Abstract
Interferon-producing killer dendritic cells (IKDC) represent a recently discovered cell type in the immune system that possesses a number of functions contributing to innate and adaptive immunity, including production of type 1 and 2 IFNs, IL-12, natural killing, and ultimately antigen presentation to naïve T-cells. Here, we compared in vitro and in vivo responses of mouse IKDC, conventional dendritic cells and natural killer cells to murine cytomegalovirus infection and found distinct functions among these cell subsets. Upon recognition of infected fibroblasts, IKDC, as well as NK, produced high level of IFN-γ, but unlike NK, IKDC simultaneously produced IL-12p40 and upregulated MHC class II and costimulatory molecules. Using MHC-II molecule expression as a phenotypic marker to distinguish activated IKDC from activated NK, we further demonstrated that highly purified MHC-II+ IKDC but not NK, cross-present MHC class I-restricted antigens derived from MCMV-infected targets to CD8+ T-cells in vitro and in vivo. Our findings emphasize the unique nature of IKDC as a killer antigen presenting cell directly linking innate and adaptive immunity.
Keywords: IKDC and cross-presentation, Dendritic cell, Antigen presentation, Natural killer
INTRODUCTION
We and others have recently described a new subset of antigen presenting cells (APC), termed Interferon-producing Killer Dendritic Cells (IKDC) or Natural Killer Dendritic Cells (NKDC), that exhibit properties of both natural killer cells (NK) and dendritic cells (DC) (1–3). In-depth phenotypic analyses established splenic IKDC as a homogeneous CD11cloB220+CD49b+ population, distinct from murine NK by their expression of MHC class II molecules (MHC-II) and from DC by their expression of NK receptors. Upon stimulation in vitro with CpG oligodeoxynucleotides (ODN) or in vivo with Listeria monocytogenes (Lm) splenic IKDC killed typical NK targets and demonstrated the unique ability to produce interferon gamma (IFN-γ), IFN-α and interleukin (IL) 12 (1). Activated IKDC further differentiated into DC-type APC that accumulated in vivo in lymph nodes (LN) to activate CD4+ T-cells. IKDC also mediated melanoma rejection via an IFN-γ and TNF-related apoptosis inducing ligand (TRAIL) dependent mechanism (2).
The functional relationship between IKDC and DC and their distinction from NK cells has been challenged in recent reports suggesting that the phenotypic definition of IKDC based on CD11c, B220, and CD49b expression could not clearly distinguish them from activated NK (4–7). However, a recent study showed that, while sharing a lymphoid origin, IKDC and NK derive from distinct precursors (8). We demonstrate here that IKDC but not NK, cross-present mouse cytomegalovirus (MCMV)-encoded antigens, derived solely from MCMV-infected cells, in association with MHC-II and MHC-I to CD4+ and CD8+ T-cells, respectively. This functional capacity of mature IKDC, which were previously shown to accumulate in the LN (1), correlates with expression of genes encoding the MHC-II processing machinery, costimulatory molecules, and co-secretion of IL-12p40 and IFN-γ at the single cell level. The antigen cross-presentation functions, together with their natural killing activity against both virally infected and tumor cells (1), establish IKDC as a unique type of APC bridging innate and adaptive immunity.
MATERIALS AND METHODS
Mice and reagents
BALB/c and C57BL/6 were purchased from NCI and Harlan laboratories. IL-12p40−/− mice were purchased from Jackson laboratories (Bar Harbor, MA). Myd88−/−TrifLps2/Lps2 mice were obtained by crossing TrifLps2/Lps2 (Bruce Beutler, Scripps Research Institute) with Myd88−/− C57BL/6 mice (Maureen Horton, Johns Hopkins University). NOD/SCID/γc−/−, Rag2−/− OT-I, OT-II, Clone4 and 6.5 T-cell transgenic mice were maintained at JHU. Antibodies were purchased from BD Biosciences, except antibodies against NKG2D (CX5) and NKp46 (eBioscience). Intracellular staining (ICS) for cytokines was performed in presence of Brefeldin A using BD Biosciences Cytofix/Cytoperm reagent following manufacturer’s recommendations. FACS analysis was done on FACSCalibur or LSR2 (BD Biosciences) and data were analyzed using FlowJo software (Tree Star, Inc). Sorting was performed on FACSAria (BD Biosciences). For some experiments, cells were activated with 6ug/ml CpG1668 (MWG Biotech). For western blot analysis, anti-invariant chain (Ii;CD74; clone OX6, Santa Cruz) and anti-Legumain (Clone 301417, R&D systems) mAbs were used as primary antibodies, and anti-tubulin mAb as a control. Secondary antibody was sheep anti-mouse IgG labeled with horseradish peroxidase (ECL, GE Healthcare Lifesciences). IL-12p40 and IL-12p70 were measured in supernatant using ELISA (Pierce Endogen).
Viruses
MCMV virus expressing GFP (Dr. M. Messerle, Martin Luther University of Halle-Winttenberg, Germany) and MCMV expressing HA (A/PR/8/34) or TfrOVA were previously described (9–11). Primary fibroblasts were infected at multiplicity of infection (MOI) of 3 for 1.5h. Cells were washed and co-incubated with splenocytes at a ratio 1fibroblast:10splenocytes for 12h. Infection was monitored by cytopathic effect and GFP expression.
Cell Isolation
Cellular isolation was previously described (1). Briefly, spleens and livers were digested with DNAse and collagenase (Liberase2 blendzyme, Roche-Applied-Science). Leukocytes were separated through Lympholyte gradient (Accurate Chemical and Scientific, Inc) and depleted for CD3+ T-cells, CD19+ B-cells, and granulocytes using purified anti-CD3, anti-CD19, GR-1 mAbs and anti-rat IgG beads (Qiagen). CD11c+ cells were further enriched using CD11c+ beads (Miltenyi Biotech). IKDC (CD11cloB220+CD49b+) and CDC (CD11chiB220−CD49b−) were sorted from CD11c+ fraction and NK (CD11c−B220CD49b+) were sorted from CD11c− fraction. In C57BL/6 mice, CD49b was substituted with NK1.1. For in vitro and in vivo presentation assay activated IKDC and NK, which both express B220 (Fig.S1), were sorted based on the differential expression of MHC-II as CD11c+NK1.1/CD49b+IAb/IEk+ and CD11c+NK1.1/CD49b+IAb/IEk−, respectively. In some experiments, we used NKp46 instead of CD49b as NK marker (Fig.2D).
Figure 2. IKDC up-regulated MHC-II upon recognition of MCMV-infected targets.
A, confocal microscopy of C57BL/6 IKDC (CD11cintB220+CD49b+), NK (CD11c-B220-CD49b+), and CDC (CD11chiB220-CD49b-) labeled with anti-IA/IE (red) and anti-CD49b (green) antibodies. Blue, nuclei. B, IKDC, NK, and CDC shown in A were stained by ICS for I-A/E (see legend) or isotype-matched control (shaded histogram) and expression was assessed by FACS. C, BALB/c CD11c+ (including CDC and IKDC) and CD11c- (including NK) splenocytes were incubated 12 h with MCMV-infected or mock-treated fibroblasts. MHC-II expression was assessed by FACS on NK (CD49b+ cells from the CD11c- fraction), IKDC (CD49b+ from the CD11c+ fraction), and CDC (CD11chiCD49b- from the CD11c+ fraction).
Gene expression profile analysis
Total RNA was isolated from cell-sorted IKDC, CDC and NK using Trizol (Invitrogen) and RNeasy MicroKit (Qiagen). For Chip analysis, RNA was processed using a two round RNA amplification protocol described by Affymetrix and Affymetrix murine genome GeneChip array M0E430-2. The statistical significance of the fold change in gene expression has been analyzed using posterior p value. Fold changes ≥2 and ≤0.5 associated with probability>0.5 were considered significant. GEO accession number: GSE11918
Confocal microscopy
Cells were fixed with 3% paraformaldehyde/sucrose and permeabilized with 0.1% Triton-X. MHC-II were detected with anti-IA/E-biotin+AF594-streptavidin, CD49b with AF488 CD49b mAb, and for OVA crosspresentation assay Kb/OVASIINFEKL with AF647-labelled 25-D1-16 mAb (Jonathan Yewdell, NIAID, NIH) (12). The labeled cells were examined using Nikon EZ-C1 microscope. Data were acquired using Nikon EZ-C1 software.
Proliferation assay
IKDC (CD11cintNK1.1+CD49b+IAb+), CDC (CD11chiNK1.1−CD49b−IAbhi) and NK (CD11cintNK1.1+CD49b+IAb−) were sorted from MCMV infected mice 2 days post-inoculation (p.i.) or from CD11c+ (IKDC and CDC) and CD11c− (NK) cell-enriched populations incubated 12h with MCMV-infected fibroblasts. T-cell proliferation was assessed by FACS analysis of CFSE dilution of labeled OVA-specific T-cells (sorted at ≥99% purity). Cell culture supernatants were assayed for IL-2 by ELISA (Pierce Endogen).
Online supplemental material
statistics of microarray, and MCMV constructions are available online on Cancer Research website.
RESULTS
IKDC exhibit the gene expression profile of MHC-II-restricted presentation
IKDC (CD11cintB220+CD49b+), NK (CD11c−B220−CD49b+), and CDC (CD11chiB220−CD49b−) isolated from LN and spleen of naïve BALB/c and purified to >98% homogeneity were assessed by RNA microarray analysis for their differential expression of genes involved in MHC-II pathway processing and presentation (Fig.1 and tableS1). Statistical computation of the fold change demonstrated the preferential expression in LN IKDC versus NK of H2-I-A/I-E alleles, Ii, C2ta and enzymes involved in Ii or antigen processing (cathepsins, IFN-γ inducible lysosomal thiol reductase (GILT)) (Fig.1) (13–15). We also detected ancillary components important for proper antigen processing, including H2-Dma and H2-Dmb1, Nox2 (Cybb), Cst3 (cystatin C) and V-ATPase subunits (Fig.1 and data not shown) (16–19). Splenic IKDC only exhibit a statistically higher expression of I-A and I-E alleles compared to NK. At the protein level, confocal microscopy confirmed that splenic IKDC, but not NK or CDC, co-expressed CD49b and MHC-II (Fig.2A). Quantification by ICS confirmed that though lower level than in splenic CDC, MHC-II expression was significantly higher in IKDC compared to NK (MFI of 16.15±3.75 for IKDC versus 5.81±0.085 for NK and 5.8±0.3 for isotype control). Western blot analysis highlighted preferential expression of Ii and Legumain in freshly sorted IKDC versus NK (Fig.1C).
Figure 1. Genomic characterization of MHC-II processing machinery.

A, Heatmaps showing the expression levels of the probe sets for the mRNA associated with MHC-II processing pathway in IKDC, NK, and CDC from spleen (two independent samples 1 and 2) and LN (two pooled samples) of BALB/c mice. The color scheme is based on the base 2 logarithmic scale. B, Fold changes of the probe set signals for IKDC versus NK and IKDC versus CDC. Up-regulated and down-regulated genes are highlighted in green and red, respectively. The criterion of significance was set as the posterior probability >0.5. Fold changes highlighted in grey are not statistically significant. Absolute value of each probe set is provided in Table S1. C, Protein extracts from cell-sorted IKDC and NK were tested by western blot for Ii and Legumain expression. Tubulin detection is shown as control.
The assertion that IKDC represent activated NK (5–7) predicts that, upon activation, NK or a subset among the total NK population will acquire properties and surface markers similar to IKDC. We co-cultured the CD11c− fraction containing naïve NK (CD11c−B220−CD49b+) or CD11c+ fraction containing IKDC (CD11cintB220+CD49b+) and CDC (CD11chiB220−CD49b−) in the presence of MCMV-infected or mock-treated primary fibroblasts. BALB/c IKDC, but not NK, strongly upregulated MHC-II molecules when cultured in presence of MCMV-infected fibroblasts (41% versus 2.9%; Fig.2C). Costimulatory molecules were also up-regulated on the surface of IKDC (data not shown and Fig.3C). These conditions induced a robust IFN-γ production (Fig.4) and upregulation of CD11c and B220 by NK (Fig.1S), indicating that the absence of MHC-II up-regulation did not reflect a failure of NK to recognize infected fibroblasts. It can be argued that NK did not up-regulate MHC-II or CD40 because of the absence of accessory cells in the CD11c− fraction. We thus performed a mixing experiment, combining Ly5.1+CD11c+ cells with Ly5.2+CD11c− cells and incubating them with uninfected or MCMV-infected fibroblasts. In this mix, activated Ly5.2+ NK did not up-regulate MHC-II above levels observed for NK derived from Ly5.2+CD11c− fraction cultured alone with MCMV-infected fibroblasts. Meanwhile, Ly5.1+ IKDC retained their ability to up-regulate MHC-II molecules (data not shown). We performed an adoptive transfer experiment in which freshly isolated naive NK (CD11c−B220−NKp46+) and IKDC (CD11cintB220+NKp46+) were FACS-sorted from Balb/c mice spleen to high purity and transferred into MCMV-infected NOD/SCID/γc−/− mice. As shown in Fig.2B, MHC-II expression on NK before transfer is essentially negative whereas expression is low on IKDC. Although transferred NK indeed upregulated B220 and CD11c upon MCMV infection (37%), they failed to express any MHC-II (Fig.2D). In contrast, 5–6% of transferred IKDC expressed intermediate to high MHC-II levels, at day 2 and 3 post infection.
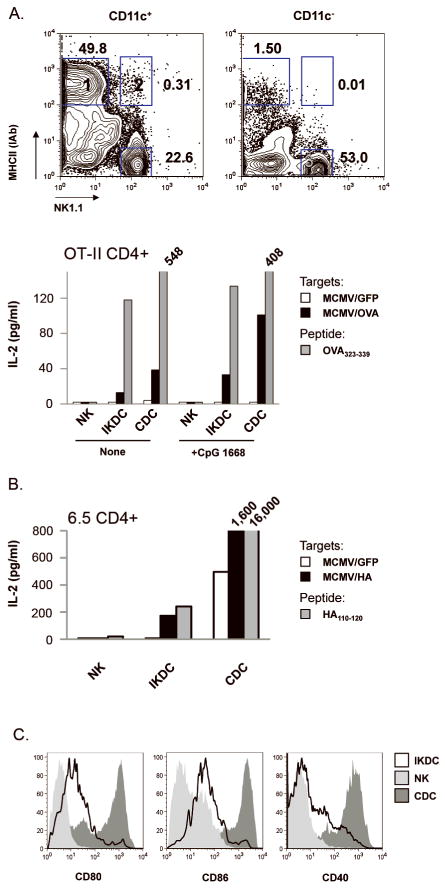
Figure 3. Upon recognition of MCMV-infected fibroblasts, IKDC differentiated into mature MHC-IIhi APC endowed with CD4+ T-lymphocyte stimulatory properties.
A, C57BL/6 CD11c+ and CD11c− splenocytes were incubated with MCMV/GFP- or MCMV/OVA-infected fibroblasts. CDC (CD11chiNK1.1−MHC-IIhi), IKDC (CD11c+NK1.1+MHC-II+) and NK (CD11c+NK1.1+MHC-II−) were FACS-sorted from gates 1, 2 and 3, respectively, and incubated with OVA-specific OT-II CD4+ T-cells. IL-2 was measured by ELISA in day 3 culture supernatants. ODN CpG 1668 was added or not to the culture to stimulate the APCs. APC were also incubated in presence of OVA323–339 to stimulate OT-II. B, Similar presentation assay was performed with HA antigenic model. The purity of sorted populations used in this assay is shown in Figure S3. C, Co-stimulatory molecules expression on CD11c+NK1.1+MHC-II+ IKDC (open) versus CD11c+NK1.1+MHC-II− NK (light grey) and CD11chiNK1.1−MHC-IIhi CDC (dark grey) in presence of infected fibroblasts.
Figure 4. IKDC activated by MCMV-infected fibroblasts simultaneously produced IFN-γ and IL-12p40 .
A, C57BL/6 CD11c+ and CD11c− fractions were incubated 12h with MCMV-infected fibroblasts. IFN-γ and IL-12p40 were detected by ICS. In the CD11c+ fraction, CD11c+NK1.1− included CDC and PDC whereas CD11c+NK1.1+ included IKDC (MHC-II+). In the CD11c− fraction, the majority of the CD11c−NK1.1+ NK upregulated CD11c after co-culture with infected fibroblasts and were CD11c+NK1.1+MHC-II−. The dot plots are representative of at least 3 experiments. B, CD11c+NK1.1+ splenocytes from TrifLps2/Lps2MyD88−/− and IL-12p40−/− mice were assessed for IFN-γ by ICS. Where indicated, rmIL-12 (100 pg/ml) was added.
Altogether, these results demonstrate that differential expression of CD11c, B220, and NK1.1/CD49b allows for the distinction between naïve IKDC, NK and CDC, while surface MHC-II expression should be included to delineate activated IKDC from activated NK. Therefore, in order to perform antigen presentation assays on sorted cell populations, we define IKDC activated during incubation with MCMV-infected fibroblasts or in vivo after MCMV injection as CD11c+CD49/NK1.1+MHC−IIhi whereas activated NK are defined as CD11c+CD49/NK1.1+MHC-II−.
IKDC, but not NK, presented antigens derived from MCMV-infected targets to CD4+ T-cells
Highly purified MHC-IIhi IKDC from BALB/c mice infected with Lm (Fig.S2A), induced a robust proliferation of HA-specific 6.5 CD4+ T-cells when loaded with the HA110–120 peptide (Fig.S2B). In order to test the hypothesis that IKDC can present antigens derived from their killed targets, we cultured CD11c− and CD11c+ fractions of C57BL/6 splenocytes with MCMV/GFP- or MCMV/OVA-infected fibroblasts (Fig.3A). After 12h incubation, activated IKDC (CD11c+NK1.1+MHC-IIhi) and CDC (CD11chiNK1.1−MHC-IIhi) were sorted from the CD11c+ fraction. Among the CD11c− fraction, NK upregulated CD11c and B220 (Fig.S1), but remained MHC-II− and, therefore, were sorted as CD11c+NK1.1+MHC-II−. IKDC derived from co-culture with MCMV/OVA- but not MCMV/GFP-infected fibroblasts induced IL-2 secretion by OT-II CD4+ T-cells (Fig.3A). Treatment of IKDC with CpG1668 stimulated a higher level of IL-2 by OVA-specific OT-II cells. CDC, but not NK, cultured with MCMV/OVA-infected fibroblasts were potent in inducing OT-II stimulation. Although low, IL-2 induction by IKDC still ranged between one third and one half of that by CDC (Fig.3A). We reproduced these findings using HA-specific 6.5 CD4+ T-cells and MCMV/HA-infected fibroblasts (Fig.3B). As a control, IKDC incubated with OVA323–339 or HA110–120 induce less IL-2 than CDC (Fig.3A and 3B). NK, which lacked MHC-II, did not stimulate CD4+ T cells. BALB/c MHC-IIhi IKDC used for the presentation assay expressed accessory molecules such as CD80, CD86, and CD40 (Fig.3C). The high purity of each FACS-sorted population (>95%) ruled out the role of contaminating CDC in assays of T-cell activation by purified IKDC (Fig.S3 and Fig.5). NK, which included similar percentages of contaminating CDC as did IKDC (1.2% for NK versus 1.0% for IKDC in fig.S3), did not stimulate T-cells to produce IL-2. No IL-2 secretion was observed when sorted CD4+ T-cells were incubated with peptide alone, ruling out their contamination with APC (data not shown). RT-PCR performed on IKDC, NK and CDC incubated with MCMV-GFP did not detect expression of viral genes ie1, m157 or GFP in IKDC (data not shown). Neither was free virus detected in the APC/fibroblast co-culture in the timeframe of experiment (data not shown) indicating that IKDC and CDC rather ingested antigens from the infected target cells and presented the processed epitope in association with MHC-II.
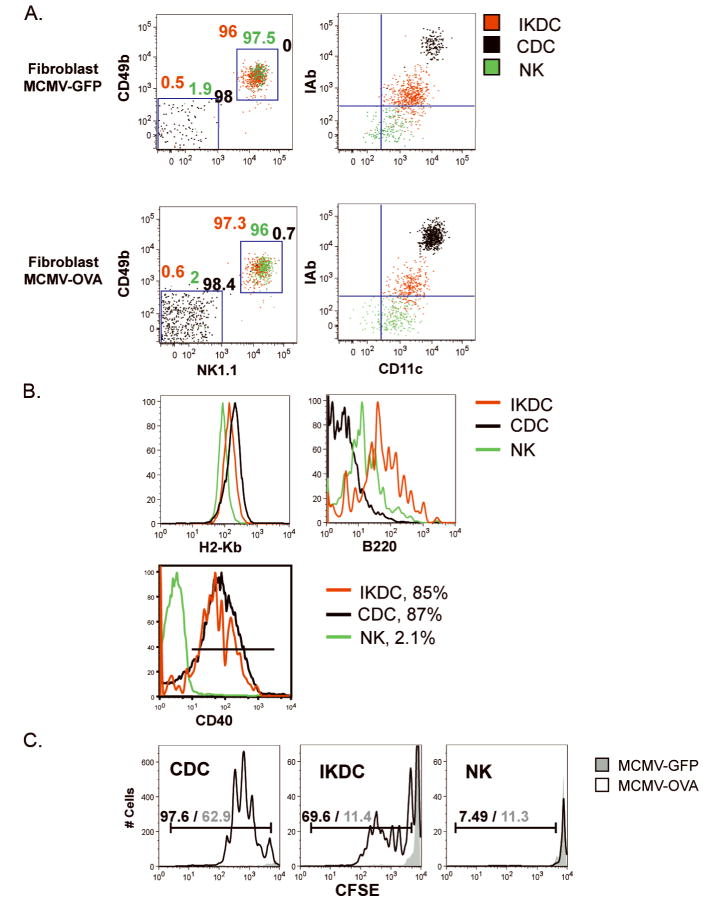
Figure 5. IKDC cross-presented viral antigens derived from MCMV-infected fibroblasts.
A, IKDC (CD11cintNK1.1+CD49b+I-Abhi, red), CDC (CD11chiNK1.1−CD49b−I-Abhi, black) and NK (CD11cintNK1.1+CD49b+I-Ab−, green) were cell sorted from 12h co-culture of CD11c+ (IKDC and CDC) or CD11c− (NK) fractions with MCMV-infected primary fibroblasts. Left dot plots represent an overlay of the CD49b/NK1.1 staining for different populations. The percentages of each population are indicated above each of the two gates CD49b+NK1.1+ (IKDC and NK) and CD49b−NK1.1− (CDC). The differential expression of CD11c and I-Ab used to sort IKDC, CDC, and NK is shown in right dot plots. B, Expression of MHC-I (H-2kb), B220, and CD40 by purified IKDC, CDC and NK. Percentages of cells expressing CD40 are indicated. C, IKDC and CDC, but not NK, cross-present OVA derived from MCMV/OVA-infected fibroblasts. Each cell population was mixed with CFSE-labeled CD8+ OT-I at a ratio of 1APC:3T-cells. CFSE dilution was assessed by FACS after 4 day-incubation. Percentages of proliferating T-cells within gate are indicated for cultures containing stimulators previously incubated with MCMV/OVA (black)- or MCMV/GFP (grey)-infected fibroblasts.
Activated IKDC simultaneously produced IFN-γ and IL-12p40
We used ICS to examine the capacity of individual IKDC to secrete IFN-γ and IL-12p40 upon activation by MCMV-infected fibroblasts (Fig.4A). Among CD11c+-enriched splenocytes, only IKDC (CD11c+NK1.1+IAbhi) secreted a large amount of IFN-γ. Notably, a significant fraction of IFN-γ+ IKDC simultaneously produced IL-12p40. All CD11c+NK1.1−IAbhi cells (CDC/PDC) secreted significant quantities of IL-12p40 but not IFN-γ whereas NK (CD11c+NK1.1+I-Ab−) produced IFN-γ but not IL-12p40. Neither IFN-γ nor IL-12p40 was detected in response to uninfected fibroblasts. Since IL-12p40 is common to IL-12p70 and IL-23, both cytokines were assessed by ELISA in supernatants of activated IKDC. Only IL-12p40 and IL-12p70 were detected (Fig.S4A; not shown for IL23). Using IL12p40−/− CD11c+ splenocytes cultured with infected fibroblasts, we found that IFN-γ secretion by IKDC was not impaired in absence of IL-12/IL-23 signaling (Fig.4B). In contrast, the absence of toll-like receptor (TLR) signaling in Trif and Myd88 deficient CD11c+ splenocytes was profoundly deleterious for production of IFN-γ by MHC-II+ IKDC as well as MHC-II− NK. Complementation with exogenous IL-12 restored some IFN-γ production by NK (MHC-II−), but not IKDC (MHC-II+). These results show that IL-12 is not primarily involved in the production of IFN-γ by IKDC. When transwells were used to separate sorted IKDC and infected fibroblasts, IFN-γ production by IKDC was also abrogated (Fig.S5), suggesting that IFN-γ secretion required cell-to-cell interaction.
Activated IKDC, distinguished from NK by upregulation of MHC-II and CD40, accumulated in spleen, LN, and liver, peaking in number at day 2 p.i. with MCMV (Fig.S6A). IKDC retained their ability to secrete IFN-γ and IL-12p40 in vivo (Fig.S6B). Quantitative real-time PCR performed on sorted activated populations detected similar IL-12p40 and IL-12p35 gene expression between IKDC and CDC, whereas only IFN-γ RNA was detectable in NK (Fig.S4B). As found with ICS, IKDC, but not CDC, simultaneously expressed IFN-γ mRNA. Furthermore, phenotyping of IKDC in spleen and LN highlighted concomitant expression of NK-associated receptors and APC markers (Fig.S6C).
Activated IKDC, but not NK, cross-primed CD8+ T-cells
Confocal microscopy using 16-D25-11 mAb demonstrated that, unlike NK, CDC and IKDC generate H2-Kb/OVASIINFEKL complexes when incubated in vitro with soluble OVA and IFN-α (Fig.S7). We further sought to determine whether IKDC were able to cross-present MHC-I-restricted OVASIINFEKL derived from MCMV/OVA-infected fibroblasts (18, 20) and stimulate OT-I Rag2−/− CD8+ T-cells. IKDC, sorted as CD11c+CD49b+NK1.1+MHC-IIhi cells from CD11c+ splenocytes cultured in the presence of MCMV/OVA-infected fibroblasts, were able to induce OT-I proliferation assessed by CFSE dilution (Fig.5 and S8A). Minimal CFSE dilution was observed when IKDC were derived from CD11c+ fraction cultured in presence of MCMV/GFP-infected fibroblasts (Fig.5C). While NK, sorted as CD11c+CD49b+NK1.1+MHC-II− after culture of CD11c− fraction in presence of MCMV/OVA-infected fibroblasts, could not cross-present OVA, CDC (CD11c+CD49b−NK1.1−MHC-IIhi) induced a strong antigen-specific proliferation of OT-I. The number of T-cells after culture with NK stimulators was one tenth that in IKDC wells. Meanwhile, the number of T-cells remaining in CDC wells was nine times that in IKDC wells. Whereas Fig.5 shows proliferation at 1:3 APC:T cell ratio, CDC but not IKDC were still able to induce OT-I proliferation at 1:10 ratio, suggesting that IKDC are less efficient in stimulating T cells (data not shown). Cell subsets were highly purified via two rounds of sorting and doublet exclusion to assure the absence of NK/DC aggregates (Fig.5A). NK included 2% CDC contamination (Fig.5A), which, nonetheless, was not sufficient to trigger OT-I proliferation (Fig.5C) indicating that T-cell proliferation cannot be attributed to the <0.7% contaminating CDC in the IKDC preparation. Homogeneous expression of CD49b and NK1.1 by sorted IKDC (>96%) excludes contamination by PDC. Figure 5B shows that MHC-IIhi IKDC, but not activated NK, also homogeneously upregulated CD40 (85%) (Fig.5B), whereas both subsets expressed B220. The inability of activated NK to stimulate OT-I T-cells does not reflect the lack of MHC-I expression since all three populations expressed comparable levels of H2-Kb (Fig.5B). Perf−/− IKDC did not activate OT-I cells indicating that killing is required for IKDC to uptake and cross-present antigen (data not shown).
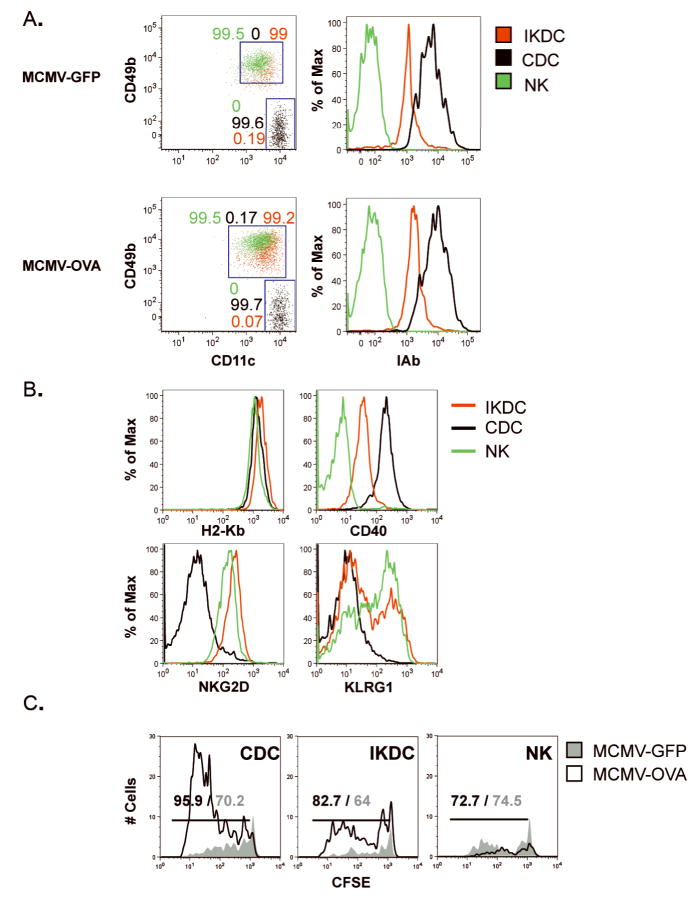
Activated IKDC, CDC, and NK sorted from C57BL/6 mice infected with MCMV/OVA- or MCMV/GFP were used in direct ex vivo antigen detection (DEAD) assays (Fig.6 and S8B). Although the proportion of MHC-IIhi IKDC was higher in LN than spleen of infected mice (Fig.S4), sorting was performed on splenocytes in order to isolate higher number of activated IKDC. Each population was subjected to a stringent two round-sorting and doublet exclusion in order to reach a ≥99% purity, especially avoiding contamination of IKDC with CDC (Fig.6A). Sorted IKDC homogeneously expressed high levels of I-Ab, CD40, CD49b and NKG2D (Fig.6A–B), ruling out the presence of putative NKG2D−CD49b+ APC (7) and CD49b−NKG2D− PDC. Both NK and IKDC expressed KLRG1 (Fig.6B). Sorted MHC-II+ IKDC and CDC, but not MHC-II− NK, triggered OT-II T-cell proliferation when pulsed with OVA323–339 (Fig.S8C). Under these conditions, IKDC and CDC, but not NK, freshly sorted from MCMV/OVA-infected mice were able to induce proliferation of CFSE-labeled Rag2−/− OT-I T-cells (Fig.6C) to a significantly greater degree than the same cells isolated from MCMV/GFP-infected mice. Here again, all populations expressed comparable levels of H2-Kb (Fig 6B). Very few viable T-cells were observed after a 4 day culture with NK (unlike IKDC and CDC) isolated from MCMV/OVA- and MCMV/GFP-infected mice, and the percentage of CFSE+ cells in these cultures (i.e. test vs background) are equivalent. The number of T-cells remaining at the end of the culture in presence of NK stimulators was one fifth that in IKDC wells. Twice as many T-cells were observed in the presence of CDC as compared to IKDC at the end of culture.
Figure 6. IKDC derived from MCMV-infected mice cross-presented viral antigens to CD8+ T-cells.
C57BL/6 mice were infected i.p. with 4×105pfu MCMV/OVA or MCMV/GFP. Splenic IKDC (CD11cintCD49b+I-Abhi), CDC (CD11chiCD49b−I-Abhi) and NK (CD11cintCD49b+I-Ab−) were sorted at day 2 p.i. A, Purity of FACS-sorted IKDC (red), CDC (black), and NK (green). Left dot plots represent an overlay of the CD49b/CD11c staining for these populations. The percentages of each population are indicated above each of the two gates CD49b+CD11cint (IKDC and NK) and CD49−CD11chi (CDC). The differential expression of MHC-II (I-Ab) used to sort IKDC, CDC and NK is shown in overlaid histograms. B, Each population was stained for MHC-I (H2-Kb), CD40, NKG2D, and KLRG1. C, DEAD assay. Ex vivo sorted cell populations were mixed with CFSE-labeled CD8+ OT-I at a ratio of 1APC:3T-cells. CFSE dilution was assessed by FACS after 4d-incubation. Percentages of proliferating T-cells within gate are indicated for cultures containing stimulators derived from mice infected with MCMV/OVA (black) or MCMV/GFP (grey).
DISCUSSION
In this report, we further delineate the phenotypic and functional differences between NK and IKDC and provide evidence that, upon killing of their targets, IKDC, but not NK, can mature into fully competent APC that cross-prime naïve CD4+ and CD8+ T-cells. First, our findings establish in vitro that cellular contact with MCMV-infected targets was mandatory for IKDC to upregulate MHC-II, costimulatory molecules, and to secrete IFN-γ and IL-12p40. Second, activated IKDC, which remained MCMV-free, presented in vivo and in vitro antigens released from killed target cells to CD4+ and CD8+ T-cells.
While IKDC have been recently affiliated to an activated NK (4, 5, 7), the present report brings forward molecular (i.e. microarray analysis) and functional (i.e. T-cell proliferation assays) evidence clearly demonstrating that MHC-II expression by activated IKDC reflects a fully functional antigen processing machinery that facilitates efficient antigen cross-presentation. Highly purified activated IKDC (MHC-IIhi) isolated from co-cultures of CD11c+ splenocytes with MCMV/OVA-infected targets process OVA antigen to consequently stimulate specific CD4+ and CD8+ T-cells. The striking discrepancy with the groups reporting a complete lack of T-cell activation by both NK and IKDC, even in presence of high doses of cognate peptide (5, 7), could be explained by the fact that we assessed highly purified FACS-sorted activated MHC-IIhiCD11cintB220+CD49b+ IKDC as APC in our antigen presentation assays since only MHC-IIhi IKDC were found to behave as mature APC (1).
Similarly to our viral infection model, Anderson’s and Zitvogel’s groups showed that cellular contact with tumor cells triggered up-regulation of MHC-II and costimulatory molecules and licensed IKDC to cross-present antigen to CD4+ and CD8+ T-cells (companion manuscripts). Although activated by the tumor or MCMV, NK failed to function as an APC in both experimental situations. While we established that killing of the target is mandatory for antigen processing and presentation by activated IKDC, the nature of the mechanism used by IKDC to capture OVA expressed by MCMV/OVA-infected fibroblasts was not addressed. However, Zitvogel and colleagues found that “licensed” CD11b+ IKDC engulfed soluble, but not cell-associated OVA (companion manuscript).
We carefully monitored the purity of MHC-IIhi IKDC, which homogeneously co-expressed NK1.1, CD40 and NKG2D (Fig.6B) formally excluding contaminating PDC, CDC, or putative CD49b+ APC as previously suspected to be responsible for stimulating CD4+ T-cells (7). We also showed that IKDC activated both in vitro with MCMV-infected fibroblasts and in vivo after i.p. injection of MCMV were unique in their ability to produce both IFN-γ and IL-12p40 at the single cell level. While expression of MHC-II, costimulatory molecules such as B7 family members (CD80, CD86 or B7-H1) and TNF family members (OX40L) has already been reported on mouse and/or human NK, the up-regulation of CD40 and production of IL-12p40 have been classically considered as the hallmarks of professional APC (4, 21–23). Contrary to recent reports (3, 24, 25), we have demonstrated that IFN-γ secretion by IKDC, but not NK, was IL-12-independent, but required TLR signaling since Myd88/Trif deficiency abrogated the production of IFN-γ by IKDC. Altogether, these features demonstrate that IKDC represent a unique population functionally distinct from activated NK.
While CDC stand as the archetypal professional APC (26), which induce the activation of naïve T-lymphocytes, additional innate immune cells including PDC, macrophages, neutrophils, and NK can potentially accomplish this mission (21, 23, 27–31). Although less efficient than CDC, we showed that IKDC express a variety of co-stimulatory and accessory molecules (CD80, CD86, CD40, and IL-12p40) and induce the cross-priming of naïve T-cells. It is unclear why and how IKDC as well as other innate effectors exercise their APC properties in vivo to complement CDC functions. IKDC and the related NKDC dramatically proliferated and accumulated in the LN in response to pathogen infections including Lm (unpublished data and (32)) and MCMV (Fig.S4). IKDC were also found in the tumor bed of B16 melanoma-bearing mice treated with GleevecR/IL-2 and improved animal survival (2). Activated IKDC re-circulate to the LN where, as mature APC, they engage directly in cognate interactions with CD4+ and possibly CD8+ T-cells [(1) and companion manuscript by Anderson and coll.]. Thus, IKDC may benefit the host via their ability to rapidly present antigens from infected or malignant cells that they kill. By comparison, CDC are mainly dependent on their cooperation with killer cells, including NK, IKDC, neutrophils, and macrophages, to obtain cell debris (33) or alternatively on the late necrosis or apoptosis processes triggered by “suicidal” stressed cells (34). We observed a strong upregulation on IKDC of B7-H1 (Fig.S6C), described to interact with PD1 on activated T-cells and regulate the immune response (35). Zitvogel and colleagues have shown that blockade of B7-H1 dramatically enhances the capacity of IKDC to stimulate effector functions of cognate T-cells (companion manuscript). This phenomenon suggests a putative role for IKDC in the regulation of T-cell function.
Activated human NK upregulate MHC-II and stimulate antigen-specific CD4+ T-cells (21, 23, 31). Strikingly, our study demonstrated that activated IKDC, unlike human NK, were additionally able to cross-present antigens to CD8+ T-cells. Therefore, we anticipate that putative activated human IKDC would be functionally distinct from activated NK. Recently, IFN-DC, generated in vitro by treatment of human monocytes with IFN-α, were shown to express CD56 and IFN-γ (36). These CD56+ IFN-DC exhibited potent cytotoxic activity and appeared to stimulate in vivo antigen-specific CD8+ T-cells. Another study described DC expressing perforin at the vicinity of tumor in vivo and demonstrated their cytotoxic activity in vitro (37). These cells represent potential human homologs of IKDC. However, only the identification of specific markers will allow the translation to human and the assessment of their therapeutic implications.
Supplementary Material
Acknowledgments
We thank Leslie Meszler and Lillian Dasko-Vincent for confocal microscopy technique, Lee Blosser and Ada Tam for cell sorting.
Financial Support: NIH grant (5U19CA113 341), the Janey Fund and Seraph Foundation, and gifts from Bill and Betty Topecer and Dorothy Needle.
Footnotes
The authors declare that they have no competing financial interests.
References
- 1.Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nature medicine. 2006;12(2):207–13. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 2.Taieb J, Chaput N, Menard C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nature medicine. 2006;12(2):214–9. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 3.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005;174(5):2612–8. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Lanier LL. Natural killer or dendritic: what's in a name? Immunity. 2007;26(1):11–6. doi: 10.1016/j.immuni.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Vosshenrich CA, Lesjean-Pottier S, Hasan M, et al. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. The Journal of experimental medicine. 2007;204(11):2569–78. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. The Journal of experimental medicine. 2007;204(11):2561–8. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caminschi I, Ahmet F, Heger K, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. The Journal of experimental medicine. 2007;204(11):2579–90. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welner RS, Pelayo R, Garrett KP, et al. Interferon-producing killer dendritic cells (IKDCs) arise via a unique differentiation pathway from primitive c-kitHiCD62L+ lymphoid progenitors. Blood. 2007;109(11):4825–931. doi: 10.1182/blood-2006-08-043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Messerle M, Sapinoro R, et al. Murine cytomegalovirus abortively infects human dendritic cells, leading to expression and presentation of virally vectored genes. Journal of virology. 2003;77(13):7182–92. doi: 10.1128/JVI.77.13.7182-7192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathys S, Schroeder T, Ellwart J, Koszinowski UH, Messerle M, Just U. Dendritic cells under influence of mouse cytomegalovirus have a physiologic dual role: to initiate and to restrict T cell activation. The Journal of infectious diseases. 2003;187(6):988–99. doi: 10.1086/368094. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol Reprod. 2003;68(6):2024–32. doi: 10.1095/biolreprod.102.012880. [DOI] [PubMed] [Google Scholar]
- 12.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6(6):715–26. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 13.Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Current opinion in immunology. 2004;16(1):96–102. doi: 10.1016/j.coi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Cresswell P, Bangia N, Dick T, Diedrich G. The nature of the MHC class I peptide loading complex. Immunological reviews. 1999;172:21–8. doi: 10.1111/j.1600-065x.1999.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 15.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 16.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299(5611):1400–3. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 17.Savina A, Jancic C, Hugues S, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126(1):205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 19.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Current opinion in immunology. 2008;20(1):89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 21.Hanna J, Gonen-Gross T, Fitchett J, et al. Novel APC-like properties of human NK cells directly regulate T cell activation. The Journal of clinical investigation. 2004;114(11):1612–23. doi: 10.1172/JCI22787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Saudemont A, Jouy N, Hetuin D, Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105(6):2428–35. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- 23.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-Talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173(6):3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 24.Chan CW, Housseau F. The 'kiss of death' by dendritic cells to cancer cells. Cell death and differentiation. 2008;15(1):58–69. doi: 10.1038/sj.cdd.4402235. [DOI] [PubMed] [Google Scholar]
- 25.Spits H, Yssel H, Takebe Y, et al. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987;139(4):1142–7. [PubMed] [Google Scholar]
- 26.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 27.Beauvillain C, Delneste Y, Scotet M, et al. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–73. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 28.Hoeffel G, Ripoche AC, Matheoud D, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27(3):481–92. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol. 2001;167(5):2538–46. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 30.Pozzi LA, Maciaszek JW, Rock KL. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol. 2005;175(4):2071–81. doi: 10.4049/jimmunol.175.4.2071. [DOI] [PubMed] [Google Scholar]
- 31.Roncarolo MG, Bigler M, Haanen JB, et al. Natural killer cell clones can efficiently process and present protein antigens. J Immunol. 1991;147(3):781–7. [PubMed] [Google Scholar]
- 32.Plitas G, Chaudhry UI, Kingham TP, Raab JR, DeMatteo RP. NK dendritic cells are innate immune responders to Listeria monocytogenes infection. J Immunol. 2007;178(7):4411–6. doi: 10.4049/jimmunol.178.7.4411. [DOI] [PubMed] [Google Scholar]
- 33.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural killer cells and dendritic cells: "l'union fait la force". Blood. 2005;106:2252–8. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 34.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature medicine. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110(1):186–92. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papewalis C, Jacobs B, Wuttke M, et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol. 2008;180(3):1462–70. doi: 10.4049/jimmunol.180.3.1462. [DOI] [PubMed] [Google Scholar]
- 37.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. The Journal of experimental medicine. 2007;204(6):1441–51. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.