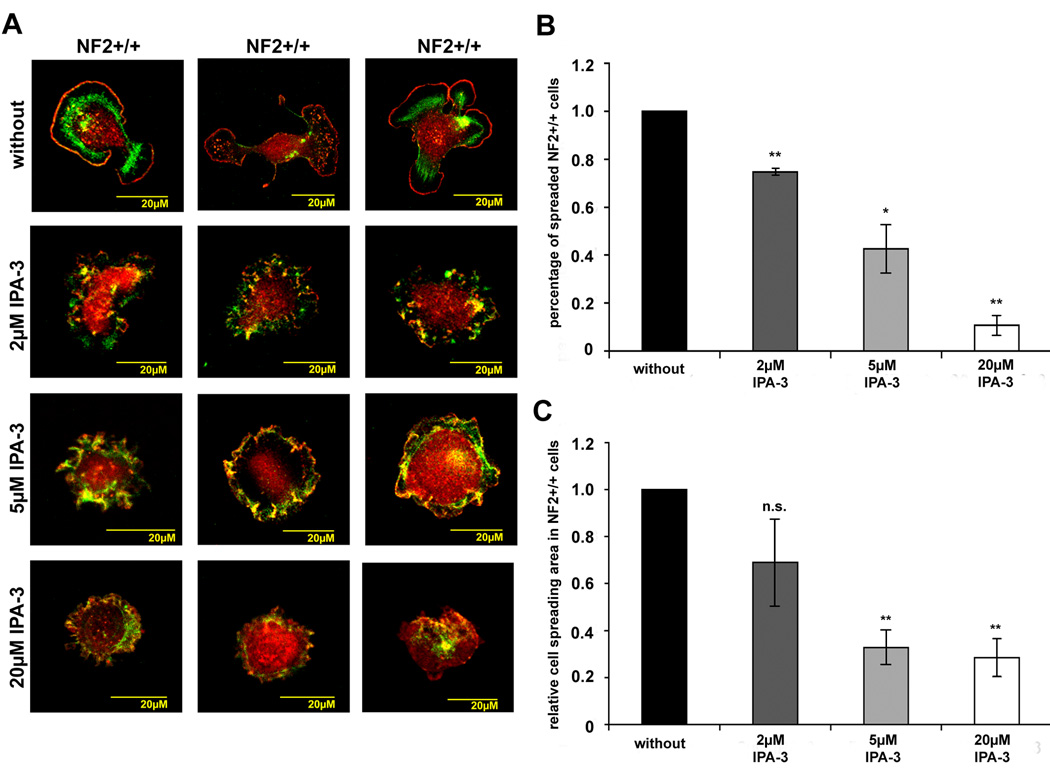
To investigate if IPA-3 also affects normal cell spreading in healthy human primary Schwann cells we investigated this cellular process, using the same experiments as described above. Untreated Schwann cells that were allowed to spread for 30 minutes on poly-L-lysine/laminin already displayed the characteristic bipolarity, with protrusive areas at either end (Fig.5 A). Ruffling could not been observed in the untreated Schwann cells in accordance with previous data (supplementary figure 4). Treatment with different concentrations of IPA-3 (2 µM, 5 µM and 20 µM) reduced number of spreaded cells and cell spreading area (Fig.5 B, C). Schwann cells show ruffling around the cell periphery in the presence of 5µM IPA-3 (Fig.5 A and supplementary figure 5 and supplementary figure 6). This ruffling after loss of spreading area is not as intense as in untreated schwannoma cells and could not be observed with higher concentrations of IPA-3 (20µM). According to these results, PAK activity seems to play a role in Schwann cell spreading
Figure 5.
IPA-3 effect on cell spreading in human primary Schwann cells (NF2+/+) Schwann cells that were allowed to spread for 30 minutes in the absence or presence of the PAK inhibitor IPA-3 on poly-L-lysine/laminin coated dishes were stained for the Arp2/3 complex (red) a marker for lamellipodia and ruffles and F-actin (green) to visualise cell morphology. The picture gallery shows three representative cells (A). Number of spreaded cells (B) and cell spreading area (C) were determined and normalised to values of untreated Schwann cells. Cell spreading is reduced in the presence of IPA-3 in a dose-dependent manner. Schwann cells treated with 2 µM and 5 µM IPA-3 showed mild ruffling. (Scale bars represent standard error of the mean; *: p<0.05 significant, **: p<0.01 highly significant, n.s. : not significant in student’s t-test).
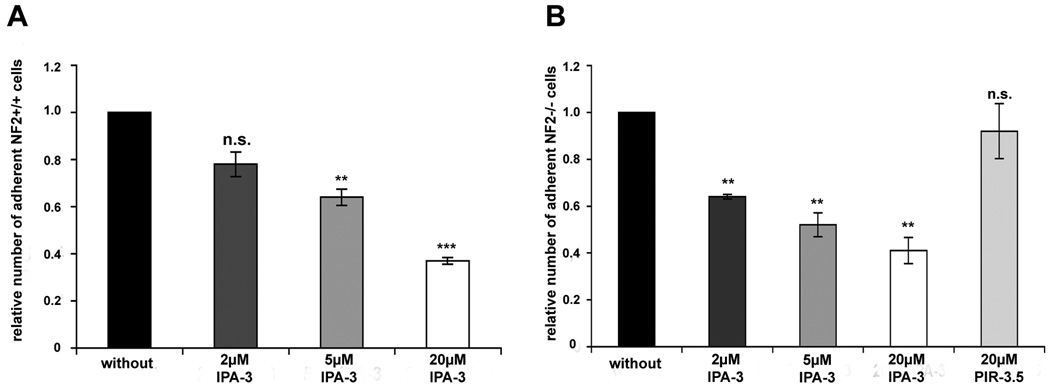
IPA-3 reduces cell adhesion in Schwann and schwannoma cells
The fact that IPA-3 clearly affects cell spreading suggests that PAK and/or Pix-PAK-mediated Rac activation are involved in the schwannoma cells ability to adhere to the extracellular matrix. We therefore conducted an adhesion assay in which Schwann and schwannoma cells were left untreated or treated with 2 µM, 5 µM, 20 µM IPA-3 or 20 µM of the control substance PIR-3.5 prior to seeding to poly-L-lysine/laminin coated dishes for 3h. Loose cells were washed away and adherent cells manually counted. IPA-3 treatment significantly reduced the number of adherent Schwann and schwannoma cells in a dose-dependent manner, whereas PIR-3.5 had no marked effect (Fig.6 A, B). We conclude that PAK activation has a clear effect on adhesion to the extracellular matrix in both human primary Schwann and schwannoma cells.
Figure 6.
IPA-3 effect on adhesion in human primary Schwann (NF2+/+) and schwannoma cells (NF2−/−).
Human primary Schwann (NF2+/+) and schwannoma cells (NF2−/−) were allowed to adhere to poly-L-lysine/laminin coated dishes for 3 hours in the absence or presence of the PAK inhibitor IPA-3 or the control substance PIR-3.5. IPA-3 reduced the number of adherent Schwann (A) and schwannoma cells (B) in a dose-dependent manner. PIR-3.5 did not affect adhesion to the extracellular matrix. (Scale bars represent standard error of the mean; *: p<0.05 significant, **: p<0.01 highly significant, ***: p<0.001 very highly significant, n.s. : not significant in student’s t-test).
Discussion
In this study we investigated the role of PAK (p21-activated kinase) in human schwannoma cells to unravel its role on Rac activation and on cell spreading, ruffling and adhesion using the novel small-molecule Pak inhibitor IPA-3. Activation of the GTPase Rac at least partly occurs through beta-Pix at the levels of focal adhesions as our previous work showed (Flaiz et al. 2009). PAK (Bokoch 2003;Zhao and Manser 2005) localises to focal adhesions (Manser et al. 1998), where it competes with Rac for binding to beta-Pix (ten Klooster et al. 2006). According to this model (ten Klooster et al. 2006) phosphorylation of PAK induces a dissociation of PAK from beta-Pix which subsequently allows activation of Rac. To investigate the role of PAK in the Pix-mediated Rac1 activation (Flaiz et al. 2009) we used the inhibitor IPA-3 (Deacon et al. 2008) which blocks PAK autophosphorylation at Ser199/204(PAK1)/Ser192/197(PAK2). As IPA-3 is ineffective in inhibiting pre-activated PAK, we starved our cells and preincubated them with IPA-3 prior to stimulation, in all experiments.
We first investigated PAKs role in Rac activation. IPA-3 effectively inhibits phosphorylation of PAK2 at Ser192/197 that is known to induce a dissociation of PAK from beta-Pix (Zhao and Manser 2005;ten Klooster et al. 2006). Phospho-PAK1 Ser199/204 is at much lower amounts in schwannoma cells in accordance with previous studies (data not shown, Flaiz et al. 2007). Previous studies have shown that beta-Pix localises to focal adhesions, whereas phospho-PAK can be found all around the membrane in schwannoma cells (Flaiz et al. 2007;Flaiz et al. 2009). Inhibition of PAK2 by IPA-3 as shown here suggests that phospho-PAK stays associated with beta-Pix. Due to antibody incompatibility co-localisations are not possible and we were unable to get a quantitative immunoprecipitation. However we showed that phospho-PAK can no longer be found at the membrane. In addition, this stable interaction of beta-Pix with PAK would then block beta-Pix, thereby preventing an activation of Rac and accordingly, Rac-GTP levels would decrease after IPA-3 treatment. Indeed, IPA-3 treatment of human primary schwannoma cells reduced Rac-GTP levels in a dose-dependent manner. Rac inhibition was already significant at 5µm where PAK inhibition was not yet significant. This could be explained by the sensitivity of the assays and the PAK antibodies known to be of low affinity. Another explanation could be that PAK1 although expressed at very low levels in schwannomas is inhibited by IPA-3 efficiently and results in Rac inhibition as IPA-3 inhibits PAK1 even stronger than PAK2 (Deacon et al. 2008). However this is an important finding, since it puts PAK upstream of Rac in human schwannomas. Even when treated with higher amounts of IPA-3 (20 µM), a small amount of activated Rac-GTP (around 20 % of the Rac-GTP levels in untreated schwannoma cells) remains. This could be because part of the Rac activation occurs through other guanine exchange factors like Tiam and Vav. The major part of the pathological Rac activation in schwannoma, however, occurs through Pix and seems to be regulated by PAK.
To test if PAK acts also upstream of Cdc42, which is pathologically activated in schwannoma cells as well (Flaiz et al. 2007), we investigated Cdc42-GTP levels in schwannoma after IPA-3 treatment. Even a high concentration (20 µM) of IPA-3 that significantly reduced Rac-GTP levels did not significantly inhibit Cdc42 activation. Rather a slight increase in Cdc42-GTP-levels after IPA-3 treatment can be observed occasionally. One possible explanation for this slight increase which can also be seen in a different experiment in schwannoma cells (Ammoun et al. 2008) could be the phenomenon of hormesis (Calabrese 2008). Thus Cdc42 is unlikely to act downstream of PAK in schwannoma cells. This is supported by findings showing that merlin phosphorylation in Schwann cells is regulated by Cdc42 (but not Rac) that is induced by activation of PAK downstream of integrins (Thaxton et al. 2007;Thaxton et al. 2008).Taken together we suggest that the model for Rac activation (ten Klooster et al. 2006) in fibroblasts could be true and relevant in human schwannoma cells. Integrin engagement would activate Cdc42 leading to PAK autophosphorylation in schwannoma. This is supported by previous findings (Flaiz et al. 2007; Kaempchen et al. 2003; Nakai et al. 2006). Phospho-PAK dissociates from beta-Pix at focal adhesions and localises to the membrane (Flaiz et al. 2007). Rac be subsequently activated by beta-Pix and also localises to the membrane (Kaempchen et al. 2003;Nakai et al. 2006). This study puts PAK upstream of Rac and reveals that Rac activation in schwannoma at least partially occurs through Pix and PAK. Recently our group has shown that PDGFRβ-mediated ERK1/2 activation is involved in schwannoma cell proliferation. We showed that PAK acts as a scaffold and is not an Erk1/2 activator in human schwannoma cells and IPA-3 does not inhibit ERK activation (Ammoun et al. 2008). As it is therefore unlikely that PAK is involved in schwannoma cell proliferation and as schwannomas are benign tumours with only slightly increased proliferation rates, we focused on PAKs role in schwannoma cell spreading and adhesion, which are major pathological characteristics of schwannoma.
To investigate cellular consequences of PAK activation and the Pix-PAK-mediated Rac-activation in schwannoma we used cell spreading/ruffling and adhesion assays. Characteristically schwannoma cells show increased cell spreading (Pelton et al. 1998;Utermark et al. 2005b;Flaiz et al. 2007) a process that is controlled by Rac (Ridley and Hall 1992) and increased integrin-dependent adhesion to the extracellular matrix (Utermark et al. 2003). We therefore first conducted cell spreading/ruffling assays and adhesion assays that have been described before (Utermark et al. 2003;Nakai et al. 2006). IPA-3 efficiently blocks cell spreading and adhesion on poly-L-lysine/laminin. This implicates Pix-PAK-mediated Rac activation and therefore PAK activity in general to be involved in cell spreading and adhesion in schwannoma cells. Washout experiments restored the normal schwannoma phenotype and indicate that IPA-3 effects are reversible. As a control, cell spreading and adhesion assays were also conducted in healthy Schwann cells that display normal Rac activity (Kaempchen et al. 2003). Cell spreading and adhesion are also markedly reduced in Schwann cells when treated with IPA-3. This is not surprising as a certain amount of Rac activation can be detected in human Schwann cells (Kaempchen et al. 2003). This Rac activity is important for axonal myelination (Benninger et al. 2007) and occurs in an integrin-dependent fashion after adhesion to the extracellular matrix (Nodari et al. 2007). The Rac inhibitor NSC23766 did not inhibit cell spreading clearly (Nakai et al. 2006). This can be explained by the fact that NSC23766 binds Rac and inhibits its activation by a subset of guanine exchange factors (GEFs). In contrast, IPA-3 directly targets PAK, preventing its autophosphorylation and thereby sequestering the GEF Pix from Rac. Both, PAK and beta-Pix localise to focal adhesions (Manser et al. 1998;Rosenberger and Kutsche 2006) and are therefore involved in cell spreading and adhesion. NSC23766 effectively suppresses membrane ruffling, whereas IPA-3 reduces but not completely abolishes this process in schwannoma cells. In the presence of 5µM IPA-3, detaching Schwann cells display ruffling. Ruffles can be seen as lamellipodia that lost the ability to adhere to the extracellular matrix (Small et al. 2002). As IPA-3 reduces the cell’s adhesion potential, ruffles could evolve as a secondary effect. Interpreting IPA-3’s influences on ruffling in Schwann and schwannoma cells is therefore difficult. Our results suggest that at least part of the Rac activation in Schwann cells occurs through beta-Pix and PAK at focal contacts. This findings fit nicely to previous reports in Schwann cells, showing that merlin phosphorylation and therefore inactivation occurs at paxillin-containing membrane domains (which we showed before to be the small and short-living focal complexes in Schwann cells) through PAK that has been activated by Cdc42 (Thaxton et al. 2007). This means that a locally restricted merlin inactivation and subsequent Rac activation occurs at the levels of focal complexes in Schwann cells. Focal contacts are short-lived in Schwann cells, thereby guaranteeing that Rac activation stays within normal limits. In schwannoma cells, however, focal contacts are very stable and long-lived (Flaiz et al. 2009) leading to a constantly increased Pix-PAK-mediated Racactivation susceptible to inhibition with IPA-3. Thus PAK seems to be a reasonable target in schwannomas.
On a side line we showed that IPA-3 is an easy-to-use, efficient PAK inhibitor that can be used even in delicate primary cells to investigate cellular processes. Moreover, our human schwannoma in vitro model has been proven to be a good model system to investigate PAK effects.
Supplementary Material
Acknowledgement
Work was supported by Peninsula College of Medicine and dentistry and Fritz Thyssen foundation (COH) and Department of Defence NF Research Program and a Career Development Award from the AACR (JP and JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 2.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J. Biol. Chem. 2005;280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 3.Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokoch GM. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ. Toxicol. Chem. 2008;27:1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- 6.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaiz C, Ammoun S, Biebl A, Hanemann CO. Altered adhesive structures and their relation to RhoGTPase activation in merlin-deficient Schwannoma. Brain Pathol. 2009;19:27–38. doi: 10.1111/j.1750-3639.2008.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaiz C, Kaempchen K, Matthies C, Hanemann CO. Actin-Rich Protrusions and Nonlocalized GTPase Activation in Merlin-Deficient Schwannomas. J. Neuropathol. Exp. Neurol. 2007;66:608–616. doi: 10.1097/nen.0b013e318093e555. [DOI] [PubMed] [Google Scholar]
- 9.Flaiz C, Utermark T, Parkinson DB, Poetsch A, Hanemann CO. Impaired intercellular adhesion and immature adherens junctions in merlin-deficient human primary schwannoma cells. GLIA. 2008;56:506–515. doi: 10.1002/glia.20629. [DOI] [PubMed] [Google Scholar]
- 10.Hanemann CO, Bartelt-Kirbach B, Diebold R, Kampchen K, Langmesser S, Utermark T. Differential gene expression between human schwannoma and control Schwann cells. Neuropathol. Appl. Neurobiol. 2006;32:605–614. doi: 10.1111/j.1365-2990.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirokawa Y, Tikoo A, Huynh J, Utermark T, Hanemann CO, Giovannini M, Xiao GH, Testa JR, Wood J, Maruta H. A clue to the therapy of neurofibromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J. 2004;10:20–26. doi: 10.1097/00130404-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum. Mol. Genet. 2003;12:1211–1221. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 13.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol. Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 14.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 16.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 17.Nakai Y, Zheng Y, MacCollin M, Ratner N. Temporal control of Rac in Schwann cell-axon interaction is disrupted in NF2-mutant schwannoma cells. J. Neurosci. 2006;26:3390–3395. doi: 10.1523/JNEUROSCI.4865-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J. Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA, Ratner N. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene. 1998;17:2195–2209. doi: 10.1038/sj.onc.1202141. [DOI] [PubMed] [Google Scholar]
- 21.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Mueller HW, Hanemann CO. Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiology of disease. 1998;5:55–64. doi: 10.1006/nbdi.1998.0179. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberger G, Kutsche K. AlphaPIX and betaPIX and their role in focal adhesion formation. Eur. J. Cell Biol. 2006;85:265–274. doi: 10.1016/j.ejcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O'Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 25.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 26.ten Klooster JP, Jaffer ZM, Chemoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaxton C, Lopera J, Bott M, Baldwin ME, Kalidas P, Fernandez-Valle C. Phosphorylation of the NF2 tumor suppressor in Schwann cells is mediated by Cdc42-Pak and requires paxillin binding. Mol. Cell Neurosci. 2007;34:231–242. doi: 10.1016/j.mcn.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Thaxton C, Lopera J, Bott M, Fernandez-Valle C. Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene. 2008;27:2705–2715. doi: 10.1038/sj.onc.1210923. [DOI] [PubMed] [Google Scholar]
- 29.Utermark T, Kaempchen K, Antoniadis G, Hanemann CO. Reduced apoptosis rates in human schwannomas. Brain Pathol. 2005a;15:17–22. doi: 10.1111/j.1750-3639.2005.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utermark T, Kaempchen K, Hanemann CO. Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol. 2003;13:352–363. doi: 10.1111/j.1750-3639.2003.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utermark T, Schubert SJ, Hanemann CO. Rearrangements of the intermediate filament GFAP in primary human schwannoma cells. Neurobiol. Dis. 2005b;19:1–9. doi: 10.1016/j.nbd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated Kinase Links Rac/Cdc42 Signaling to Merlin. J. Biol. Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 33.Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem. J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.