Abstract
Production of mature mRNAs that encode functional proteins involves highly complex pathways of synthesis, processing and surveillance. At numerous steps during the maturation process, the mRNA transcript undergoes scrutiny by cellular quality control machinery. This extensive RNA surveillance ensures that only correctly processed mature mRNAs are translated and precludes production of aberrant transcripts that could encode mutant or possibly deleterious proteins. Recent advances in elucidating the molecular mechanisms of mRNA processing have demonstrated the existence of an integrated network of events, and have revealed that a variety of human diseases are caused by disturbances in the well-coordinated molecular equilibrium of these events. From a medical perspective, both loss and gain of function are relevant, and a considerable number of different diseases exemplify the importance of the mechanistic function of RNA surveillance in a cell. Here, mechanistic hallmarks of mRNA processing steps are reviewed, highlighting the medical relevance of their deregulation and how the understanding of such mechanisms can contribute to the development of therapeutic strategies.
INTRODUCTION
Messenger RNA (mRNA) mediates the transfer of genetic information from the cell nucleus to the cytoplasm (1). The development of a mature mRNA molecule requires several multiple-step processes that proceed through a progressive sequence of interdependent events: transcription, capping, splicing, edition, 3′-end processing, and numerous events dependent on RNA-binding proteins that bind to the transcript, forming an mRNA ribonucleoprotein (mRNP) complex (2). The perfect synchrony of those events is maintained by cellular quality control machinery that assures tight regulation of gene expression and consequently a healthy organism.
Because the molecular aspects of mRNA metabolism have been extensively investigated, many points concerning this metabolic pathway are known. After processing, identification of potential errors in the transcripts by cellular surveillance machinery (3,4) can avoid disastrous consequences to the organism, because the defective transcripts have the potential to be translated into aberrant and deleterious proteins. This quality control machinery serves a critical role in maintaining health, and studies have linked error-induced mRNA metabolism to several human diseases (5–7). Notable examples of such failures in the mRNA quality control mechanisms are β-thalassemia (8) and Marfan syndrome (9). Certain kinds of cancers and heritable genetic diseases may also be associated with mistakes in mRNA editing and/or processing (5). For instance, a variety of clinical diseases are due to inappropriate 3′-end processing of the transcripts (10–12).
Several laboratories are investigating RNA to understand all the molecular aspects of mRNA processing and surveillance. This research will lead to the development of more sophisticated clinical diagnostic and therapeutic approaches. This review discusses key mechanistic features of mRNA processing and surveillance and then discusses the clinical perspective of those mechanisms, which illustrate how diseases can be caused by errors/mutations of important RNA sequence elements and/or by the pathological expression of proteins whose corresponding mRNA contains serious mistakes. Also presented here is a brief overview of the influence of circadian rhythms on RNA processing and surveillance.
MESSENGER RNA PROCESSING AND SURVEILLANCE
Cotranscriptional mRNA Processing: Integrated Events That Control Gene Expression
In eukaryotes, including humans, DNA is initially transcribed in the nucleus as a precursor mRNA by RNA polymerase II (RNAP II) (13). Soon after RNAP II initiates transcription, the nascent molecule is modified by the addition of a 7-methyl guanosine cap structure at the 5′ end of the RNA. The introns are also cotranscriptionally removed from the precursor mRNA, the body of this molecule has its sequence edited, and the cleavage and the polyadenylation of the 3′-end also take place (2). During all the processing events, a variety of mRNA-binding proteins and other factors interact with the maturing transcript (14,15), assuming a critical function in the mRNA surveillance context.
Understanding of the cotranscriptional processing events requires a mechanistic description of the involved steps. As soon as the nascent transcript is 20–30 nucleotides long, the capping addition (the first mRNA processing event to be analyzed here) happens in the nucleus by a sequential action of three enzymes: RNA 5′-triphosphatase, guanylyltransferase and N7G-methyltransferase (16,17). Both guanylyltransferase and N7G-methyltransferase bind to the phosphorylated C-terminal domain of RNAP II, which increases the efficacy of the capping process (18). After the addition of the 5′ end to the precursor mRNA, proteins get assembled on that structure; these proteins include the cap-binding complex (CBC20 and CBC80) (19), which protects the molecule from a 5′→3′ exonucleolytic degradation. Interestingly, once the transcript is exported to the cytoplasm, the CBC proteins bound to it are usually replaced by the 4E subunit of the eukaryotic initiation factor (eIF4E) (17), promoting the ribosome attachment (20).
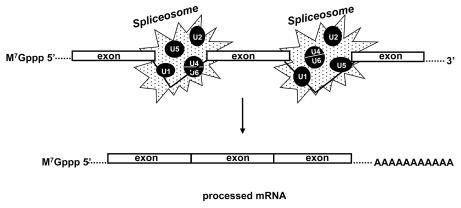
In eukaryotes, a second mechanistic approach to be considered in the context of pre-mRNA processing and surveillance is the intron removal process (Figure 1). In mammals the majority of the genes go through this process. Three canonical splicing signals are required to remove most introns: a 5′ splice site (AG|GURAGU, where R = purine), a 3′ splice site (YAG|RNNN, where Y = pyrimidine and N = any base), and a branch point sequence located within 50 nucleotides upstream of the 3′ end of the intron. For splicing to occur, two consecutive transesterefication reactions take place that result in the fusion of two exons (21). In those events the small ribonucleoproteins U1, U2, U4, U5 and U6 and the Lsm proteins 2–8 assemble together in a complex called a spliceosome, which acts directly on the exon joining process (22). Moreover, several studies have shown that the CBC proteins enhance the interaction of the small ribonucleoprotein U1 at the 5′ splice site (11,23), demonstrating the existence of interconnected mechanisms in mRNA processing (21). In addition, conserved sequences characterized as positive-acting elements within the exons (the exonic splicing enhancers [ESE]) are recognized by specific proteins such as those rich in serine and arginine, whose interaction modulates the efficacy of the splicing mechanism. The cotranscriptional splicing processing is undoubtedly an important cellular tool that contributes to the final quality of the mRNA in several different organisms (23,24). In humans the mechanism seems to be extremely complex because of the extension length of some of their genes, such as the dystrophin gene (14,000 bp) (25). The huge introns must be precisely removed to maintain a healthy organism.
Figure 1.
Schematic representation of mRNA splicing. Exons are joined by removal of in-tronic sequences mediated by the spliceosome assembly.
After the studies of cotranscriptional modification of the precursor mRNA reviewed here, RNA editing was ignored by investigators for a long time, possibly because of difficulties in identifying regions containing a single nucleotide edition (26). The enzymatic modification of nucleotide sequences was originally identified in the mitochondrial RNA of trypanosomes (27). Since then this mechanism of RNA processing has been extensively explored and described in a wide range of species. In mammals there are two main classes of editing enzymes. One of those classes is involved in the editing of cytidine to uridine in RNA molecules through the activity of APOBEC-1 (apolipoprotein B–editing catalytic subunit 1) (28). In humans, proteins from the APOBEC family have been identified, and interestingly, most of them are involved in DNA edition of immunological system cells (29) that contribute to adaptive immunity. A second class of those editing enzymes is responsible for the edition of adenine to inosine; in humans, adenosine is frequently edited to inosine by ADAR (adenosine deaminases that act on RNA) enzymes, whose activity is more intensive in the nervous system (30). The phylogenetic analyses of the two classes of proteins have indicated that they evolved from a common ancestral cytosine deaminase involved in pyrimidine metabolism (31).
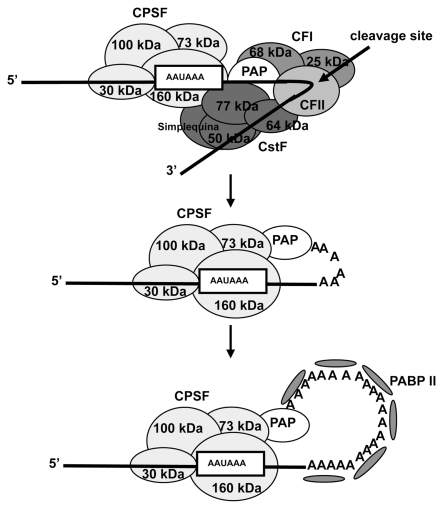
The final step in cotranscriptional mRNA processing is 3′-end formation (Figure 2). With the exception of some histone mRNAs, the eukaryotic mRNA possess poly(A) tails at their 3′ end, which are produced by a two-step reaction involving endonucleolytic cleavage and a subsequent poly(A) tail addition. In mammals the formation of the mRNA 3′ end requires the enzymes poly(A) polymerases (PAPs) plus four multi-meric complexes: cleavage and poly-adenylation specificity factor (CPSF), cleavage stimulatory factor (CstF), cleavage factor I (CFI), and CFII. CPSF and its subunits bind to the canonical poly-adenylation signal AAUAAA, which is located upstream of the cleavage site (32). CstF contains three subunits (CstF-50, CstF-64, and CstF-77) and recognizes the so-called downstream U- or G/U-rich element of the cleavage site in the mRNA precursor (33). Working in a synchronous way, the complexes CPSF and CstF recruit the cleavage factors to the precise location of the cleavage. Poly(A) polymerase is usually required for the cleavage reaction and, together with CPSF, directs poly(A) addition. Poly(A) binding protein (PABP) II binds to the emerging poly(A) tail and in turn enhances the processivity of the poly(A) polymerase (34). Poly(A) tails usually reach 200 nucleotides in length in different mRNAs, and upon export to the cytoplasm, the nuclear PABP 1 that interacts with the poly(A) tails is replaced by the cytosolic PABP (35), which interacts with the translation initiation factor eIF4G that associates with eIF4E. This process stimulates translation and regulates mRNA stability (36).
Figure 2.
mRNA polyadenylation requires the subsequent steps of cleavage and poly(A) addition.
All of these findings support the existence of a complex and integrated network of cotranscriptional mRNA-processing events. Different inherited and acquired human diseases exemplify errors in such events, and from a medical perspective, either loss or gain of function can be relevant for maintaining a healthy organism. Therefore, the understanding of mechanistic hallmarks of mRNA processing and surveillance highlights the relevance of these phenomena and illustrates their implications for clinical diagnostic and therapeutic strategies.
RNA Surveillance: Molecular Mechanisms That Ensure mRNA Quality Control
RNA performs multiple diverse functions in a cell, and therefore plays important roles in human diseases. Because of the interconnection of the cotranscriptional events, the surveillance mechanisms are compounded by several distinct elements. In a cell, the exosome can be considered one important component of the surveillance system. The exosome machinery comprises a versatile complex of 3′→5′ exonucleases that degrade mRNA in both the nucleus and cytoplasm (3,37–39). This complex is also required for processing small nuclear RNA (snRNA), ribosomal RNA (rRNA), and small nucleolar RNA (snoRNA) (40). Researchers studying yeast have demonstrated that normal or aberrant transcripts that extend time in the nucleus become a target for degradation by the nuclear exosome (38,41). In the defective mRNAs, processing could take a longer time owing to defects in the body of the transcripts, which in turn can activate the nuclear RNA surveillance machinery.
It is intriguing to consider the possibility that all RNA transcripts may be subject to extensive surveillance mechanisms. Mechanistic details concerning the RNA processing and degradation involved in surveillance have been described. It is possible that all transcripts are potential substrates for the exosome, but those that are correctly processed interact with a bulk of proteins that protect them from exosome-mediate degradation (42). Researchers studying human exosome components reported that in patients with autoimmune scleroderma syndrome the PM-Scl75 and the PM-Scl100 exosomal subunits are recognized and destroyed by autoantibodies (37,43). This finding illustrates the importance of the exosome complex in organism integrity. However, the details concerning exosome activity in distinguishing mRNA substrates and directing them to degradation remain to be investigated.
Nonsense-Mediated Decay: How Does This Mechanism Work in Quality Control Checking?
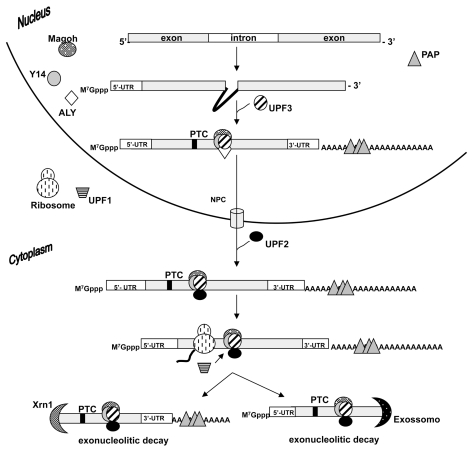
Once the transcripts are synthesized and processed within the nucleus, they must be transported to the cytoplasm. There they have several potential fates, and one of them is to become a target for nonsense-mediated decay (NMD). This process was initially described in Saccharomyces cerevisiae (44), and it is known that one-third of inherited genetic disorders and many forms of cancers are caused by frameshift or nonsense mutations, which generate premature termination codons (PTCs). Contrary to intuition, the predominant consequence of nonsense mutations is not the synthesis of truncated proteins, whose deleterious effect could be crucial to the organism. The NMD surveillance mechanism accelerates the degradation process by its ability to bypass the rate-limiting step of deadenylation prior to decapping, and may perform 5′→3′ mRNA decay (45) (Figure 3). Indeed, the phenotypic severity of selected diseases caused by nonsense mutations can be predicted by the extent of reduction in the mRNA level of the mutant allele (5,7). Chain termination mutation reduces mRNA abundance by decreasing its half-life. For example, a recessive form of β-thalassemia common in the Mediterranean population results from a mutation that generates a premature UAG stop codon, leading to a reduction of β-globin mRNA accumulation (8).
Figure 3.
Simplified view of the eukaryotic NMD process. After splicing, the nuclear EJC proteins deposit onto an mRNA. Once this mRNA reaches the cytoplasm, after the nuclear export, some nuclear EJC components dissociate and the cytoplasmic NMD proteins bind to the mRNA. During the first round of translation, NMD is triggered by the recognition of PTC upstream of the exon-exon junction and the EJC-associated NMD proteins. This PTC-containing mRNA can be degraded either from the 5′-end by the Xrn1-mediated 5′→3′ exonucleolytic decay, or from the 3′-end by deadenylation and exo-some-mediated 3′→5′ exonucleolytic decay.
Studying NMD, researchers have demonstrated the existence of mechanistic particularities of this mRNA-decaying process in various organisms. A combination of two cis elements is always required to trigger the process: an abnormality in the mRNA sequence, such as PTC, and a second RNA element, such as a downstream sequence element (DSE) in yeast or an exon-exon junction in mammals. Particularly in mammals, the NMD process is marked by the assemblage of many proteins, which form a complex known as the exon junction complex (EJC) (46). Those proteins are loaded on the transcript during splicing at 20–24 nucleotides upstream of the exon-exon junction. In mammals the exon-exon junction is the cis-acting element that acts in conjunction with the PTC-triggering NMD (47,48). Parallel PTCs are located at 50–55 nucleotides upstream from the exon-exon junction and are usually recognized by Upf proteins (49). A current model suggests that during the first round of translation the relative position of the PTC from the EJC is recognized and the transcript is targeted for degradation. Studies on the EJC of Hela cells have demonstrated the existence of a central complex composed by REF/ALY, Y14, and MAGOH proteins (50). After the initial binding of those central proteins on the mRNA, Upfs are recruited and act on the induction of NMD in the presence of a PTC. In humans, the medical significance of NMD was first described in β-thalassemia, which leads to β-globin mRNA degradation. Scientists now know that several other diseases can be explained by NMD, including brachy-dactyly type B (51), von Willebrand disease (52), factor x deficiency (53), and retinal degeneration (54). These and several other diseases mediated by NMD demonstrate the importance of surveillance mechanisms in protecting the organism against aberrant protein forms.
MOVING FURTHER: miRNA MEDIATING POSTTRANSCRIPTIONAL PROCESSING
MicroRNAs (miRNA) are small endogenous noncoding mRNAs of about 21–23 nucleotides that control the fundamental cellular process in a variety of organisms. Since the discovery of miRNA in the 1990s a multitude of basic information has accumulated, which has identified their function in posttran-scriptional control, either via degradation or translational inhibition of target mRNAs. miRNAs fine-tune gene expression, working in parallel with transcriptional regulatory processes (55,56). In the current model, miRNAs are initially formed by long precursors known as primary miRNAs (57,58), which are processed in the nucleus by Drosha. This process forms double-stranded fragments that are exported to the cytoplasm and there they are further processed by a second RNAse type III endonuclease, termed Dicer. This enzyme produces double-stranded RNAs, which contain 21–22 nucleotide fragments, paired in the 19 central nucleotides with 2 overhanging ends (59). These small molecules are characterized as mature miRNAs and can be subsequently incorporated into a complex termed RNA-induced silencing complex (RISC). An miRNA incorporated into an RISC can guide the RNA interference (RNAi) machinery to its target and complementary mRNAs by forming RNA duplexes, which result in sequence-specific translation repression (60,61) or mRNA decay (55,62,63). Interestingly, computational analyses indicate that 20–30% of protein-coding genes are likely targets of miRNAs (64), suggesting that these molecules are important in the surveillance mechanism owing to their ability to degrade the mRNA when it is claimed by the quality control mechanism.
miRNA expression profiles are highly dynamic during embryonic development as well as in adulthood. The misexpression of miRNAs can disrupt embryogenesis and tissue homeostasis. Moreover, evidence from gain- and loss-of-function studies indicates roles for miRNAs in pathophysiologic states, including cardiac hypertrophy, muscle dystrophy, hepatitis infection, diabetes, Parkinson disease, hematological malignancies and several types of cancers, but the miRNA mechanistic processes in diseases are still largely unknown. Preliminary studies concerning the therapeutic potential of miRNA and the RNAi processes were initially done in 2001 (65) and are currently being further developed. The use of miRNA is still a promising therapeutic strategy, but more clarification is needed of the mechanistic details mediated by miRNA and how they fit into the quality control mechanisms. Moreover, the establishment of an efficient system to deliver those small RNAs to the desired tissue is considered the biggest challenge to their clinical application (66); therefore, much effort has been directed at improving this approach.
HOW ARE CIS AND TRANS ELEMENTS INTERCONNECTED WITH THE mRNA SURVEILLANCE PROCESS?
mRNA turnover helps the cell to reach its own biochemical equilibrium. In mammalian cells, this mechanism requires a precise interconnection of physiological signals (5,67). The degradation rate of a specific transcript is always modulated by both cis-acting elements within the mRNA body sequence and trans-acting factors that bind to the cis-acting elements (67,68). In the cis-acting elements, sequences rich in A and U nucleotides, also called AU-rich elements (AREs), have been demonstrated to be involved in mRNA rapid degradation. Those elements are regularly found in the 3′-UTR (untranslated region) of several short half-life mRNAs such as those that encode cytokines, growth factors and oncogenic proteins (69,70,71). Frequently, those elements are recognized by specific ARE-binding proteins (AREBPs) that modulate the kinetic rates of mRNA degradation. For the last two decades, researchers have been conducting studies to clarify mechanistic details of ARE and AREBPs in mediating mRNA processing. Many of those results have demonstrated a direct correlation between an unbalanced mRNA turnover of ARE-containing mRNAs and the development of cancer, inflammatory processes, arthritis and other organic dysfunction (72,73). Examples of the broadly studied proteins AREBP, HUR and TTP are considered next.
HUR is a member of a super family of elav-related proteins, which are differentially expressed during embryonic development (74). HUR has a predominantly nuclear localization and can shuttle between the nucleus and cytoplasm (75), serving as an adaptor for the nuclear export of some mRNA containing ARE (76) and contributing to the surveillance process mechanism within a cell. In addition, through its binding as a trans element this AREBP contributes to mRNA stability (77,78), and in an unbalanced environment HUR, through cyclin mRNA stabilization, can be directly involved in the development of cancers such as colorectal carcinoma (79).
On the other hand, TTP (tristetrapolin protein) is a predominant cytoplasmic molecule, and TTP binding can cause degradation of the target mRNA. Containing two classical zinc fingers, TTP protein binds to mRNAs classified as class II ARE, such as tumor necrosis factor-α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-3 (IL-3) (80,81). Research conducted with transgenic mice, in which the TTP gene was previously knocked down, demonstrated an increased level of TNF-α protein, and consequently the mice developed several clinical symptoms such as erosive arthritis, dermatitis and myeloid hyperplasia (82). This study suggested the relevance of TTP in the control of the development of several clinical diseases, which are mediated by the action of the protein in the regulation of the mRNA level of several important molecules.
The above observations demonstrate that mammalian cells are able to interconnect cis and trans elements to modulate the rate of mRNA turnover and to regulate important physiological and pathophysiological functions. Myotonic dystrophy and several other diseases that are related to the mRNA metabolism will be addressed in the following sections. The majority of them demonstrate the existence of a disrupted balance between the elements involved in the mRNA turnover events.
How Can a Cell Assure RNA Surveillance? A Kinetic Energetic Conformation-Based Model
To assure a homeostatic state in a cell, aberrant RNAs must be distinguished from normal ones and rapidly degraded by the cellular machinery. In all living organisms, the efficiency and the accuracy of such biochemical mechanisms are the result of billions of years of evolution. Particularly in eukaryotes, mRNA undergoes maturation through several steps, and it seems imperative that the mRNA surveillance process occurs in parallel to each of those steps. As previously described, mRNA molecules play a key role in cellular biology, and to fulfill their functions, they must fold into complex tertiary structures. Given the dynamic biological systems in a cell, mRNA folding happens cotranscriptionally and is able to influence the general folding process of the mature mRNA molecule, either positively or negatively, thus either preventing or favoring the generation of nonnative trapped intermediates (83), impacting the general RNA maturation process and cellular homeostasis. During transcription RNA molecules are dynamically refolded several times, and surveillance can help in the quality control of those transcripts. However, how can the mRNA surveillance machinery efficiently differentiate the corrected and well-processed molecules from the aberrant ones in the complex cellular environment? Here is a discussion of a model of the system that may safeguard the surveillance mechanism in a cell, thus helping to maintain the complexity of life.
Since initial X-ray diffraction studies of biological molecules were performed in the first decades of the 20th century, scientists have sought to understand how molecular structures can explain the biological function of the molecules. Currently, X-ray diffraction, nuclear magnetic resonance, computer structure prediction based on sequence homology and other techniques have been helping researchers to elucidate the molecular structure of biological compounds. As a result of these investigations, pharmacological agents have been developed to target specific regions of molecules, facilitating the mechanistic action of the drugs. However, the knowledge of the tridimensional structure of such molecules by no means constitutes the full information about their biological activity. In general, large molecules in a solution are not rigid (84), and during their life cycle in a cell, biological molecules can actively interact among themselves by forming functional complexes that require conformational changes of the constituents, as seen in the components of the mRNA surveillance machinery. Based on those two observations, one can conclude that the dynamics of the conformational rearrangements, and consequently the thermodynamics, significantly contribute to building molecular models for every step of the gene expression process in a kinetic energetic conformational model. Moreover, several studies have demonstrated that the overall stability of complex interactions are dictated by the standard Gibbs free energy change (ΔG), which involves both enthalpic and entropic contributions, giving the thermodynamic parameters an important position in the study of molecular interactions and their functions in a cell (84–87).
Regarding mRNA surveillance, sets of protein complexes are required all along the pathway. These proteins, working in an orchestrated way, follow the rules of thermodynamics in assembling at their substratum. As in most biological processes, mRNA surveillance has a higher degree of accuracy in its mechanistic procedure than could be reasonably explained by the kinetic proofreading processes. First proposed by Hopfield (1974) (88) and next by Thompson and Stone (1977) (89), the kinetic proofreading model is an established and accepted mechanism that explains the accuracy of most biological reactions, including those involving nucleic acid interactions (88,90–92). In the proposed model, the highly selective recognitions in molecular reactions are carried out enzymatically and are strongly driven by the hydrolysis of nucleoside triphosphates. The mechanisms happen in two distinct irreversible steps, which increase the specificity of the interactions between molecules that are conducted by the difference in free energy. Interestingly, the kinetic proofreading model involves the formation of an intermediate product. In the initial step of the reactions, noncognate molecules are rejected, but near-cognate ones could be involved in the binding reactions between two molecules. During the second step of the reaction, after the nucleoside triphosphate hydrolysis, the kinetic proofreading model allows a time delay in order for the equilibrium in the reaction system to be reached. This delay makes it possible to discriminate between the correct and the incorrect substrate based on the energy differences between them, which increase the dissociation rate of the noncognate substrates for binding and, in some circumstances, the degradation of RNA. The second step of the kinetic proofreading reactions expends energy, but minimizes the binding of incorrect molecules in the reaction. This step has a fine-tuning approach to the effectiveness of the biological reactions in a cell that explores the general fold of the molecules and their thermodynamic interactions.
During the surveillance process, in which a series of concatenated reactions are performed and the RNA is matured and processed, a minor error along the pathway could be disastrous to cellular homeostasis. The kinetic proofreading provides a potential rationale for the complexity and efficacy of the mechanism. Along with the correct molecular interactions mediated by hydrogen bonding, salt bridges, π-π– and π-cation–stacking interactions, and van der Waals contacts, plus hydrophobic interactions of aliphatic molecular parts (84) between the mRNA surveillance machinery and RNA, the overall stability requires a thermodynamic approach to distinguish the correct binding without mistakes. Those incorrect molecules facilitate neither correct molecular interactions with the surveillance proteins nor a correct thermodynamic event, which favors the identification of the mistakes on the mRNA molecule by the kinetic proofreading mechanisms. Once the errors are identified, they can lead to mRNA body degradation, which safeguards health.
LOOKING AHEAD: CLINICAL APPROACHES
Failure of Quality Control in mRNA Biogenesis: How Can it Influence Human Clinical Diseases?
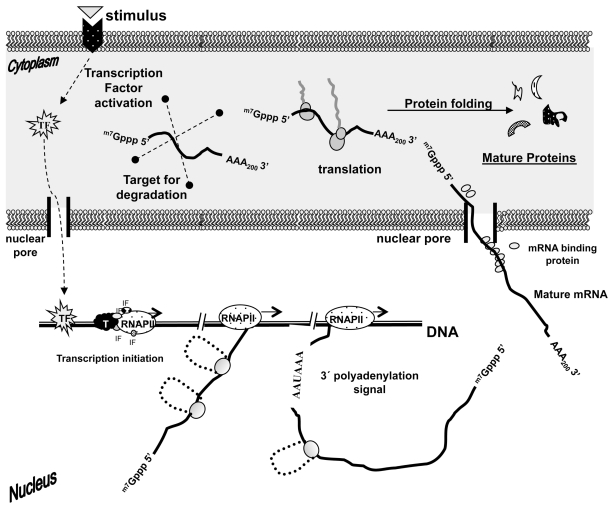
The regulation of gene expression occurs at multiple stages and, as previously discussed, transcription and RNA processing have major functions in the quality control mechanisms of an organism (Figure 4). So far, a lot of effort has been directed to the understanding of how errors in RNA processing could be related to the development of diseases. From the medical point of view, biomarkers have been investigated to help diagnose important clinical diseases such as those related to mutations in the 20210G→A (F220210*A) of the prothrombin gene (93). This mutation raises prothrombin plasma concentrations and predisposes carriers to develop thromboses (94). Next this review will briefly describe examples of defects in RNA processing mechanisms and how the RNA quality control machinery works in the surveillance process in a cell.
Figure 4.
A contemporary view of gene expression and its surveillance processes. Research conducted in the field suggests the subdivision of continuous stages, which are physically and functionally connected to each other, assuring an efficient process and the organism steady–state.
Mutations in Sequence-Conserved Elements
Mutations can be potentially harmful to an organism, if transcripts containing such aberrant sequences are not recognized by the mRNA surveillance machinery. Several studies have been performed on globin mRNA related to mutations and their side effects on an organism. Globins were the first human genes to be cloned (95) and are the earliest medically important genes that illustrate how their expression pathways can be inactivated by naturally occurring mutations. The remarkable phenotypic diversity reflects the heterogeneity of mutations on the globin loci (96). Of the globin diseases, thalassemia is one of the best characterized, and is characterized by several molecular abnormalities caused by punctual mutations on α- or β-globin genes. Thalassemic patients with hemolysis and ineffective erythropoiesis may have a complete lack of symptoms or display a variety of symptoms, including severe transfusion- dependent anemia (97). Based on the investigation of the 3′-UTR of globin mRNAs from thalassemic patients, different mutations on the canonical AAUAAA polyadenylation signal of both α-globin (98) and β-globin genes (99) were described. Interestingly, the different types of mutations found by investigators demonstrated a clear ethnic distribution that correlated with the existence of a founder effect and natural selection, which in some cases works positively on the side of the heterozygote.
In addition to globin mRNA analysis, other studies involving mutation in the canonical AAUAAA region have also been performed. In these investigations, mutations in the AAUAAA region of the Foxp3 mRNA were identified and shown to be related to the development of IPEX syndrome (100), a rare and fatal disorder characterized by polyendocrinopathy, enteropathy and immune system dysfunction. Fabry disease, another clinical manifestation, is attributable to a severe X-linked recessive inborn error of glycosphingolipid catabolism that results from a mutation on the lysosomal α-galactosidase A (α-Gal A) gene. α-Gal A is a typical gene that bears its polyadenylation signal within the coding region and lacks the 3′-UTR (101). The deletion of an AA dinucleotide on the internalized poly(A) site leads to an aberrant 3′ end, which leads to the generation of several different nonfunctional transcripts and complete inactivation of the gene. Furthermore, other diseases can be taken as examples to illustrate mutations on the RNA-conserved poly (A) signal AAUAAA, such as the acetyltransferase 1 polymorphism in colorectal cancer (102), metachromatic leukodystrophies (103), and X-linked severe combined immunodeficiency (104). These examples clarify the functional importance of the mRNA conserved poly(A) region in maintaining equilibrium in the organism. However, regardless of the kind of mutation, most of them may alter the quality and level of the protein that will be translated, and a mutation does not necessarily abolish the production of the final product. But this unbalance in the cellular equilibrium may initiate an illness process.
Remarkable studies have been performed by investigators seeking to describe and understand the influence of other mutations on the mRNA processes and their clinical relevance. Several mRNA cis-acting elements have been investigated, and clinically relevant mutations have been found. Some of these, however, need more investigation concerning their biochemical and phenotypical effects on an organism. Many of these elements are directly correlated with the 3′-end processing of the mRNAs, and investigators reported a mutation between them in the cleavage mRNA site of the prothrombin, coagulation factor II (F2) (105). The cleavage mRNA region is localized 10 to 30 nucleotides upstream of the polyadenylation site and in a mutated F2 gene there is a CA→CG transition that is responsible for raising the thrombin plasma concentration. This increment in the protein level disturbs the finely tuned balance between pro- and anticoagulatory activities, resulting in an increased risk for development of trombophilia (106), which can lead to several endothelial injuries and hypercoagulation disorder. Subsequently other mutations on the F2 gene were identified, and all of them are related to the mRNA cleavage site at the 3′-UTR, leading to an unbalanced regulation of gene expression.
Investigations in RNA processing have revealed different elements that work in an integrated network in the context of the mRNA surveillance steps to monitor mRNA structure and function (1,11). Medical perspectives on applying such knowledge for therapeutic procedures are in high demand; difficulty resides, however, in the identification of a common mechanistic pathway or a key biochemical element that works on the recognition and surveillance of such aberrant molecules. On the basis of various studies, scientists have realized that the mechanistic processes related to mRNA surveillance in a cell have evolved independently, possibly in parallel with the evolution and complexity of the genome structures and transcript-processing pathways (107). A more detailed understanding of mRNA surveillance systems is therefore likely to reveal previously unknown determinants of gene regulation, and these findings will facilitate the development of clinical approaches against the defects in the surveillance mechanisms.
mRNA Metabolism and Cancer
Cancer is the most serious clinical manifestation in our modern world. In a cancer cell several mutations are present in its nuclear material, but in order for the disease to start developing intracellular changes must be modulated by extra-cellular signals that turn on the oncogenic machinery. In an attempt to find key elements that lead to cancer, signal transduction pathways were elucidated and a correlation between errors in mRNA metabolism and uncontrolled cell growth was found (108–110).
A strict regulation of mRNA level is absolutely required for the maintenance of the cellular steady–state, and in a cancer cell the mRNA turnover is frequently altered. Transcripts that usually have a 30-minute half-life, such as the mRNAs of protooncogenes and cytokines, which contain the cis-acting ARE at the 3′-UTR, have a significant increase in their half-lives in cancer cells. In classic studies, Hollis et al. (1998) (111) demonstrated in myeloma plasma cells a seven-fold increase in the c-myc mRNA half-life, due to a chromosomal translocation that disrupts the ARE region at the 3′-UTR of the transcript. Interestingly, the same effect of chromosomal translocation on the c-myc gene is found in Burkitt lymphoma (112). Other defects in mRNA metabolism can also lead to serious health problems, such as those related to the alternative splicing of the CD44 gly-coprotein mRNA. CD44 protein is a cellular adhesion molecule involved in cell-cell interaction and in cell adhesion and migration, and is also characterized as a receptor for hyaluronic acid and other ligands such as collagens and matrix metalloproteinases. These characteristics allow this protein to participate in a wide variety of cellular functions (113,114). The alternative mRNA splicing is the basis for this functional diversity of the CD44 proteins, and studies have demonstrated that this protein may be related to tumor metastasis, because different isoforms were identified in some types of tumors such as lymphomas (115) as well as cervical (116), breast and prostate cancer cells (117). We still do not understand, however, what controls the production of problematic CD44 mRNA isoforms and how they evade the cellular surveillance machinery. Furthermore, investigators studying the transcriptional machinery in certain human cancers such as ovary, colon, pancreas and breast cancer (118) detected an over-expression of poly(A) polymerases, which favors the use of CD44 as a molecular biomarker in certain kinds of cancers (119,120). Other types of bio-markers are currently being investigated and may provide powerful tools for the clinical diagnosis of this problematic threat.
From a medical perspective, more investigation is needed to explain all the mechanistic details in a cancer cell. Once these details are clarified, more therapeutic decisions can be made in a strict and realistic way.
Differential mRNA Processing in Human Diseases and RNA Pathogenesis as the Focus of Medical Research
Several neurodegenerative diseases are also caused by mistakes in the mRNA processing steps, due to an exaggerated nucleotide repetition in the mRNA sequencing. Those expansions usually originate during mitosis and/or meiosis and in general are present in a normal cell. Under pathogenic conditions, however, the number of nucleotide repeats is considerably high, turning the transcript into a toxic molecule to the cell. In 1981 the first two of those pathogenes were identified, fragile X mental retardation (FMR) (121) and spinal and bulbar muscular atrophy (SBMA) (122). FMR disease is an inherited mental impairment that can range from learning disabilities to severe cognitive and intellectual disabilities. In this disease there is a trinucleotide (CGG) repetition at the 5′-UTR of the FMR1 gene. In a healthy person this gene contains fewer than 44 trinucleotide repeats; however, FMR patients have a hyperexpansion of a polymorphic CGG repeat in the gene. Expansions of 55–200 repeats are called premutations and characterize carriers, who usually have no mental impairment. A full mutation exceeds 200 CGG hypermethylated repeats, which leads to a transcriptional silencing of the gene and absence of the fragile X mental retardation protein. Owing to the considerable number of patients, diagnostic methodologies have been developed involving molecular and immunocytochemical approaches. The other clinical condition initially reported is spinal and bulbar muscular atrophy, also an X-linked threat, which causes degeneration of motor neurons, with adult onset and slow progression. The main symptoms are weakness and atrophy of bulbar, facial and limb muscles. Sensory disturbances also are frequently found in SBMA patients. This disease, like FMR, involves hyperexpansion of trinucleotide repeats (CAG) in the coding region of the androgen receptor (AR) gene, and the length of expansions is correlated with the intensity of the disease symptomatology. Patients with longer CAG repeats (=47) will present a more accentuated clinical phenotype than patients with shorter expansion. In any case, the problematic transcript is not recognized by the quality-control machinery and leads to the production of a toxic molecule protein that curiously aggregates inside the cell.
Since these initial studies, several pathogenies due to trinucleotide-repeat expansion have been described, such as Huntington disease (123,124), spinocerebellar ataxias (125), myotonic dystrophy (126) and many others. Interestingly, most of those diseases have a founder effect (127), and several studies have suggested that new mutations occur in a subgroup of unstable high-normal triplet repeat alleles with a particular founder chromosomal haplotype (124,128).
The trinucleotide repeat disorders are considered serious health problems demanding much effort to elucidate their molecular mechanisms. Among all the clinical manifestations due to nucleotide expansion, some muscular diseases require special attention owing to their differential level of clinical manifestation, including life impairment. Myotonic dystrophy (DM), also known as Steiner disease, has been broadly investigated. It is a progressive disease characterized by multisystemic manifestations. Affected patients develop skeletal muscle weakness, myotonia, cardiac conduction defects, dilated cardiomyopathy, endocrinopathy, alteration in smooth muscle function and cognitive impairment (129). DM is an autosomal dominant trait, and two genetic loci presenting nucleotide repeat expansion have been associated with the disease phenotype: DM1 in the chromosome 19q13.2–q13.3 and DM2 in the chromosome 3q13.3–q24. DM1 is caused by expanded (CUG)n repeats in the 3′-UTR of the myotonic dystrophy protein kinase (DMPK) gene (130,131). As with other trinucleotide expansion disorders, unaffected individuals have from 5 to 40 repeats. Patients with 41–180 repeats have mild symptoms, and those with >1500 repeats have congenital DM, a severe form of the disease characterized by mental retardation, hypotonia and symptoms associated with severe muscle weakness. Many congenital DM patients die soon after birth from respiratory problems due to underdeveloped diaphragm and intercostal muscles (129). The mechanism associated with the nontranslated CUG repeat in a single allele, which results in the severe dominant phenotype of DM1, remains unclear. One possible explanation is that this series of repeats in the 3′-UTR of the DMPK gene may disrupt the expression of other genes at either the DNA or RNA level (132). It is possible that the triplet expansion is bound in a competitive way by proteins that are directly involved with mRNA processing, which affects the expression of a number of genes by a trans-dominant effect (133). DM2, on the other hand, closely mimics the phenotype of adult-onset DM1, but DM2 is not associated with severely atrophic facial and forearm muscles. DM2 is due to the (CCUG)n nucleotides in intron 1 of the zinc finger 9 gene (134), and as in the case of DM1, studies suggest that the disease is correlated with the disruption of certain kinds of molecular signaling pathways, and the direct involvement of CUG binding proteins has been reported. Special attention has been given to the CUG–binding protein isoforms as key mediators of the pathogenic effect of DM disease (135), due to its direct influence on mRNA metabolism (136–138). More biochemical mechanistic investigation must be performed to develop clinical palliative treatments for DM patients.
DM and several other pathologies represent a broken balance in the cellular mRNA metabolism that is very important in the context of a healthy life, and thus the focus of medical research on RNA pathogenesis has become more intense. Furthermore, as mentioned before, considering the complexity of the mRNA surveillance processes, it is extremely important to keep developing clinical and methodological approaches for detecting changes in the surveillance system under pathological conditions. Several laboratories have been trying to improve their strategies on those approaches. Progress requires interdisciplinary cooperation and the use of up-to-date methods such as DNA amplification and sequencing, microarrays, and mass spectrometry, as well as several other modern techniques currently applied in molecular biology laboratories. As a result, strategies have been developed to identify disease genes that correlate with failures in the RNA surveillance machinery (139–141). However, in spite of all the research being conducted in the field, scientists do not yet understand all the details, for example, what directs the recruitment of NMD factors at the premature termination site (142). Details like this negatively impact the development of a universal methodological view to detect pathologies due to mechanistic problems in the RNA surveillance machinery. Maybe in the near future a particular and unique kinase that mediates mRNA processing and surveillance will be identified and a sophisticated diagnostic procedure able to differentiate normal from aberrant RNA surveillance mechanisms will be developed. This possibility is suggested based on the knowledge that the phosphorylation mechanism has been identified as an important factor in posttranscription quality control of mRNA (143,144). For the time being, mRNA surveillance is still an enigmatic and intricate puzzle for molecular biologists, human geneticists and the medical community.
MODERN THERAPEUTIC STRATEGIES FOR RNA-BASED DISEASES
mRNA target-based therapy is an emerging and powerful alternative for the treatment of genetic disorders because of mutations or RNA processing defects that directly correlate with RNA surveillance mechanisms. Although an emerging field, RNA therapy has the potential to modify native mRNA transcripts within a normal regulatory environment. The approaches range from complete degradation of specific mRNA targets to modification of mature mRNA molecules. Advances in knowledge of the structure and function of RNA biology are leading to increased development of RNA-based therapeutic strategies. Many of the effector molecules underpinning these novel methods have their origins in natural biochemical pathways that have been discovered in recent years. Since the approval of the first RNA-based drug for the treatment of a human disease by the US Food and Drug Administration in 1998, there has been an increasing biotech company “gold rush” for patents on efficient drug delivery systems that target the desired tissues. Here two major RNA-based strategies to treat human diseases are briefly described.
Antisense Oligonucleotides
Initially designed to downregulate gene transcription, antisense oligonucleotides (AO) are currently used to bind a complementary sequence in a target pre-mRNA by altering its processing. Specifically, AO are currently being employed to block sequences critical for splicing events in a target mRNA to prevent the production of undesired disease-causing mRNA products. The synthetic molecules have been used in studies of therapeutic strategies for the treatment of diseases such as β-globin, β-thalassemia, Hutchinson–Gilford progeria syndrome, Duchenne muscular dystrophy, spinal muscular atrophy and amyotrophic lateral sclerosis (145,146). Human clinical trials have been performed and several drugs are currently available for the treatment of human diseases. These drugs include formivirsen, an AO that blocks the synthesis of important cytomegalovirus proteins that are responsible for certain kinds of human eye inflammations, and mipomersen, an antisense RNA drug that reduces the production of Apo-100 in patients with hypercholesterolemia (147). Readministration of the AO may be necessary with these treatments, because these compounds have a limited biological half-life in transiently targeting transcription and/or mRNA processing. To eliminate this problem, recombinant adeno-associated viruses have been used as delivery vehicles to transduce anti-sense RNAs to the target cells (148). However, problems such as tissue- specific targeting, toxicity and immune response to the viral vectors still pose difficulties in correcting the RNA errors and their surveillance machinery.
RNA Interference
Based on the natural characteristics of miRNAs that are able to interfere in post-transcriptional control via degradation or translation of target mRNA, RNAi mechanisms have been studied and developed as promising therapeutic methods for knocking down genes whose activity correlates with pathogenesis. The key effector molecules of the RNAi are 21–23 ribonucleotides long, are able to target a unique mRNA sequence or even specific splice variants (149) and, as a consequence, to decrease mRNA expression. RNAi-based therapy has been used in clinical trials for the treatment of HIV (150), hepatitis B virus (151), macular degeneration (152), Alzheimer disease (153), liver cancer (154) and other disorders. Moreover, the specificity of the RNAi also opens the possibility of targeting specific mutant alleles associated with dominant genetic diseases (155).
However, while RNAi offers a novel therapeutic strategy for several diseases, the delivery of such molecules is still the major hurdle for their clinical use as a regular pharmaceutical agent. The non-specific off-target sequence recognition for the small 21–23 ribonucleotides continues to be a significant concern (156). On the other hand, the historic success of small molecules as pharmacological agents makes them attractive for use in therapeutic trials (146). More investigation is needed to elucidate particular mechanistic details of the RNA surveillance pathways to progress with RNA-based therapeutic strategies.
CONNECTING THE WHOLE TALE: mRNA SURVEILLANCE UNDER DIFFERENT CELLULAR ENVIRONMENTAL CONDITIONS. HOW IS IT POSSIBLE TO ASSURE HOMEOSTASIS?
Many behavioral, physiological and biochemical actives in a variety of organisms have been reported to show a circadian rhythm driven by endogenous biological clocks. Circadian rhythms allow organisms to coordinate their physiology with day-night cycles and environmental changes, and may have first evolved to control cellular metabolism (157). To date, studies have focused on the understanding of the molecular mechanisms that underlie those rhythmic clocks, and genetic analyses have identified numerous clock genes in different species. Across evolution, the molecular circadian clock is self sustained and typically consists of autoregulatory loops regulated by specific proteins that are rhythmically translated in an integrated manner (158), and transcription is considered the most critical step in the control of such circadian rhythms (159,160). In mammals, the core molecular clock components are the period genes, Per (Per1, Per2, and Per3); cryp-tochrome genes, Cry (Cry1 and Cry2); two helix-loop-helix transcription factors (Clock and Bmal1 genes); casein kinase I epsilon (Csnk1e); and two nuclear hormone receptor genes (RevErbAa and Rora). Other clock-controlled genes like the transcription factors Dbp and Nfil3 have also been identified (159,161). The basic mechanism of the circadian rhythm consists of the transcriptional activation of Per and Cry genes by CLOCK/BMAL1 heterodimers. Then the increased concentration of PER and CRY blocks the transcription regulated by CLOCK/BMAL1, which closes the autoregulatory feedback loop (162). Thus, the balance between transcription and translation of certain genes keeps the core clock strictly regulated and remarkably plastic, thus supporting circadian rhythmicity (163,164).
In mammals, the suprachiasmatic nucleus (SCN) is considered the center of the circadian clock in the body (162), which coordinates peripheral oscillators through neural and endocrine pathways (165). Circadian oscillators have been identified in peripheral tissues such as the heart (166), liver (167), bone marrow (168), endocrine tissues (169) and pancreas (170), and they must respond promptly to initial stimuli captured by the SCN. Tight and precise control of circadian rhythms in an organism assures its homeostasis; alterations in such rhythms can lead to serious pathologies such as sleep disorders, cardiovascular diseases, depression and even cancer (171–173). However, in order for a body to interconnect all molecular signals generated by initial stimuli, in every single cell the transcriptional, translational and surveillance machineries take on major responsibilities. As previously discussed in this review, the amount of mRNA in a cell is regulated at many levels; in this context mRNA processing and surveillance significantly contribute to changes in gene-expression patterns in response to external stimuli. More recently, experiments to determine circadian transcription on a large scale are currently being performed in several labs (174). Some evidence indicates that only a few particular mRNAs present a degradation pattern in the form of a circadian rhythm (175,176) in various organisms, despite the rhythmic increase in levels of some proteins, findings that suggest an alternative control of the posttranscriptional and posttranslational mechanisms. Several investigations point to a direct involvement of signaling cascades of protein phosphorylation being directly involved during the course of the day (177), events that either stabilize a required protein for cellular mechanistic adjustments to the circadian clock or stabilize proteins that are able to bind mRNA molecules. This avoids their premature degradation and makes them available for more translational rounds. MicroRNAs were also identified as molecular elements that help in the regulation of the circadian machine (178,179).
Due to the complexity of the circadian rhythms and their direct connection to the quality control of gene expression, scientists seek to reveal details in the circadian clock to understand how such events modulate the homeostasis of an organism. Actually, there is growing evidence that circadian rhythms are connected with the physiological state of an organism, such as its nutritional condition, hormonal fluctuation, aging and even diseases. These physiological conditions act positively or negatively on biological clocks, but the events must be integrated to maintain the organism. Also, the genetic background of an individual supports the plasticity of the molecular circadian mechanisms. In 2004 Rudic et al. (180) demonstrated impaired insulin responsiveness and reduced gluconeogenesis in Bmal1 −/− mice; other studies have shown that those mutant mice present early aging and reduced lifespan. Those mutants also have a high accumulation of reactive oxygen species, supporting the idea that the circadian protein BMAL1 may participate in the oxidative stress responses (181) frequently observed in age-related neurode-generative diseases such as Alzheimer and Parkinson (182). Failures in the transcription and/or surveillance machinery may block the correct expression of bmal1, which favors the development of those neurodegenerative diseases. This corroborates the observation that the circadian clock must be tightly regulated to modulate a state of dynamic homeostasis in the body to assure the maintenance of life.
In addition, more and more studies have demonstrated that the outside signals are captured by the SCN in the bodies of mammals. These signals are decoded as molecular messages that regulate the integrity of the organism and its surveillance at the cellular and molecular levels. Examples of such integrity have been revealed in investigations that demonstrated that the levels of the metabolic hormones glucagon, insulin, ghrelin, leptin and corticosterone oscillate in a circadian rhythm along with oscillation of the level of the peroxisome proliferator-activated receptor-γ coactivator-1α, which is an essential activator of gluconeogenesis in organisms in a nutritionally deprived state (183–185). The molecular interplay between the circadian clock regulators mediates the circadian oscillation of those important functional molecules and fine-tunes the final amount of every single metabolite in a cell. Transcription absolutely turns out to be a central mediator and along with its surveillance machinery assures the presence of correct temporal and physical amounts of mRNA molecules during circadian rhythms. Once available, those mRNA molecules can be translated when required, contributing to maintenance of the body’s homeostasis. Therefore, understanding the details of this molecular, fine-tune regulation of the circadian clock will provide a lot of information and insight as to how to deal with pathological conditions related to disturbances in those circadian rhythms.
PERSPECTIVE
Studies of the RNA surveillance mechanisms in eukaryotes are constantly reshaping our thinking about how errors in gene expression are detected and how they are controlled at the co- and post-transcriptional level. Eventually the growing knowledge of these processes is likely to have an important impact on clinical medicine as the era of genetic intervention develops.
The regulation of mRNA processing and stability are nature’s intriguing and very effective molecular adaptation mechanisms, which enable a cell to maintain a translatable transcript throughout all the energy-demanding steps of mRNA quality control surveillance. Moreover, the intricate mRNA quality control mechanism permits a cell to respond rapidly to changes in intrinsic and extrinsic stimuli, maintaining an equilibrated cellular environment. Failures in such molecular quality control mechanisms usually lead to the development of diseases. Considering the medical and clinical relevance of mRNA metabolism, the 3′-UTR has clearly emerged as a unique region that controls important cellular function such as morphogenesis, metabolism, cell proliferation and apoptosis. Actually, several ongoing studies are trying to identify novel sequence elements in the 3′-UTR that can modulate the mRNA surveillance processes. Unfortunately, many details concerning the mechanism of mRNA quality control are still undiscovered. These details need to be clarified to allow coherent combination of the available information and development of therapeutic strategies. In addition, future research addressing the key changes in mRNP composition at each critical remodeling step of an mRNA, as it goes on its journey from the nucleus to the cytoplasm, will be crucial for understanding how mRNA decay, translation, RNA quality control and circadian rhythms are regulated through interplay of various mechanisms.
Currently, all of the efforts in this field of investigation contribute to a general expectation that in the near future molecular biologists, human geneticists and the medical community will incorporate the new knowledge of mRNA processing and surveillance mechanisms into the design of novel and realistic approaches to clinical interventions for a variety of abnormalities.
ACKNOWLEDGMENTS
I am in debt to CP Soares and NS da Silva for all the assistance and friendship at UNIVAP. I am also in debt to AE Alder Rangel for assistance with the English revision of the text. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant 2009/ 07671-2) and Fundação Valeparaibana de Ensino.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The author declares that she has no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–51. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 2.Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ. Nuclear RNA turnover. Cell. 2002;108:431–4. doi: 10.1016/s0092-8674(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 4.Vasudevan S, Peltz SW. Nuclear mRNA surveilance. Curr. Opin. Cell Biol. 2003;15:332–7. doi: 10.1016/s0955-0674(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 5.Culbertson MR. RNA surveillance. Unforeseen consequences for gene expression, inherited genetic disorders and cancer. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 6.Hentze MW, Kulozik AE. A perfect message: RNA surveillance and nonsense mediated decay. Cell. 1999;96:307–10. doi: 10.1016/s0092-8674(00)80542-5. [DOI] [PubMed] [Google Scholar]
- 7.Frischmeyer PA, Dietz HC. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 1999;8:1893–1900. doi: 10.1093/hmg/8.10.1893. [DOI] [PubMed] [Google Scholar]
- 8.Baserga SJ, Benz EJ., Jr β-globin nonsense mutation: deficient accumulation of mRNA occurs despite normal cytoplasmic stability. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2935–9. doi: 10.1073/pnas.89.7.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum. Mol. Genet. 1995;4:1799–1809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 10.Barabino SM, Keller W. Last but not least: regulated poly (A) tail formation. Cell. 1999;99:9–11. doi: 10.1016/s0092-8674(00)80057-4. [DOI] [PubMed] [Google Scholar]
- 11.Proudfoot NJ, Furger A, Dye MJ. Integrating mRNA processing with transcription. Cell. 2002;108:501–12. doi: 10.1016/s0092-8674(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 12.Danckwardt S, Hentze MW, Kulozik AE. 3′ end mRNA processing: molecular mechanisms and implications for health and disease. EMBO J. 2008;27:482–98. doi: 10.1038/sj.emboj.7601932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornberg RD. The eukaryotic gene transcription machinery. Biol. Chem. 2001;382:1103–7. doi: 10.1515/BC.2001.140. [DOI] [PubMed] [Google Scholar]
- 14.Stutz F, Izaurralde E. The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 2003;13:319–27. doi: 10.1016/s0962-8924(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 15.Jensen TH, Dower K, Libri D, Rosbah M. Early formation of mRNAP: license for export or quality control? Mol. Cell. 2003;11:1129–38. doi: 10.1016/s1097-2765(03)00191-6. [DOI] [PubMed] [Google Scholar]
- 16.Shuman S. Capping enzyme in eukaryotic mRNA synthesis. Prog. Nucleic Acid Res. Mol. Biol. 1995;50:101–129. doi: 10.1016/s0079-6603(08)60812-0. [DOI] [PubMed] [Google Scholar]
- 17.Shatikin AJ, Manley JL. The ends of the affair: Capping and polyadenylation. Nat. Struct. Biol. 2000;7:838–42. doi: 10.1038/79583. [DOI] [PubMed] [Google Scholar]
- 18.Hirose Y, Manley JL. RNA polymerase II and the integration of nuclear events. Genes. Dev. 2000;14:1415–29. [PubMed] [Google Scholar]
- 19.Moteki S, Price D. Functional coupling of capping and transcription of mRNA. Mol. Cell. 2002;10:599–609. doi: 10.1016/s1097-2765(02)00660-3. [DOI] [PubMed] [Google Scholar]
- 20.Niedzwiecka A, et al. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J. Mol. Biol. 2002;319:615–35. doi: 10.1016/S0022-2836(02)00328-5. [DOI] [PubMed] [Google Scholar]
- 21.Hastings ML, Krainer AR. Pre-mRNA splicing in the new millennium. Curr Opin Cell Biol. 2001;13:302–09. doi: 10.1016/s0955-0674(00)00212-x. [DOI] [PubMed] [Google Scholar]
- 22.Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 23.Gornemann J, Kotovic KM, Hijer K, Neugebauer KM. Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol Cell. 2005;19:3–63. doi: 10.1016/j.molcel.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Beyer AL, Osheim YN. Splice site selection, rate of splicing and alternative splicing on nascent transcripts. Genes Dev. 1988;2:754–65. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- 25.Tennyson C, Klamut H, Worton R. The human dystrophin gene requires 16 hours to be transcribed and is cotranscriptionally spliced. Nat. Genet. 1995;9:184–90. doi: 10.1038/ng0295-184. [DOI] [PubMed] [Google Scholar]
- 26.Hoopengardner B, Bhalla T, Staber C, Reena R. Nervous system targets of RNA editing identified by comparative genomics. Science. 2003;301:832–6. doi: 10.1126/science.1086763. [DOI] [PubMed] [Google Scholar]
- 27.Smith HC, Gott JM, Hanson MR. A guide to RNA editing. RNA. 1997;3:1105–23. [PMC free article] [PubMed] [Google Scholar]
- 28.Anant S, et al. Novel role for RNA-binding protein CUGBP2 in mammalian RNA editing. J. Biol. Chem. 2001;276:47338–51. doi: 10.1074/jbc.M104911200. [DOI] [PubMed] [Google Scholar]
- 29.Turelli P, Trono D. Editing at the crossroad of innate and adaptive immunity. Science. 2005;307:1061–5. doi: 10.1126/science.1105964. [DOI] [PubMed] [Google Scholar]
- 30.Reenan RA. The RNA world meets behavior: A→ I pre-mRNA editing in animal. Trends Genet. 2001;17:53–6. doi: 10.1016/s0168-9525(00)02169-7. [DOI] [PubMed] [Google Scholar]
- 31.Gerber AP, Keller W. RNA editing by base deamination: more enzymes, more targets, new mysteries. Trends Biochem. Sci. 2001;26:376–84. doi: 10.1016/s0968-0004(01)01827-8. [DOI] [PubMed] [Google Scholar]
- 32.Murthy KG, Manley JL. The 160-kD subunit of human cleavage-polyadenylation specific factor coordinates pre-mRNA 3′-end formation. Genes. Dev. 1995;9:2672–83. doi: 10.1101/gad.9.21.2672. [DOI] [PubMed] [Google Scholar]
- 33.Mac Donald CC, Wilusz J, Shenk T. The 64-kilodalton subunit of the CstF polyadenylation factor binds to pre-mRNAs downstream of the cleavage site and influences cleavage site location. Mol. Cell. Biol. 1994;14:6647–54. doi: 10.1128/mcb.14.10.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wahle E, Ruegsegger U. 3′-end processing of pre-mRNA in eukaryotes. FEMS Microbiol. Rev. 1999;23:277–95. doi: 10.1111/j.1574-6976.1999.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 35.Mangus DA, Evans MC, Jacobson A. Poly (A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003;4:223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahvejian A, Roy G, Sonenberg N. The mRNA closed-loop model: the function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb. Symp. Quant. Biol. 2001;66:293–300. doi: 10.1101/sqb.2001.66.293. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell P, Petfaski E, Shevenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′ → 5′ exoribonucleases. Cell. 1997;91:457–66. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 38.Hilleren P, McCarthy T, Rosbash M, Parker R, Jensen TH. Quality control of mRNA 3′-end processing is linked to the nuclear exossome. Nature. 2001;413:538–42. doi: 10.1038/35097110. [DOI] [PubMed] [Google Scholar]
- 39.Raijmakers R, Schildeers G, Pruijn GJ. The exosome, a molecular machine for controlled RNA degradation in both nucleus and cytoplasm. Eur. J. Cell Biol. 2004;83:175–83. doi: 10.1078/0171-9335-00385. [DOI] [PubMed] [Google Scholar]
- 40.Allmang C, et al. Functions of the exosome in rRNA, snoRNA, snRNA synthesis. EMBO J. 1999;18:5399–410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das B, Butler S, Sherman F. Degradation of normal mRNA in the nucleus of Saccharomyces cerevisiae. Mol. Cell. Biol. 2003;23:5502–15. doi: 10.1128/MCB.23.16.5502-5515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fasken MB, Corbett AH. Process or perish: quality control in mRNA biogenesis. Nat. Struc. Mol. Biol. 2005;12:482–8. doi: 10.1038/nsmb945. [DOI] [PubMed] [Google Scholar]
- 43.Allmang C, et al. The yeast exosome and human PM-Scl are related complexes of 3′ → 5′ exonucleases. Genes. Dev. 1999;13:2148–58. doi: 10.1101/gad.13.16.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leeds P, Wood JM, Lee BS, Culbertson MR. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:2165–77. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caponigro G, Parker R. Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol. Rev. 1996;60:233–49. doi: 10.1128/mr.60.1.233-249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tange TO, Nott A, Moore MJ. The ever- increasing complexities of the exon junction complex. Curr. Opin. Cell Biol. 2004;16:279–84. doi: 10.1016/j.ceb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Reichert VL, Le Hir H, Jurica MS, Moore MJ. 5′ exon interaction within the human spliceosome establish a framework for exon junction complex structure and assembly. Genes. Dev. 2002;16:2778–91. doi: 10.1101/gad.1030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat. Rev. Mol. Cell. Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 49.Kashima I, et al. Binding of a novel SMG-1-Upf1-eRF1–eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes. Dev. 2006;20:355–67. doi: 10.1101/gad.1389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–17. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 51.Schwabe GC, et al. Distinct mutations in the receptor thyrosine kinase gene ROR2 cause brachydachtyly type B. Am. J. Hum. Genet. 2000;67:822–31. doi: 10.1086/303084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneppenheim R, et al. Expression and characterization of von Willebrand factor dimerization effects in different types of von Wille-brand disease. Blood. 2001;97:2059–66. doi: 10.1182/blood.v97.7.2059. [DOI] [PubMed] [Google Scholar]
- 53.Millar DS. Molecular analysis of the genotype-phenotype relationship in factor X deficiency. Hum. Genet. 2000;106:249–57. doi: 10.1007/s004390051035. [DOI] [PubMed] [Google Scholar]
- 54.Rivolta C, Berson EL, Dryja TP. Dominant leber congenital amaurosis, cone-rod degeneration and retinitis pigmentosa caused by mutant versions of the transcription factor CRX. Hum. Mutat. 2001;18:488–98. doi: 10.1002/humu.1226. [DOI] [PubMed] [Google Scholar]
- 55.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 56.Lin LP, et al. Microarrays analysis shows that some microRNAs down regulate large numbers of target mRNA. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 57.Cai X, Hagedorn CH, Xand Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–66. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–60. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–9. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 60.Pillai RS, Bhattacharya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–26. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Chendrimada TP, et al. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447:823–8. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 62.Bagga S, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–63. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Eulalio A, et al. Target-specific requirements for enhancers of decapping in miRNA- mediated gene silencing. Genes. Dev. 2007;21:2558–70. doi: 10.1101/gad.443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajewisky N. microRNA target predictions in animals. Nat. Genet. 2006;38:S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 65.Griffiths-Jones S, Grocock RJ, vanDongen S, Bate-man A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonetta L. RNA-based therapeutics: Ready for delivery? Cell. 2009;136:581–4. doi: 10.1016/j.cell.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Ross J. mRNA stability in mammalian cells. Microbiol. Rev. 1995;59:423–50. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beelman CA, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–83. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- 69.Caput D, et al. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatatory mediators. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1670–4. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–67. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 71.Zubiaga AM, Belasco JG, Greenberg ME. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol. Cell. Biol. 1995;15:2219–30. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 73.Galbán S, et al. Influence of the RNA-binding protein HuR in pVHL-regulated p53 expression in renal carcinoma cells. Mol. Cell. Biol. 2003;23:7083–95. doi: 10.1128/MCB.23.20.7083-7095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antic D, Knee JD. Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am. J. Hum. Genet. 1997;61:273–8. doi: 10.1086/514866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao FB, Knee JD. Hel-N1/Hel-N2 proteins are bound to poly(A)+ mRNA in granular RNP structures and are implicated in neuronal differentiation. J. Cell Sci. 1996;109:579–89. doi: 10.1242/jcs.109.3.579. [DOI] [PubMed] [Google Scholar]
- 76.Gallouzi IE, Steitz JA. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science. 2001;294:1895–901. doi: 10.1126/science.1064693. [DOI] [PubMed] [Google Scholar]
- 77.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–60. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng SS, Chen CY, Xu N, Shyu A–B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–70. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–50. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carballo E, Lai WS, Blackshear PJ. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood. 2000;95:1891–9. [PubMed] [Google Scholar]
- 81.Stoecklin G, Ming XF, Looser R, Moroni C. Somatic mRNA turnover mutants implicate tristetraprolin in the interleukin-3 mRNA degradation pathway. Mol. Cell. Biol. 2000;20:3753–63. doi: 10.1128/mcb.20.11.3753-3763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carballo E, Gilkeson GS, Blackshear PJ. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (−/−) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFalpha overproduction. J. Clin. Invest. 1997;100:986–95. doi: 10.1172/JCI119649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schroeder R, Grossberger R, Pichler A, Waldsich C. RNA folding in vivo. Curr. Opin. Struct. Biol. 2002;12:296–300. doi: 10.1016/s0959-440x(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 84.Stolarski R. Thermodynamics of specific protein-RNA interactions. Acta Biochim Pol. 2003;50:297–318. [PubMed] [Google Scholar]
- 85.Rassokhin TI. Study of the binding of the S7 protein with 16S rRNA fragment 926–986/1219–1393 as a key step in the assembly of the small subunit of prokaryotic ribosomes. Mol. Biol. (Mosk.) 2001;35:617–27. [PubMed] [Google Scholar]
- 86.Tang Z, et al. Real-time monitoring of nucleic acid ligation in homogenous solutions using molecular beacons. Nucleic Acids Res. 2003;31:e148. doi: 10.1093/nar/gng146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jason-Moller L, Murphy M, Bruno J. Overview of Biocore systems and their applications. Curr. Protoc. Protein Sci. 2006 Sep; doi: 10.1002/0471140864.ps1913s45. Chapter 19:Unit 19.13. [DOI] [PubMed] [Google Scholar]
- 88.Hopfield JJ. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl. Acad. Sci. U. S. A. 1974;71:4135–9. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson RC, Stone PJ. Proofreading of the codon-anti-codon interaction on ribosomes. Proc. Natl. Acad. Sci. U. S. A. 1977;74:198–202. doi: 10.1073/pnas.74.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hopfield JJ. The energy relay: a proofreading scheme based on dynamic cooperativity and lacking all characteristic symptoms of kinetics proofreading in DNA replication and protein synthesis. Proc. Natl. Acad. Sci. U. S. A. 1980;77:5248–52. doi: 10.1073/pnas.77.9.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–76. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 92.Zaher HS, Green R. Fidelity at the molecular level: lessons from protein synthesis. Cell. 2009;136:746–62. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood. 2002;99:3102–10. doi: 10.1182/blood.v99.9.3102. [DOI] [PubMed] [Google Scholar]
- 94.Cattaneo M, Chantarangkul V, Taioli E, Santos JH, Tagliabue L. The G20210A mutation of the prothrombin gene in patients with previous first episodes of deep-vein throbosis: prevalence and association with factor V G1691A, methylenetetrahydrofolate reductase C677T and plasma prothrombin levels. Tromb Res. 1999;93:1–8. doi: 10.1016/s0049-3848(98)00136-4. [DOI] [PubMed] [Google Scholar]
- 95.Maniatis T, Kee SG, Efstratiadis A, Kafatos FC. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976;8:163–82. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- 96.Weatherall DJ. Phenotype-genotype relationship in monogenic disease: lessons from thalassemia. Nat Rev Genet. 2001;2:245–55. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- 97.Nussbaum RL, McInnes RR, Willard HF. Thompson & Thompson Genetics in Medicine. 6th ed. Philadelphia: Saunders; 2004. p. 444. [Google Scholar]
- 98.Higgs DR, et al. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983;306:398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- 99.Orkin SH, Cheg TC, Antonarakis SE, Kazazia HH., Jr Thalassemia due to mutation in the cleavage-polyadenylation signal of the human beta-globin gene. EMBO J. 1985;4:453–6. doi: 10.1002/j.1460-2075.1985.tb03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bennett CL, et al. A rare polyadenylation signal mutation of the FOX3 gene (AAUAAA→AAUGAA) leads to the IPEX syndrome. Immunogenetics. 2001;53:435–9. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 101.Bishop DF, Kornreich R, Desnick RJ. Strutuctural organization of the human alpha-galactosidase A gene: further evidence for the absence of a 3′ untranslated region. Proc. Natl. Acad. Sci. U. S. A. 1988;85:3903–7. doi: 10.1073/pnas.85.11.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bell DA, et al. Polyadenylation polymorphism in the acetyltransferase 1 gene (NAT1) increases risk of colorectal cancer. Cancer Res. 1995;55:3537–42. [PubMed] [Google Scholar]
- 103.Giselmann V, Polten A, Kreysing J, von Figura K. Arylsulfatase A pseudoeficiency: loss of polyadenylation signal and N-glycosylation site. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9436–40. doi: 10.1073/pnas.86.23.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hsu AP, et al. Unusual X-linked SCID phenotype due to mutation of the poly-a addition signal of IL2RG (abstract 206) Am. J. Hum. Genet. 2000;67:A2. [Google Scholar]
- 105.Chen F, Macdonald CC, Wilusz J. Cleavage site determinants in the mammalian polyadenylation signal. Nucleic Acids Res. 1995;23:2614–20. doi: 10.1093/nar/23.14.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′untranslated region of the prothrombin gene variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–703. [PubMed] [Google Scholar]
- 107.Kong J, Liebhaber SA. A cell type-restricted mRNA surveillance pathway triggered by ribo-some extension into the 3′ untranslated region. Nat. Struct. Mol. Biol. 2007;14:670–6. doi: 10.1038/nsmb1256. [DOI] [PubMed] [Google Scholar]
- 108.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 109.Zhao H, et al. Reduced expression of N-Myc downstream-regulated gene 2 in human thyroid cancer. BMC Cancer. 2008;8:1–9. doi: 10.1186/1471-2407-8-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu B, et al. Activation of signal transducers and activators of transcription 3 and overexpression of its target gene CyclinD1 in laryngeal carcinomas. Laryngoscope. 2008;118:1976–80. doi: 10.1097/MLG.0b013e31817fd3fa. [DOI] [PubMed] [Google Scholar]
- 111.Hollis GF, Gazdar AF, Bertness V, Kirsch IR. Complex translocation disrupts c-myc regulation in a human plasma cell myeloma. Mol. Cell. Biol. 1988;8:124–9. doi: 10.1128/mcb.8.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eick D, et al. Aberrant c-myc RNAs of Burkitt’s lymphoma cells have longer half-lives. EMBO J. 1985;4:3717–25. doi: 10.1002/j.1460-2075.1985.tb04140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lesley J, Hyman R, Kincade PW. A CD44 and its interaction with extracellular matrix. Adv. Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 114.Tanabe KK, Saya H. The CD44 adhesion molecule and metastasis. Crit. Rev. Oncog. 1994;5:201–12. doi: 10.1615/critrevoncog.v5.i2-3.50. [DOI] [PubMed] [Google Scholar]
- 115.Koopman, et al. Activated human lymphocytes and aggressive non-Hodgkin’s lymphomas express a homologue of the rat metastasis-associated variant of CD44. J. Exp. Med. 1993;177:897–904. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dall P, Heider KH, Hekele P. Surface protein expression and messenger RNA-splicing analysis of CD44 in uterine cervical cancer and normal cervical epithelium. Cancer Res. 1994;54:3337–41. [PubMed] [Google Scholar]
- 117.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 118.Pendurthi UR, Alok D, Rao LVM. Biding of factor VII a to tissue factor induces alterations in gene expression in human fibroblast cells: upregulation of poly(A) polymerase. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12598–603. doi: 10.1073/pnas.94.23.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jacob ST, Terns MP, Marguire KA. Polyadenylated polymerases from normal and cancer cells and their potential role in messenger RNA processing: a review. Cancer Res. 1989;49:2827–33. [PubMed] [Google Scholar]
- 120.Scorilas A. Polyadenylated polymerase (PAP) and 3′ end pre-mRNA processing: function assays, and association with disease. Cri. Rev. Clin. Lab. Sci. 2002;39:193–224. doi: 10.1080/10408360290795510. [DOI] [PubMed] [Google Scholar]
- 121.Verkerk AJMH, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 122.La Spada AR, Wilson EM, Lubahn DB, Hardin AE, Fischbeck KH. Androgen receptor gene mutation in X-linked spinal and bulbar muscular atrophy. Nature. 1991;3532:77–9. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 123.MacDonald ME, et al. The Huntington’s disease candidate region exhibits many different haplotypes. Nat. Genet. 1992;1:99–103. doi: 10.1038/ng0592-99. [DOI] [PubMed] [Google Scholar]
- 124.Rubinsztein DC, Leggo J, Goodburn S, Barton DE, Ferguson-Smith MA. Haplotype analysis of the [Delta] 2642 and (CAG)n polymorphisms in the Huntington’s disease (HD) gene provides an explanation for an apparent “founder” HD haplotype. Hum. Mol. Genet. 1995;4:203–6. doi: 10.1093/hmg/4.2.203. [DOI] [PubMed] [Google Scholar]
- 125.Lunkes A, et al. Autosomal dominant spinocerebellar ataxia (SCA) in a Siberian founder population: assignment to the SCA1 locus. Exp. Neurol. 1994;126:310–12. doi: 10.1006/exnr.1994.1070. [DOI] [PubMed] [Google Scholar]
- 126.Harley HG, et al. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am. J. Hum. Genet. 1993;52:1164–74. [PMC free article] [PubMed] [Google Scholar]
- 127.Takanaka F, et al. Founder effect in spinal and bulbar muscular atrophy (SBMA) Hum. Mol. Genet. 1996;5:1253–7. doi: 10.1093/hmg/5.9.1253. [DOI] [PubMed] [Google Scholar]
- 128.Squitieri F, et al. DNA haplotype analysis of Huntington disease reveals clues to the origins and mechanisms of CAG expansion and reasons for geographic variations of prevalence. Hum. Mol. Genet. 1994;3:2103–14. doi: 10.1093/hmg/3.12.2103. [DOI] [PubMed] [Google Scholar]
- 129.Harper PS. Myotonic Dystrophy. 3rd ed. London: WB Saunders Press; 2001. p. 113. [Google Scholar]
- 130.Brook JD, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 131.Fu YH, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–8. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 132.Harris S, Moncrieff C, Johnson K. Myotonic dystrophy: will the real gene please step forward! Hum. Mol. Genet. 1996;5:1417–23. doi: 10.1093/hmg/5.supplement_1.1417. [DOI] [PubMed] [Google Scholar]
- 133.Caskey CT, Swanson M, Thimchenko LT. Myotonic dystrophy: discussion of molecular mechanism. Cold Spring Harb. Symp. Quant. Biol. 1996;61:607–14. [PubMed] [Google Scholar]
- 134.Liquori CL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in inton 1 of ZNF9. Science. 2001;293:864–7. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 135.Timchenko LT, et al. Identification of a (CUG)n triplet repeat RNA-biding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–14. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Philips AV, Thimchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–41. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- 137.Ranum LPW, Day JW. Myotonic systrophy: RNA pathogenesis comes into focus. Am. J. Hum. Genet. 2004;74:793–804. doi: 10.1086/383590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moraes KCM, Wilusz CJ, Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA. 2006;12:1084–91. doi: 10.1261/rna.59606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Noensie EN, Dietz HC. A strategy for disease gene identification through nonsense-mediated mRNA decay inhibition. Nat. Biotechnol. 2001;19:434–9. doi: 10.1038/88099. [DOI] [PubMed] [Google Scholar]
- 140.Bateman JF, Freddi S, Nattrass G, Savarirayan R. Tissue-specific RNA surveillance? Nonsense-mediated mediated mRNA decay causes collagen X haploinsufficiency in Schmid metaphyseal chondrodysplasia cartilage. Hum. Mol. Genet. 2003;12:217–25. doi: 10.1093/hmg/ddg054. [DOI] [PubMed] [Google Scholar]
- 141.Qian DZ, et al. Prostate cancer-associated gene expression alterations determined from needle biopsies. Clin. Cancer Res. 2009;15:3135–42. doi: 10.1158/1078-0432.CCR-08-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brogna S, Wen J. Nonsense-mediated mRNA decay (NMD) mechanisms. Nat. Struct. Mol. Biol. 2009;16:107–13. doi: 10.1038/nsmb.1550. [DOI] [PubMed] [Google Scholar]
- 143.Ohnishi T, et al. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol. Cell. 2003;12:1187–200. doi: 10.1016/s1097-2765(03)00443-x. [DOI] [PubMed] [Google Scholar]
- 144.Vargas NB, Brewer BY, Rogers TB, Wilson GM. Protein kinase C activation stabilizes LDL receptor mRNA via the JNK pathway in HepG2 cells. J. Lipid Res. 2009;50:386–97. doi: 10.1194/jlr.M800316-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wood M, Yin H, McClorey M. Modulating the expression of disease genes with RNA-based therapy. PLOS Genet. 2007;3:845–54. doi: 10.1371/journal.pgen.0030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–93. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bonetta L. RNA-based Therapeutics: Ready for delivery? Cell. 2009;136:581–4. doi: 10.1016/j.cell.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 148.Danos O. AAV vectors for RNA-based modulation of gene expression. Gene Ther. 2008;15:864–9. doi: 10.1038/gt.2008.69. [DOI] [PubMed] [Google Scholar]
- 149.Grimm D, Kay MA. Therapeutic application of RNAi: is mRNA targeting finally ready for time prime? J. Clin. Invest. 2007;117:3633–41. doi: 10.1172/JCI34129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rossi JJ. RNAi as a treatment for HIV-1 infection. Biotechniques. 2006;(Suppl):25–9. doi: 10.2144/000112167. [DOI] [PubMed] [Google Scholar]
- 151.Morrissey, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–7. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 152.Shen J, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–34. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 153.Singer, et al. Targeting BACE1 with siRNA ameliorates Alzheimer disease neuropathology in a transgenic model. Nat. Neurosci. 2005;8:1343–9. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 154.Pappas TC, Bader AG, Andruss BF, Brown D, Ford LP. Applying small RNA molecules to the directed treatment of human diseases: realizing the potential. Expert Opin. Ther. Targets. 2008;12:115–27. doi: 10.1517/14728222.12.1.115. [DOI] [PubMed] [Google Scholar]
- 155.Rodriguez-Lebron E, Paulson HL. Allele-specific RNA interference for neurological disease. Gene Ther. 2006;13:576–81. doi: 10.1038/sj.gt.3302702. [DOI] [PubMed] [Google Scholar]
- 156.Pei Y, Tuschl T. On the art of identifying effective and specific siRNAs. Nat Methods. 2006;3:670–6. doi: 10.1038/nmeth911. [DOI] [PubMed] [Google Scholar]
- 157.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem. 2002;71:307–31. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 158.Zheng X, Sehgal A. Probing the relative importance of molecular oscillations in the circadian clock. Genetics. 2008;178:1147–55. doi: 10.1534/genetics.107.088658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ueda HR, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005;37:187–92. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 160.Palumbo MC, et al. Collective behaviour in gene regulation: post-transcriptional regulation and the temporal compartmentalization of cellular cycles. FEBS J. 2008;275:2364–71. doi: 10.1111/j.1742-4658.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- 161.Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 2005;174:7618–24. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 162.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 163.Lee C, Weaver DR, Reppert SM. Direct association between mouse PERIOD and CK-Iepsilon is critical for a functioning circadian clock. Mol. Cell. Biol. 2004;24:584–94. doi: 10.1128/MCB.24.2.584-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Etchegaray JP, et al. The polycomb group protein EZH2 is required for mammalian circadian clock function. J Biol. Chem. 2006;281:21209–15. doi: 10.1074/jbc.M603722200. [DOI] [PubMed] [Google Scholar]
- 165.Albrecht U, Oster H. The circadian clock and behaviour. Behav. Brain Res. 2001;125:89–91. doi: 10.1016/s0166-4328(01)00288-1. [DOI] [PubMed] [Google Scholar]
- 166.Young ME, Razegui P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ. Res. 2001;88:1142–50. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 167.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen YG, Mantalaris A, Bourne P, Keng P, Wu JH. Expression of mPer1 and mPer2, two mammalian clock genes, in murine bone marrow. Biochem. Biophys. Res. Commun. 2000;276:724–8. doi: 10.1006/bbrc.2000.3536. [DOI] [PubMed] [Google Scholar]
- 169.Bittman EL, Doherty L, Huang L, Paroskie A. Period of gene expression in mouse endocrine tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R561–9. doi: 10.1152/ajpregu.00783.2002. [DOI] [PubMed] [Google Scholar]
- 170.Muhlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–6. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- 171.Bovbjerg DH. Circadian disruption and cancer: sleep and immune regulation. Brain Behav. Immun. 2003;17(Suppl 1):S48–50. doi: 10.1016/s0889-1591(02)00066-1. [DOI] [PubMed] [Google Scholar]
- 172.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat. Rev. Cancer. 2003;3:350–61. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 173.Kumar VM. Sleep and sleep disorders. Indian J. Chest Dis. Allied Sci. 2008;50:129–35. [PubMed] [Google Scholar]
- 174.Jacobshagen S, Kessler B, Rinehart CA. At least four distinct circadian regulatory mechanisms are required for all phases of rhythms in mRNA amount. J. Biol. Rhythms. 2008;23:511–24. doi: 10.1177/0748730408325753. [DOI] [PubMed] [Google Scholar]
- 175.Lidder P, Gutierrez RA, Salome PA, McClung CR, Green PJ. Circadian control of messenger RNA stability: association with a sequence-specific messenger RNA decay pathway. Plant Physiol. 2005;138:2374–85. doi: 10.1104/pp.105.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim T-D, et al. Rhythmic serotonin N-acetyltransferase mRNA degradation is essential for the maintenance of its circadian oscillation. Mol. Cell. Biol. 2005;25:3232–46. doi: 10.1128/MCB.25.8.3232-3246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Crosthwaite SK. Circadian clocks and natural antisense RNA. FEBS Lett. 2004;567:49–54. doi: 10.1016/j.febslet.2004.04.073. [DOI] [PubMed] [Google Scholar]
- 178.Cheng HY, et al. MicroRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–29. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pegoraro M, Tauber E. The role of mi-croRNAs (miRNA) in circadian rhythmicity. J. Genet. 2008;87:505–11. doi: 10.1007/s12041-008-0073-8. [DOI] [PubMed] [Google Scholar]
- 180.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gorbacheva VY, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3407–12. doi: 10.1073/pnas.0409897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat. Struct. Mol. Biol. 2009;16:462–7. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hansen AP, Johansen K. Diurnal patterns of blood glucose, serum free fatty acids, insulin, glucagon, and growth hormone in normals and juvenile diabetics. Diabetologia. 1970;6:27–33. doi: 10.1007/BF00425888. [DOI] [PubMed] [Google Scholar]
- 184.Sinha MK, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Invest. 1996;97:1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu C, Li S, Liu T, Borigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–81. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]