Abstract
Background
Pro-inflammatory cytokines are associated with sickness behaviors, a set of behaviors including low mood, which are orchestrated by the brain and described as shift in motivational state. The present study investigated the hypothesis that local inflammation is associated with greater subgenual anterior cingulate cortex (sACC) activation in persons undergoing chronic stress.
Methods
Women undergoing the emotional stress of bereavement had fMRI scans during a grief-elicitation task. Local inflammation was measured by salivary concentrations of two markers of proinflammatory cytokine activity (e.g., interleukin-1β and soluble tumor necrosis factor receptor II).
Results
Analyses revealed that both inflammatory markers were positively associated with ventral prefrontal activation (e.g., sACC and orbitofrontal cortex) as well as other regions important in the emotional task such as noun retrieval (e.g., temporal cortex), and visual processing (e.g., cuneus and fusiform gyrus). In separate analyses, the ventral prefrontal activations correlated with free recall of grief-related word stimuli, but not neutral word stimuli.
Conclusions
This is the first study to demonstrate the relationship between emotional processing, regional brain activation and localized inflammation in a chronically stressed population of adults.
Keywords: Grief, cytokines, inflammation, emotion, neuroscience, psychoneuroimmunology
Introduction
It has become increasingly clear that the symptoms associated with inflammation (e.g., reduced appetite, social withdrawal) are part of a full-body orchestration described as “sickness behavior”, which together have overlapping features with depressive symptoms in humans (Dantzer et al., 2008). This sickness behavior arises from a change in motivational state that occurs when the brain receives signals of peripheral inflammation, which yields “a re-organization of perceptions and actions (Dantzer et al., 2008, p. 46)”. However, what is not known is what brain regions in humans are responsible for this change in motivational state associated with high levels of peripheral inflammation.
To investigate the relationship between peripheral inflammation and “sickness behavior”, attention has recently focused on the influence of inflammation that is found within peripheral, localized sites such as the mouth. Indeed, several types of evidence have provided the background rationale for this relationship, including experimental and naturalistic correlational studies. This literature can be grouped into 1) the relationship between chronic stress and poor oral health, 2) the relationship between acute and chronic stress and proinflammatory cytokines in oral fluids (other than saliva).
First, gingival inflammation is correlated with depression (Klages et al., 2005) and periodontal disease is increased in chronic stress (e.g., exam stress and widow(er)hood) (Deinzer et al., 2005; Hugoson et al., 2002). Marcenes and Sheiham (1992) showed that in 158 participants, marital quality and work demand were the best predictors of periodontal disease in multiple linear regression analysis, even after including a number of other predictors, such as frequency of dental appointments and tooth brushing frequency.
Second, the localized expression of inflammatory markers in the mouth (i.e., gingival crevicular fluid) occurs following acute, experimental social stress (Deinzer et al., 2004; Weik et al., 2008). Localized expression of inflammatory markers in crevicular fluid has also been found in chronic stress (e.g., examination periods) and depression (Johannsen et al., 2007; Waschul et al., 2003). These findings are similar to the well-recognized impact of psychological stress on cellular inflammatory signaling, activation of nuclear factor κB, and expression of systemic markers of inflammation (Bierhaus et al., 2003; Pace et al., 2006). The mouth is a critical aspect of immune surveillance and response, as it is constantly in contact with bacterial, viral and other airborne pollutants. The variation in inflammatory response to these immune challenges is varied based upon the level of concurrent stress.
The strongest evidence for the relationship between salivary cytokines and sickness behavior symptoms in humans is from a study comparing participants with periodontitis who were exposed to war and those who were not (all from Croatia) (Aurer et al., 1999). Irritability, anxiety, and tiredness were symptoms associated with the stress of war exposure. These are symptoms of sickness behavior (Dantzer et al., 2008), and in this report there was higher IL-6 in saliva in a group exposed to war stress (with sickness behavior symptoms) in comparison to a group not exposed to war.
Neural pathways involving the trigeminal nerve directly communicate local inflammation from the mouth to the brain (Navarro et al., 2006). In rats, surgical transection of the trigeminal nerve abrogated the pyrogenic effects of Escherichia coli lipopolysaccharide (E. coli LPS) when doses were injected into periodontal tissues of the mouth. In addition, immunohistochemistry demonstrated neuronal activation where the trigeminal makes its first synapse in the brain. In humans, it is not known whether peripheral, localized increases of inflammatory markers in the mouth are associated with behavioral changes and/or alterations in brain activation.
Given the association between psychological stress and increases of inflammatory markers in the mouth, we investigated the influence of bereavement, the most significantly rated life stressor (Hobson et al., 1998), on localized levels of inflammation in saliva and whether varying levels of markers of proinflammatory cytokine activity are associated with brain activation during an emotion paradigm. To our knowledge, no study has examined the relationship between local inflammation (i.e., oral cavity) and brain activation as measured by fMRI in humans. A few studies have investigated the relationship between systemic inflammation and regional brain activation (Brydon et al., 2008; Capuron et al., 2005; Rocca et al., 2007). Because of our interest in the change in motivational state associated with sickness behavior, emotion tasks during neuroimaging are most relevant to the present study. A study that has used an emotion paradigm demonstrated that increases of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) correlated with activation of the subgenual anterior cingulate cortex (sACC) (Rosenkranz et al., 2005). Rosenkranz and colleagues interpreted this data as evidence that the sACC could integrate the afferent information regarding homeostatic processes in the periphery such as inflammation and emotion processing.
In addition to this data, we developed an a priori hypothesis for the brain regions that would be correlated with local inflammation based on the sickness behavior literature. Because the sickness behavior evidence suggests that mood regulation is perturbed during higher levels of inflammation, we hypothesized a priori that the brain regions involved in mood regulation would be positively correlated with higher levels of oral inflammation. Specifically, the ventral prefrontal cortex (PFC) has been shown to be associated with mood regulation in a number of types of neuroimaging (Drevets et al., 1997). The sACC is frequently found to have higher metabolism in affective disorders (for a review, see Drevets et al., 2008), and this region participates in a network of structures that modulate neuroendocrine responses during emotion processing (Drevets et al., 2002). For all of these reasons, we hypothesized that the ventral PFC would be associated with local inflammation.
Therefore, in the present study, we hypothesize that higher levels of markers of proinflammatory cytokine activity, as measured by the levels of the cytokine receptor soluble tumor necrosis factor receptor II (sTNFrII) and interleukin-1β (IL-1β), are associated with activation of the sACC in persons undergoing the chronic emotional stress of bereavement. IL-1β and TNF-α were chosen because they are implicated in sickness behavior (Dantzer et al., 2008). In contrast with IL-1β, levels of TNF-α are not routinely detected in saliva; hence levels of its cytokine receptor (i.e., sTNFrII), which correlate with cytokine levels (Schuld et al., 1999), was measured. Whole saliva has been used for the measurement of local pro-inflammatory cytokines in the mouth, including IL-1β (Miller et al., 2006; Tobon-Arroyave et al., 2008) and sTNFrII (Al-Harthi et al., 2000; Nishanian et al., 1998; Winkler et al., 2001).
Methods and Materials
Eighteen women who had experienced the death of a mother or a sister to breast cancer in the past 5 years participated in an event-related fMRI study. The Institutional Review Board at UCLA approved the study and all participants gave written informed consent. All participants were screened for mood disorders through a structured clinical interview, and any with Axis I disorders (including major depression) were excluded. Smoking and diseases of the immune system (e.g., rheumatoid arthritis) were exclusion criteria, due to their potential impact on pro-inflammatory cytokines, although the oral health of the study participants was not systematically rated. Participants mean age was 44.3 years (range 25-60 years), average education was 16.7 years (range 12-22 years) and average length of time since the death was 30.0 months (range 2-59 months). The sample was 22.2% non-white, and 16.7% had lost sisters (vs. mothers).
The fMRI task has previously been described (O'Connor et al., 2008) and has been validated for grief-elicitation through skin conductance responses and subjective report (Gündel et al., 2003). A summary of the method is listed here. Each participant provided a photograph of her deceased loved one and were asked to describe how their loved ones died in an open-ended format. The photos were matched for age, sex, environment (indoor/outdoor) and type of photo (snapshot/portrait) with photos of a stranger. In addition, 15 words were chosen from each participant's narrative that was ideographically specific to the death (e.g., cancer, tumor, dying, ambulance, morphine). These were matched for part of speech, number of letters and frequency of usage in the English language with 15 neutral words (e.g., ginger, entry, simple, directory, footpath). These photos and words were made into 60 composites, each consisting of one picture (deceased or stranger) and one word (grief-related or neutral). The composites were presented in random order, for a total task time of 6 minutes and 10sec. Composites were shown for 4.5 seconds, with a jittered interstimulus interval (minimum interval: 0.5 seconds, maximum interval: 10 seconds). Participants viewed the task through MR-compatible video goggles during scanning. Participants were instructed to allow any thoughts, feelings or memories to arise naturally during the task.
MRI data were acquired on a Siemens Allegra 3T scanner. For each participant, a high-resolution structural T2-weighted echo-planar image (spin-echo; TR = 5000 ms; TE=33 ms; matrix size 128 × 128; 36 axial slices; FOV = 20cm; 3-mm thick, skip 1-mm) was acquired coplanar with the functional scan. A functional scan, lasting 6 minutes and 10 seconds, was acquired (echo planar T2*-weighted gradient-echo, TR = 2500 ms, TE = 25 ms, flip angle = 90, matrix size 64 × 64, 36 axial slices, FOV = 20cm; 3-mm thick, skip 1-mm).
Following the scan, participants provided a saliva sample with Salivettes (Sarstedt, Inc., Newton, NC), which are comprised of a cotton swab (without citric acid preparation) and tube. IL-1β was measured by using Quantikine Human IL-1β enzyme immunoassay (ELISA) kit manufactured by R&D Systems (Minneapolis, MN). Participants were instructed to keep the cotton swab in their mouth until it felt full like a sponge, about one minute. sTNFrII was measured by using Quantikine Human sTNFrII enzyme immunoassay (ELISA) kit manufactured by R&D Systems (Minneapolis, MN). The measurement of IL-1β and sTNFrII were performed according to the manufacturer's instructions. All samples were run in duplicate and averaged. The saliva samples were diluted with diluents RD5-5 and tested. The minimum detectable dose (MDD) of IL-1β was less than 1 pg/ml and the interassay and intraassay coefficients of variation were less than or equal to 4% and 7% respectively. The MDD of sTNFrII was 0.6 pg/ml and the interassay and intraassay coefficients of variation were less than or equal to 5%.
A manipulation check was conducted following scanning to document that subjects were attending to the composites during the scan. After participants exited the scanner and provided their saliva sample, they were given an incidental memory task. Without being instructed prior to the scan, they were asked to recall any words that they could from the task in a free recall format. The number of grief-related words and neutral words that were correctly recalled were summed separately.
Data analysis
The imaging data were analyzed using statistical parametric mapping (SPM'99; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images were realigned, normalized to the MNI template, and smoothed with an 8mm Gaussian kernel, full width at half maximum. Analysis used the general linear model in an event-related analysis. Effects at each voxel were estimated using linear contrasts to compare regionally specific effects. The contrasts for the individual subjects were aggregated for group analysis according to the random effect model in SPM. The data were first analyzed for main effects and are reported by O'Connor and colleagues (2008). The four composites were modeled as separate regressors (deceased/grief word, stranger/grief word, deceased/neutral word, stranger/neutral word). In the previous report, data were also grouped by the diagnosis of Complicated Grief. However, because the inflammatory markers were not significantly different between the Complicated and Noncomplicated Grief (data not shown), between-group analyses are not included in the present paper.
All non-MRI data was analyzed in SPSS 16.0. IL-1β and sTNFrII were log transformed since the distribution of raw values is typically skewed. To assess covariation between individual differences in IL-1β and neural activation, IL-1β values were entered in a second-level analysis as a between-subject regression analysis (separately for Deceased > Stranger and Grief Word > Neutral Word contrasts). These analyses were performed separately for sTNFrII. All analyses were thresholded using an uncorrected p-value of .001 combined with a cluster size threshold of 10 voxels. All coordinates are reported in MNI format.
Results
Pro-inflammatory cytokine results
Results from the ELISA analyses showed that participants had a raw IL-Iβ level of 198.2 pg/mL with a standard deviation (SD) of 264.2. The ELISA for sTNFRII had a mean of 251.3 pg/mL and SD of 271.2 (Table 3).
Table 3.
Raw values (pg/mL) of interleukin-1β (IL-1β) and soluble tumor necrosis factor receptor II (sTNFrII). Values of IL-1β for comparison from whole saliva samples include: Miller et al, 2006 (control = 212.8 ±167.4; periodontitis 753.7 ±1022.4), Tobón-Arroyave et al, 2008 (control = 295.8pg/mL ± 175.0; periodontitis = 543.8 pg/mL ± 243.9).
| Subject | IL-1β | sTNFRII |
|---|---|---|
| 1 | 243.4 | 233.0 |
| 2 | 60.5 | 213.2 |
| 3 | 85.8 | 58.8 |
| 4 | 199.0 | 1148.6 |
| 5 | 193.5 | 287.1 |
| 6 | 232.6 | 429.0 |
| 7 | 9.3 | 26.3 |
| 8 | 58.7 | 264.5 |
| 9 | 309.7 | 487.9 |
| 10 | 58.5 | 268.3 |
| 11 | 6.1 | 22.9 |
| 12 | 1147.9 | 5.3 |
| 13 | 153.3 | 489.4 |
| 14 | 65.6 | 157.1 |
| 15 | 66.5 | 126.4 |
| 16 | 14.9 | 95.7 |
| 17 | 233.3 | 51.8 |
| 18 | 428.9 | 157.3 |
| Mean (SD) | 198.2 ± 264.2 | 251.3 ± 271.2 |
Neuroimaging results
The main effects of the study replicate prior grief neuroimaging results (Gündel et al., 2003) and are reported elsewhere (O'Connor et al., 2008). Results from the covariate analyses with Grief Word > Neutral Word and the pro-inflammatory markers are shown in Table 1. Of note, separate analyses using IL-1β and sTNFrII both demonstrated activation in the temporal cortex and the ventral prefrontal cortex (Figure 1). Results from the covariate analyses with Deceased > Stranger and levels of IL-1β and sTNFrII are shown in Table 2. In these separate analyses, cuneus activation was seen in association with both IL-1β and sTNFrII.
Table 1.
Brain region activation associated with IL-1β and sTNFrII pro-inflammatory markers during grief elicitation (Grief Word > Neutral Word contrast) (N = 18). All regions reported at the threshold of .001, uncorrected, 10 voxels. ACC = anterior cingulate cortex.
| Region | MNI Coordinates (x,y,z) | Z | T | p-value (voxels) | ||
|---|---|---|---|---|---|---|
| IL-1β | ||||||
| Inferior temporal (BA 20/21) | 40 | -6 | -24 | 4.51 | 6.58 | <0.001 (63) |
| Subgenual ACC (BA 32) | -2 | 26 | -6 | 4.43 | 6.37 | <0.001 (70) |
| Precentral gyrus (BA 4/6) | -60 | 0 | 16 | 3.69 | 4.73 | <0.001 (22) |
| Medial pons | 10 | -34 | -46 | 3.63 | 4.63 | <0.001 (18) |
| Precentral gyrus (BA 4) | 8 | -30 | 52 | 3.59 | 4.55 | <0.001 (27) |
| Lateral pons | 2 | -26 | -44 | 3.40 | 4.20 | <0.001 (14) |
| sTNFrII | ||||||
| Inferior temporal (BA 20) | 56 | -14 | -28 | 3.55 | 4.48 | <0.001 (12) |
| Orbitofrontal cortex (BA 47) | 38 | 36 | -10 | 3.49 | 4.36 | <0.001 (12) |
Figure 1.
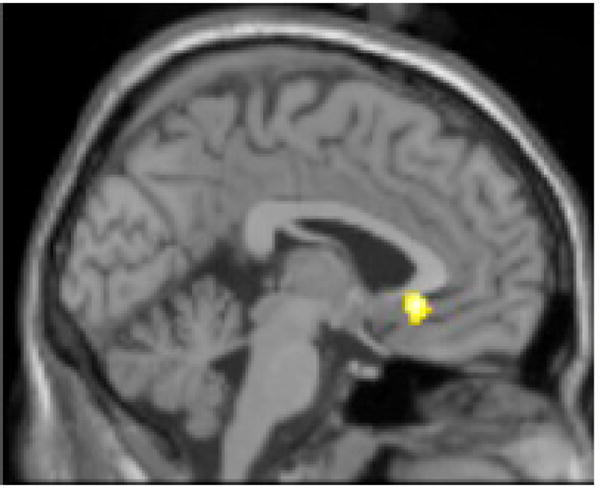
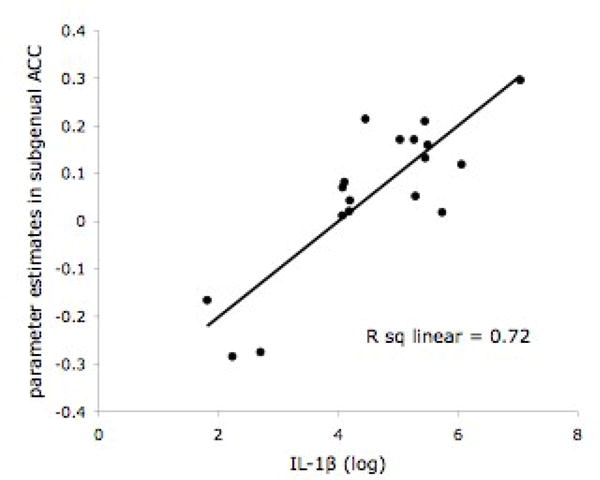
A. Subgenual anterior cingulate (sACC) activity (-2, 26, -6; t = 6.37, p < 0.001 uncorrected, 70 voxels) in response to Grief-related > Neutral Words in 18 bereaved participants. B. Scatter plot of the positive correlation between IL-1β (-2, 26, -6) and BOLD activity in the sACC.
Table 2.
Brain region activation associated with IL-1β and sTNFrII pro-inflammatory markers during grief elicitation (Deceased > Stranger contrast) (N = 18). All regions reported at the threshold of .001, uncorrected, 10 voxels.
| Region | MNI Coordinates (x,y,z) | Z | T | p-value (voxels) | ||
|---|---|---|---|---|---|---|
| IL-1β | ||||||
| Cuneus (BA 19) | 22 | -68 | 32 | 4.16 | 5.72 | <0.001 (55) |
| Inferior frontal (BA 46) | -46 | 36 | 8 | 3.95 | 5.26 | <0.001 (31) |
| Inferior frontal (BA 44) | 48 | 16 | 16 | 3.71 | 4.78 | <0.001 (12) |
| Inferior frontal (BA 44) | -56 | 24 | 26 | 3.62 | 4.60 | <0.001 (20) |
| Inferior frontal (BA 46) | 32 | 26 | 14 | 3.44 | 4.28 | <0.001 (11) |
| Inferior frontal (BA 44) | 42 | 16 | 30 | 3.44 | 4.27 | <0.001 (11) |
| Inferior frontal (BA 44) | 34 | 16 | 28 | 3.34 | 4.10 | <0.001 (10) |
| sTNFrII | ||||||
| Fusiform gyrus (BA 37) | -36 | -58 | -12 | 4.04 | 5.45 | <0.001 (184) |
| Putamen | -24 | -26 | -4 | 3.83 | 5.01 | <0.001 (53) |
| Putamen | 18 | -26 | 0 | 3.64 | 4.64 | <0.001 (42) |
| Cuneus (BA 19) | -28 | -86 | 18 | 3.62 | 4.59 | <0.001 (37) |
| Temporal pole (BA 38) | 28 | 14 | -24 | 3.52 | 4.42 | <0.001 (27) |
| Fusiform gyrus (BA 37) | 40 | -54 | -12 | 3.34 | 4.10 | <0.001 (18) |
| Parahippocampus (BA 36) | -30 | -36 | -24 | 3.18 | 3.83 | <0.001 (11) |
In order to test whether other variables could explain the relationships between brain region activation (sACC and OFC) and cytokines (IL-1β and sTNFrII), correlations between these variables and demographic variables (length of time since the death, age, education, ethnicity, sister vs mother loss, body mass index, alcohol use) were analyzed. No significant correlations were found.
Behavioral correlates
In order to test whether the brain regions associated with local levels of IL-1β and sTNFrII were part of a motivational state that shaped the perceptions of the participant, behavioral correlates for the regional activation were analyzed. The incident free recall of words from the scanner task, initially designed as a manipulation check of scanner behavior, was chosen as an appropriate behavioral correlate. Because of a priori hypotheses regarding activation in the ventral prefrontal cortex, parameter estimates were extracted from the Grief Word > Neutral Word contrast of sACC (in the IL-1β analysis) and OFC (in the sTNFrII analysis). This contrast was chosen because it is the contrast that elicits grief through words. In order to demonstrate the association between activation in sACC and emotion processing, the number of grief and neutral words was correlated with the parameter estimates of sACC across subjects.
The correlation between sACC and freely recalled grief-related words was r = .50, p < 0.04, and the correlation between sACC and freely recalled neutral words was r = .27, p < 0.29 (Figure 2). Multiple regression analysis with grief-related and neutral words as independent variables confirmed that grief-related words were a marginally significant predictor of sACC activity (beta weight = 0.52, p < .09) and neutral words were not (beta weight = -0.04, p < .89). In order to demonstrate discriminant validity, sACC activation did not correlate with months since death, or kinship (sister vs. mother) relationship (all r's < 0.09, p = n.s.). The correlation between OFC and freely recalled grief-related words was r = .45, p < 0.07, and the correlation between OFC and freely recalled neutral words was r = .28, p < .28. As with sACC activation, OFC did not correlate with months since death, or kinship (sister vs. mother) relationship (all r< 0.26, p = n.s.).
Figure 2.
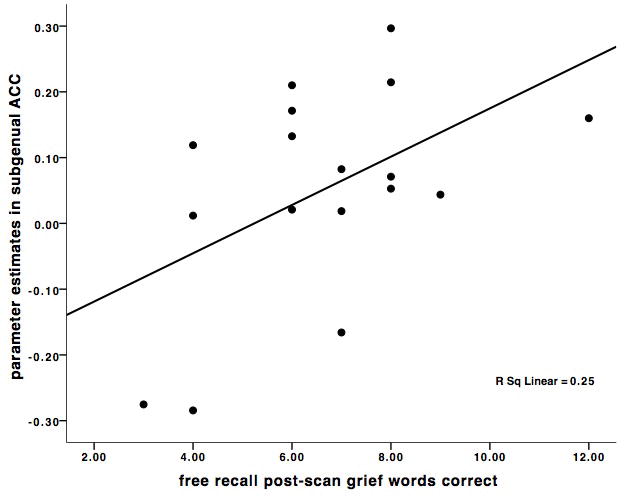
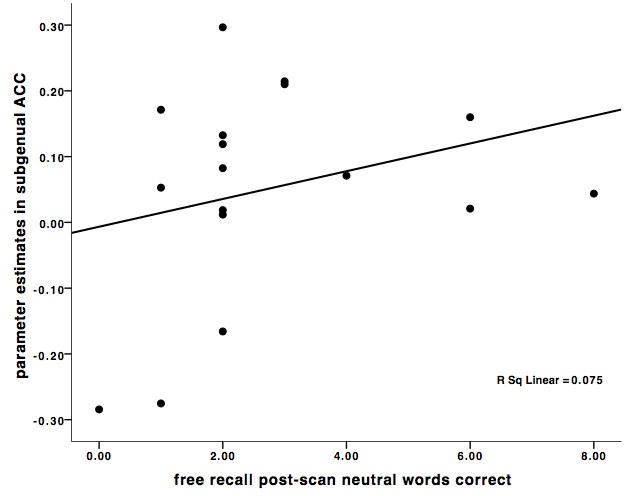
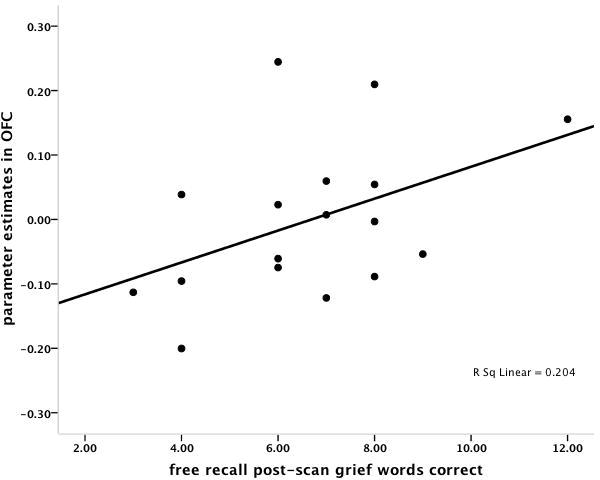
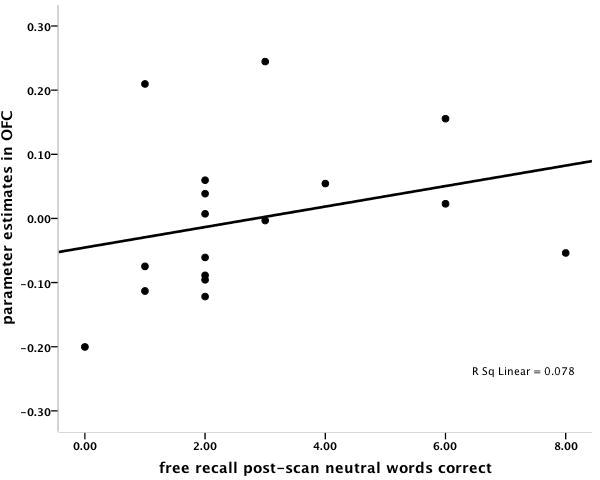
A. Scatter plot of the significant positive correlation between subgenual anterior cingulate (sACC) activations (-2, 26, -6) in the (Grief-related > Neutral Words contrast) and the number of correctly recalled grief-related words post-scanning. B. Scatter plot of the non-significant correlation between sACC activations (-2, 26, -6) in the (Grief-related > Neutral Words contrast) and the number of correctly recalled neutral words. C. Scatter plot of the marginally significant positive correlation between orbitofrontal cortex (OFC) activations (38, 36, -10) in the (Grief-related > Neutral Words contrast) and the number of correctly recalled grief-related words. D. Scatter plot of the non-significant correlation between OFC activations (38, 36, -10) in the (Grief-related > Neutral Words contrast) and the number of correctly recalled neutral words.
Discussion
The present study investigated the hypothesis that local inflammation, as measured by salivary concentrations of two markers of proinflammatory cytokine activity (e.g., IL-1β and sTNFrII), is positively associated with greater sACC activation. This hypothesis was based upon evidence that local levels (e.g., mouth) of proinflammatory cytokines are associated with sickness behaviors, (a set of behaviors including low mood) which are orchestrated by the brain and described as a shift in motivational state. In summary, the present study found that local levels of both inflammatory markers in the mouth were positively associated with ventral prefrontal activation (e.g., sACC and OFC) as well as other regions important in the emotional task such as noun retrieval (e.g., temporal cortex), and visual processing (e.g., cuneus and fusiform gyrus).
Activation of the sACC and the OFC are both related to monitoring the affective valence of stimuli (Kringelbach, 2005). The sACC is one of the most reliably activated regions in major depression, and shows a relationship to mood in other studies (for a review, see Ressler and Mayberg, 2007). The OFC is also associated with depression, showing elevated activation in depressed vs. remitted persons (Drevets, 2001). The hypothesis that a motivational state is changed under conditions of higher inflammation, and that this state includes low mood, is in keeping with the positive association between local inflammation and these regional activations.
We used a behavioral measure in order to test the association between ventral prefrontal regions and memory for grief-related words (in contrast to neutral words). The sACC and OFC activations, each associated with increased local inflammation, were correlated specifically with the recall of grief-related words. We hypothesized sACC activation, and indeed that correlation was strongest, while the correlation between grief-related words and the OFC activation was a trend. Biased memory retrieval for mood-congruent words has been found in other mood disorders, such as anxiety and depression (Mathews and MacLeod, 2005). The strong positive correlation between the recall of grief-related words and ventral prefrontal activation (and the absence of a correlation with neutral words) suggests that those with the highest local inflammation are in a motivational state that preferentially attends to mood congruent information, which could be expected from the sickness behavior hypothesis.
Other areas of activation were related to functions required during the grief-eliciting tasks. The cuneus region, activated in both IL-1β and sTNFrII analyses, is an area important in visual processing, as seen in emotion induction with other visual tasks (Ganis et al., 2004). The fusiform gyrus is a brain region specifically activated during the visual processing of human faces (Critchley et al., 2000; Kanwisher et al., 1997). These two areas were activated in the Deceased > Stranger condition. This condition relies upon visual stimuli for grief elicitation. The inferior temporal region, also activated in both IL-1β and sTNFrII analyses, functions in noun retrieval and is seen in the Grief Word > Neutral Word. This condition relies upon words for grief elicitation. The pons activation in the present study may reflect activation of the sensory pathway positively correlated with local inflammation. The trigeminal nerve carries sensory information from the mouth to the brain, and enters the brainstem at the pons (Bowsher, 1997).
The local IL-1β levels in the present study are comparable to other studies using whole saliva to investigate IL-1β (Miller et al., 2006; Tobon-Arroyave et al., 2008) and sTNFrII (Al-Harthi et al., 2000; Nishanian et al., 1998; Winkler et al., 2001). In studies comparing those with periodontal disease with normal controls, it is evident that levels vary widely, and the sample in the present study reflects this wide variability (Table 3).
Our data is not evidence that peripherally measured IL-1β or TNF-α is binding to receptors in the prefrontal cortex; however, the present results support the idea that peripheral inflammation at a local site (i.e., mouth) is communicated to the brain, and that the resulting changes in motivational state may influence emotional processing. If sickness behavior is the output of a motivational state of the brain, it may influence the regional processing of emotional stimuli (e.g., greater use of sACC) upon presentation of reminders of a recently deceased loved one.
Several limitations of the present study should be noted. While it is clear that pro-inflammatory cytokine levels in the mouth have been linked to sickness behaviors (e.g., social withdrawal, depressed mood), a limitation of the present study is that periodontal disease was not evaluated in each participant. Therefore, alternative interpretations of the data are possible. However, levels of IL-1β in the present study are in line with the levels published by other laboratories (Al-Harthi et al., 2000; Miller et al., 2006; Nishanian et al., 1998; Tobon-Arroyave et al., 2008; Winkler et al., 2001). The present study is cross-sectional, and therefore it is not possible to state a causal relationship. The reverse causation must be considered: bereaved individuals with greater medial prefrontal activation may have lower mood and motivation for health behaviors, such as oral care, and this may cause greater oral inflammation. It is impossible to rule out another interpretations, because there was only one assessment of pro-inflammatory markers. Those participants who found the task most emotionally arousing may have had both the greatest emotional recall scores and pro-inflammatory responses. Although few studies of the kinetics of oral cytokine production exist, Mastrolonardo and colleagues (2007), report a rise in IL-1β in saliva after 20 minutes following a stress task in control subjects and Dickerson and colleagues (2004) report a stress-related change in sTNFrII in other oral fluids after 30 minutes. However, samples were not taken earlier than these time points in either report, and therefore it is possible that measures taken immediately following the task in the present study could reflect the result of acute stress of the emotional arousing task or could reflect the individual variability in response to chronic bereavement stress. Finally, future research should include a nonbereaved comparison group, in order to determine whether the associations are specific to a chronically stressed state, and include men to examine generalizability.
This is the first study to demonstrate the relationship between emotional processing, brain activation and local immune system activation in a chronically stressed population of adults. Future research should investigate the direction of causality in this relationship. For example, a directional hypothesis could test whether it is more difficult for those with high levels of local inflammation to adjust to stressful life events, or conversely, whether mood-related brain activation leads to prolonged inflammation in the periphery. In summary, the present data demonstrates that higher salivary levels of sTNFrII and interleukin-1β (IL-1β), in separate analyses, are both associated with activation of the ventral prefrontal cortex in persons undergoing the chronic emotional stress of bereavement, and adds to a growing literature on the relationship between local inflammation, the brain, and behavior.
Acknowledgments
This research was supported by funds from the California Breast Cancer Research Program of the University of California, Grant Number 10IB-0048. M-F.O. was supported by a Friends of the Semel Institute Research Fellowship. This work was supported in part by NIA grant K01 AG028404, NIMH T32-MH19925, and the Cousins Center for Psychoneuroimmunology. We would like to thank the UCLA Brain Mapping Center for their assistance and Heather L. Urry for comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Harthi L, Wright DJ, Anderson D, Cohen M, Matity Ahu D, Cohn J, Cu-Unvin S, Burns D, Reichelderfer P, Lewis S, Beckner S, Kovacs A, Landay A. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res. 2000;20:719–724. doi: 10.1089/10799900050116426. [DOI] [PubMed] [Google Scholar]
- Aurer A, Aurer-Kozelj J, Stavljenić-Rukavina A, Kalenić S, Ivić-Kardum M, Haban V. Inflammatory mediators in saliva of patients with rapidly progressive periodontitis during war stress induced incidence increase. Coll Antropol. 1999;23:117–124. [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, Maximilian von E, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A Mechanism Converting Psychosocial Stress into Mononuclear Cell Activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher D. Trigeminal neuralgia: An anatomically oriented review. Clinical Anatomy. 1997;10:409–415. doi: 10.1002/(SICI)1098-2353(1997)10:6<409::AID-CA7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biological Psychiatry. 2005;58:190. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews of Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinzer R, Granrath N, Spahl M, Linz S, Waschul B, Herforth A. Stress, oral health behaviour and clinical outcome. Br J Health Psychol. 2005;10:269–283. doi: 10.1348/135910705X26858. [DOI] [PubMed] [Google Scholar]
- Deinzer R, Granrath N, Stuhl H, Twork L, Idel H, Waschul B, Herforth A. Acute stress effects on local Il-1[beta] responses to pathogens in a human in vivo model. Brain, Behavior, and Immunity. 2004;18:458–467. doi: 10.1016/j.bbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Cogniton and Brain Research. 2004;20:226–241. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gündel H, O'Connor MF, Littrell L, Fort C, Lane RD. Functional neuroanatomy of grief: An fMRI study. American Journal of Psychiatry. 2003;160:1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Hobson CJ, Kamen J, Szostek J, Nethercut CM, Tiedmann JW, Wojnarowicz S. Stressful life events: A revision and update of the Social Readjustment Rating Scale. International Journal of Stress Management. 1998;5:1–23. [Google Scholar]
- Hugoson A, Ljungquist B, Breivik T. The relationship of some negative events and psychological factors to periodontal disease in an adult Swedish population 50 to 80 years of age. Journal Of Clinical Periodontology. 2002;29:247–253. doi: 10.1034/j.1600-051x.2002.290311.x. [DOI] [PubMed] [Google Scholar]
- Johannsen A, Rydmark I, Söder B, Åsberg M. Gingival inflammation, increased periodontal pocket depth and elevated interleukin-6 in gingival crevicular fluid of depressed women on long-term sick leave. Journal of Periodontal Research. 2007;42:546–552. doi: 10.1111/j.1600-0765.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages U, Weber AG, Wehrbein H. Approximal plaque and gingival sulcus bleeding in routine dental care patients: relations to life stress, somatization and depression. J Clin Periodontol. 2005;32:575–582. doi: 10.1111/j.1600-051X.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- Marcenes WS, Sheiham A. The relationship between work stress and oral health status. Soc Sci Med. 1992;35:1511–1520. doi: 10.1016/0277-9536(92)90054-t. [DOI] [PubMed] [Google Scholar]
- Mastrolonardo M, Alicino D, Zefferino R, Pasquini P, Picardi A. Effect of psychological stress on salivary interleukin-1beta in psoriasis. Arch Med Res. 2007;38:206–211. doi: 10.1016/j.arcmed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. Journal of the American Dental Association. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- Navarro VP, Iyomasa MM, Leite-Panissi CR, Almeida MC, Branco LG. New role of the trigeminal nerve as a neuronal pathway signaling brain in acute periodontitis: participation of local prostaglandins. European Journal of Physiology. 2006;453:73–82. doi: 10.1007/s00424-006-0113-2. [DOI] [PubMed] [Google Scholar]
- Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin Diagn Lab Immunol. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain's reward center. Neuroimage. 2008;42:969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased Stress-Induced Inflammatory Responses in Male Patients With Major Depression and Increased Early Life Stress. American Journal of Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Agosta F, Colombo B, Mezzapesa DM, Falini A, Comi G, Filippi M. fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Human Brain Mapping. 2007;28:373–382. doi: 10.1002/hbm.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proceedings of the National Academy of Science. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuld A, Mullington J, Hermann D, Hinze-Selch D, Fenzel T, Holsboer F, Pollmacher T. Effects of granulocyte colony-stimulating factor on night sleep in humans. American Journla of Physiology: Regulatory, Integrative and Comparative Physiology. 1999;276:R1149–1155. doi: 10.1152/ajpregu.1999.276.4.R1149. [DOI] [PubMed] [Google Scholar]
- Tobon-Arroyave SI, Jaramillo-Gonzalez PE, Isaza-Guzman DM. Correlation between salivary IL-1beta levels and periodontal clinical status. Archives of Oral Biology. 2008;53:346–352. doi: 10.1016/j.archoralbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Waschul B, Herforth A, Stiller-Winkler R, Idel H, Granrath N, Deinzer R. Effects of plaque, psychological stress and gender on crevicular Il-1beta and Il-1ra secretion. Journal Of Clinical Periodontology. 2003;30:238–248. doi: 10.1034/j.1600-051x.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- Weik U, Herforth A, Kolb-Bachofen V, Deinzer R. Acute stress induces proinflammatory signaling at chronic inflammation sites. Psychosomatic Medicine. 2008;70:906–912. doi: 10.1097/PSY.0b013e3181835bf3. [DOI] [PubMed] [Google Scholar]
- Winkler O, Hadnagy W, Idel H. Cytokines detectable in saliva of children as appropriate markers of local immunity of the oral cavity--an approach for the use in air pollution studies. Int J Hyg Environ Health. 2001;204:181–184. doi: 10.1078/1438-4639-00092. [DOI] [PubMed] [Google Scholar]


