Abstract
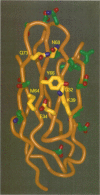
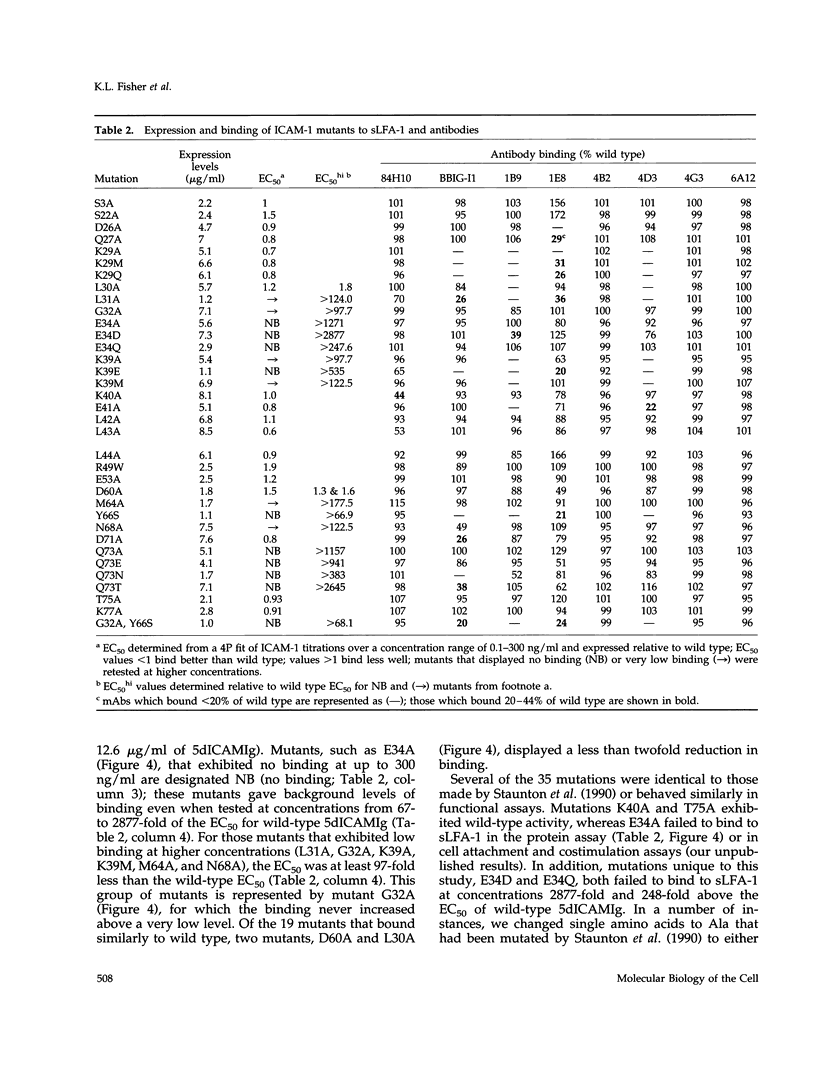
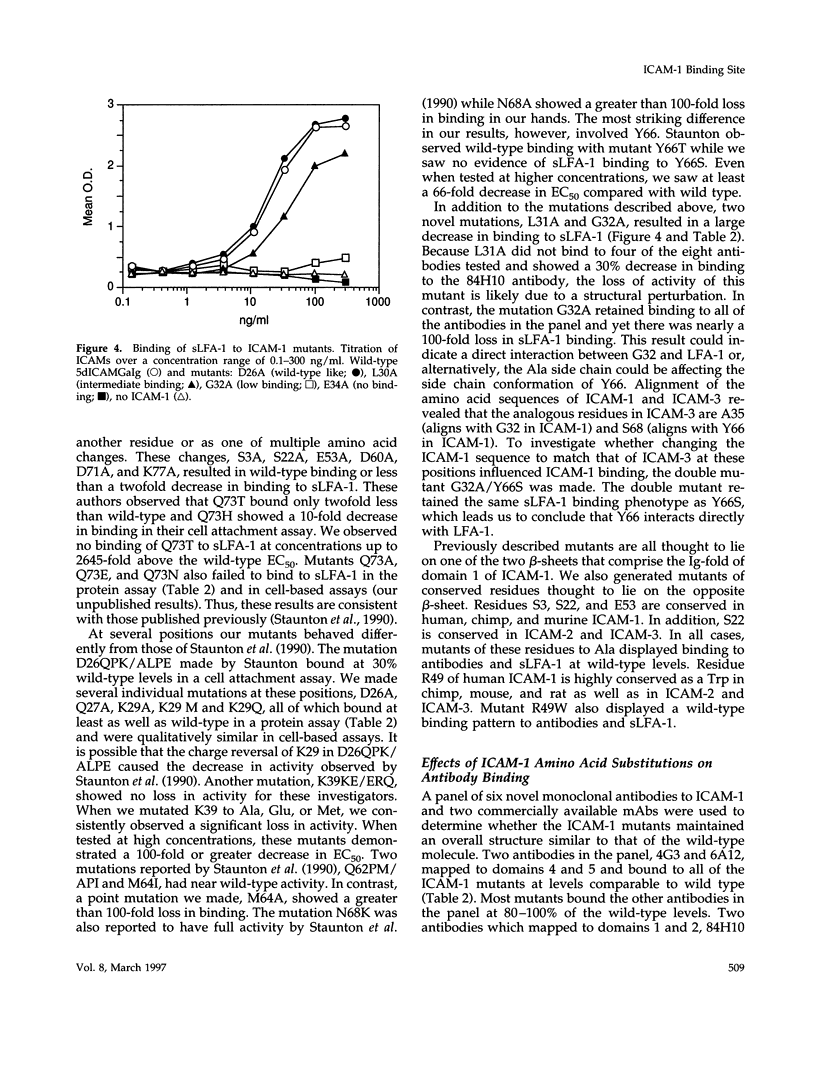
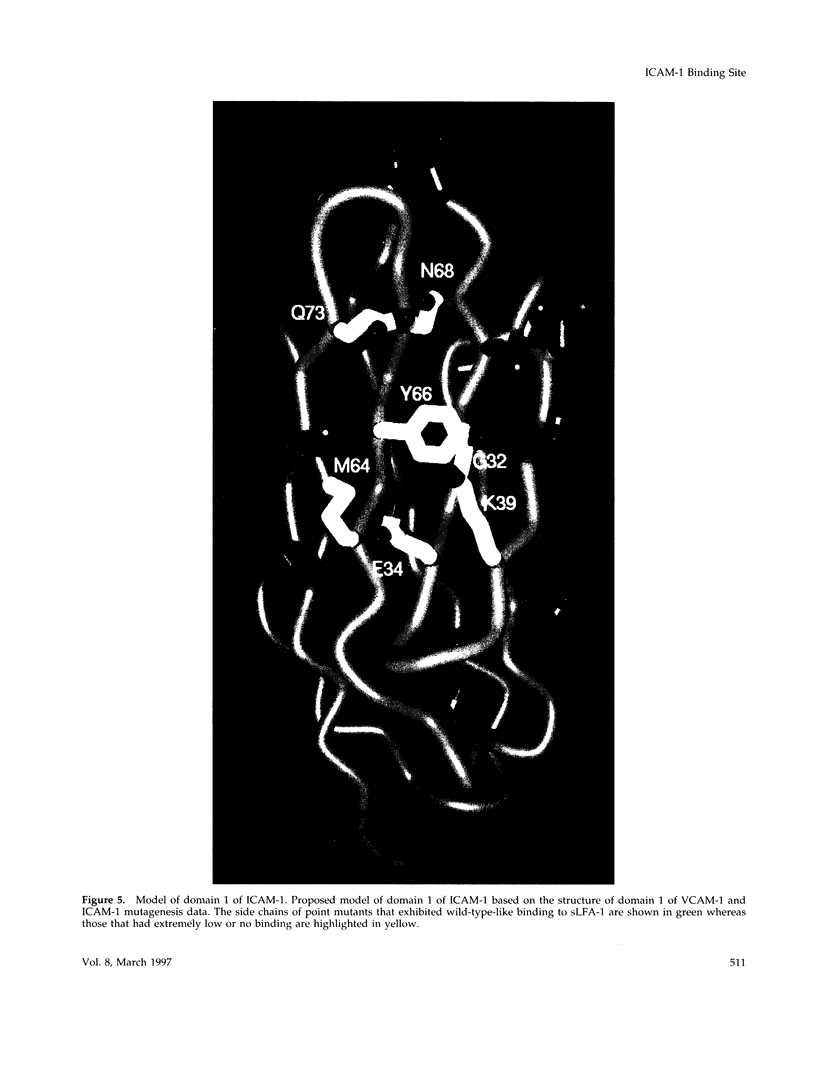
Intercellular adhesion molecule 1 (ICAM-1, CD54) is a member of the Ig superfamily and is a counterreceptor for the beta 2 integrins: lymphocyte function-associated antigen 1 (LFA-1, CD11a/CD18), complement receptor 1 (MAC-1, CD11b/CD18), and p150,95 (CD11c/CD18). Binding of ICAM-1 to these receptors mediates leukocyte-adhesive functions in immune and inflammatory responses. In this report, we describe a cell-free assay using purified recombinant extracellular domains of LFA-1 and a dimeric immunoadhesin of ICAM-1. The binding of recombinant secreted LFA-1 to ICAM-1 is divalent cation dependent (Mg2+ and Mn2+ promote binding) and sensitive to inhibition by antibodies that block LFA-1-mediated cell adhesion, indicating that its conformation mimics that of LFA-1 on activated lymphocytes. We describe six novel anti-ICAM-1 monoclonal antibodies, two of which are function blocking. Thirty-five point mutants of the ICAM-1 immunoadhesin were generated and residues important for binding of monoclonal antibodies and purified LFA-1 were identified. Nineteen of these mutants bind recombinant LFA-1 equivalently to wild type. Sixteen mutants show a 66-2500-fold decrease in LFA-1 binding yet, with few exceptions, retain binding to the monoclonal antibodies. These mutants, along with modeling studies, define the LFA-1 binding site on ICAM-1 as residues E34, K39, M64, Y66, N68, and Q73, that are predicted to lie on the CDFG beta-sheet of the Ig fold. The mutant G32A also abrogates binding to LFA-1 while retaining binding to all of the antibodies, possibly indicating a direct interaction of this residue with LFA-1. These data have allowed the generation of a highly refined model of the LFA-1 binding site of ICAM-1.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Gupta S. K., Pierce M. W., Tenen D. G. Amino acid sequence of the alpha subunit of human leukocyte adhesion receptor Mo1 (complement receptor type 3). J Cell Biol. 1988 Jun;106(6):2153–2158. doi: 10.1083/jcb.106.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt A. R., McDowall A., Craig A. G., Bates P. A., Sternberg M. J., Marsh K., Newbold C. I., Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell. 1992 Jan 10;68(1):71–81. doi: 10.1016/0092-8674(92)90207-s. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Briskin M. J., McEvoy L. M., Butcher E. C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993 Jun 3;363(6428):461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- Carter P., Nilsson B., Burnier J. P., Burdick D., Wells J. A. Engineering subtilisin BPN' for site-specific proteolysis. Proteins. 1989;6(3):240–248. doi: 10.1002/prot.340060306. [DOI] [PubMed] [Google Scholar]
- Connolly M. K., Kitchens E. A., Chan B., Jardieu P., Wofsy D. Treatment of murine lupus with monoclonal antibodies to lymphocyte function-associated antigen-1: dose-dependent inhibition of autoantibody production and blockade of the immune response to therapy. Clin Immunol Immunopathol. 1994 Aug;72(2):198–203. doi: 10.1006/clin.1994.1130. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Kishimoto T. K., Miller L. J., Springer T. A. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) alpha subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988 Sep 5;263(25):12403–12411. [PubMed] [Google Scholar]
- Corbi A. L., Miller L. J., O'Connor K., Larson R. S., Springer T. A. cDNA cloning and complete primary structure of the alpha subunit of a leukocyte adhesion glycoprotein, p150,95. EMBO J. 1987 Dec 20;6(13):4023–4028. doi: 10.1002/j.1460-2075.1987.tb02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994 Jun 1;4(6):506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989 Dec 1;8(12):3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Wood W. I., Eaton D., Hass P. E., Hollingshead P., Wion K., Mather J., Lawn R. M., Vehar G. A., Gorman C. Construction and characterization of an active factor VIII variant lacking the central one-third of the molecule. Biochemistry. 1986 Dec 30;25(26):8343–8347. doi: 10.1021/bi00374a001. [DOI] [PubMed] [Google Scholar]
- Edwards C. P., Champe M., Gonzalez T., Wessinger M. E., Spencer S. A., Presta L. G., Berman P. W., Bodary S. C. Identification of amino acids in the CD11a I-domain important for binding of the leukocyte function-associated antigen-1 (LFA-1) to intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 1995 May 26;270(21):12635–12640. doi: 10.1074/jbc.270.21.12635. [DOI] [PubMed] [Google Scholar]
- Ellison J. W., Berson B. J., Hood L. E. The nucleotide sequence of a human immunoglobulin C gamma1 gene. Nucleic Acids Res. 1982 Jul 10;10(13):4071–4079. doi: 10.1093/nar/10.13.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J., Holness C. L., Needham L. A., Turley H., Gatter K. C., Mason D. Y., Simmons D. L. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature. 1992 Dec 3;360(6403):481–484. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- Giranda V. L., Chapman M. S., Rossmann M. G. Modeling of the human intercellular adhesion molecule-1, the human rhinovirus major group receptor. Proteins. 1990;7(3):227–233. doi: 10.1002/prot.340070304. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Heagy W., Waltenbaugh C., Martz E. Potent ability of anti-LFA-1 monoclonal antibody to prolong allograft survival. Transplantation. 1984 May;37(5):520–523. doi: 10.1097/00007890-198405000-00021. [DOI] [PubMed] [Google Scholar]
- Hession C., Moy P., Tizard R., Chisholm P., Williams C., Wysk M., Burkly L., Miyake K., Kincade P., Lobb R. Cloning of murine and rat vascular cell adhesion molecule-1. Biochem Biophys Res Commun. 1992 Feb 28;183(1):163–169. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Holt V., August J. T., Pescovitz M. D. Monoclonal antibodies against porcine LFA-1: species cross-reactivity and functional effects of beta-subunit-specific antibodies. Mol Immunol. 1989 Sep;26(9):883–895. doi: 10.1016/0161-5890(89)90145-4. [DOI] [PubMed] [Google Scholar]
- Holness C. L., Bates P. A., Little A. J., Buckley C. D., McDowall A., Bossy D., Hogg N., Simmons D. L. Analysis of the binding site on intercellular adhesion molecule 3 for the leukocyte integrin lymphocyte function-associated antigen 1. J Biol Chem. 1995 Jan 13;270(2):877–884. doi: 10.1074/jbc.270.2.877. [DOI] [PubMed] [Google Scholar]
- Huang C., Springer T. A. A binding interface on the I domain of lymphocyte function-associated antigen-1 (LFA-1) required for specific interaction with intercellular adhesion molecule 1 (ICAM-1). J Biol Chem. 1995 Aug 11;270(32):19008–19016. doi: 10.1074/jbc.270.32.19008. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Davis S. J., Williams A. F., Harlos K., Stuart D. I. Crystal structure at 2.8 A resolution of a soluble form of the cell adhesion molecule CD2. Nature. 1992 Nov 19;360(6401):232–239. doi: 10.1038/360232a0. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Harlos K., Bottomley M. J., Robinson R. C., Driscoll P. C., Edwards R. M., Clements J. M., Dudgeon T. J., Stuart D. I. Crystal structure of an integrin-binding fragment of vascular cell adhesion molecule-1 at 1.8 A resolution. Nature. 1995 Feb 9;373(6514):539–544. doi: 10.1038/373539a0. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Yaoita E., Yamamoto T., Tamatani T., Miyasaka M., Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993 Feb 1;150(3):1074–1083. [PubMed] [Google Scholar]
- Kim K. J., Alphonso M., Schmelzer C. H., Lowe D. Detection of human leukemia inhibitory factor by monoclonal antibody based ELISA. J Immunol Methods. 1992 Nov 25;156(1):9–17. doi: 10.1016/0022-1759(92)90005-e. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Klickstein L. B., York M. R., Fougerolles A. R., Springer T. A. Localization of the binding site on intercellular adhesion molecule-3 (ICAM-3) for lymphocyte function-associated antigen 1 (LFA-1). J Biol Chem. 1996 Sep 27;271(39):23920–23927. doi: 10.1074/jbc.271.39.23920. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R. S., Corbi A. L., Berman L., Springer T. Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J Cell Biol. 1989 Feb;108(2):703–712. doi: 10.1083/jcb.108.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Osborn L., Vassallo C., Browning B. G., Tizard R., Haskard D. O., Benjamin C. D., Dougas I., Kirchhausen T. Arrangement of domains, and amino acid residues required for binding of vascular cell adhesion molecule-1 to its counter-receptor VLA-4 (alpha 4 beta 1). J Cell Biol. 1994 Feb;124(4):601–608. doi: 10.1083/jcb.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Qu A., Leahy D. J. Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, alpha L beta 2) integrin. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10277–10281. doi: 10.1073/pnas.92.22.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz M. E., Chiu H. H., Jones S., Fox J., Kim K. J., Presta L. G., Fong S. Structural requirements for adhesion of soluble recombinant murine vascular cell adhesion molecule-1 to alpha 4 beta 1. J Cell Biol. 1994 Jun;125(6):1395–1406. doi: 10.1083/jcb.125.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C., Lipsky B., Erickson H. P., Hayflick J., Dick K. O., Gallatin W. M., Staunton D. E. LFA-1 binding site in ICAM-3 contains a conserved motif and non-contiguous amino acids. Cell Adhes Commun. 1994 Oct;2(5):429–440. doi: 10.3109/15419069409004453. [DOI] [PubMed] [Google Scholar]
- Simmons D. L. The role of ICAM expression in immunity and disease. Cancer Surv. 1995;24:141–155. [PubMed] [Google Scholar]
- Simmons D. L., Walker C., Power C., Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990 Jun 1;171(6):2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Stockinger H., Gadd S. J., Eher R., Majdic O., Schreiber W., Kasinrerk W., Strass B., Schnabl E., Knapp W. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol. 1990 Dec 1;145(11):3889–3897. [PubMed] [Google Scholar]
- Tanaka Y., Kobayashi K., Takahashi A., Arai I., Higuchi S., Otomo S., Habu S., Nishimura T. Inhibition of inflammatory liver injury by a monoclonal antibody against lymphocyte function-associated antigen-1. J Immunol. 1993 Nov 1;151(9):5088–5095. [PubMed] [Google Scholar]
- Van der Vieren M., Le Trong H., Wood C. L., Moore P. F., St John T., Staunton D. E., Gallatin W. M. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995 Dec;3(6):683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Vazeux R., Hoffman P. A., Tomita J. K., Dickinson E. S., Jasman R. L., St John T., Gallatin W. M. Cloning and characterization of a new intercellular adhesion molecule ICAM-R. Nature. 1992 Dec 3;360(6403):485–488. doi: 10.1038/360485a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Wurm F. M., Gwinn K. A., Kingston R. E. Inducible overproduction of the mouse c-myc protein in mammalian cells. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5414–5418. doi: 10.1073/pnas.83.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]