Abstract
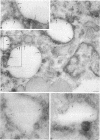
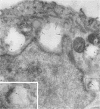
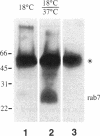
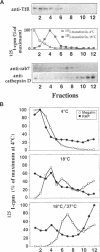
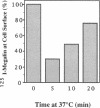
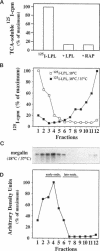
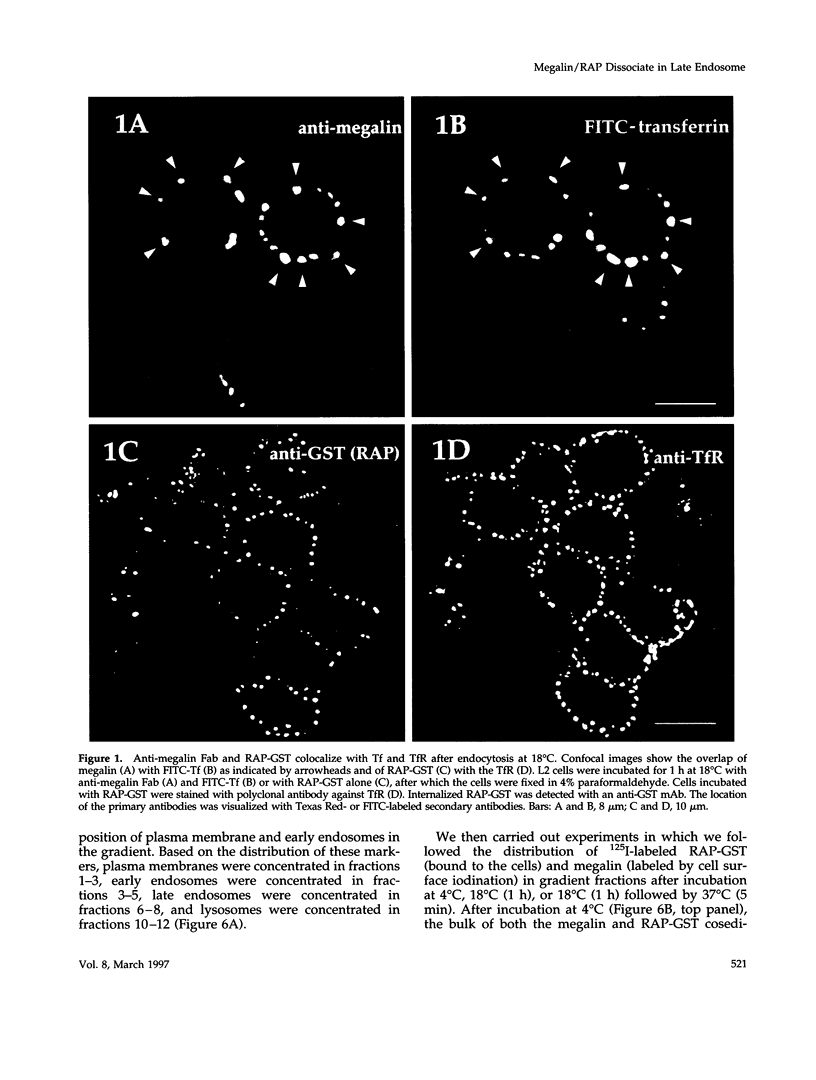
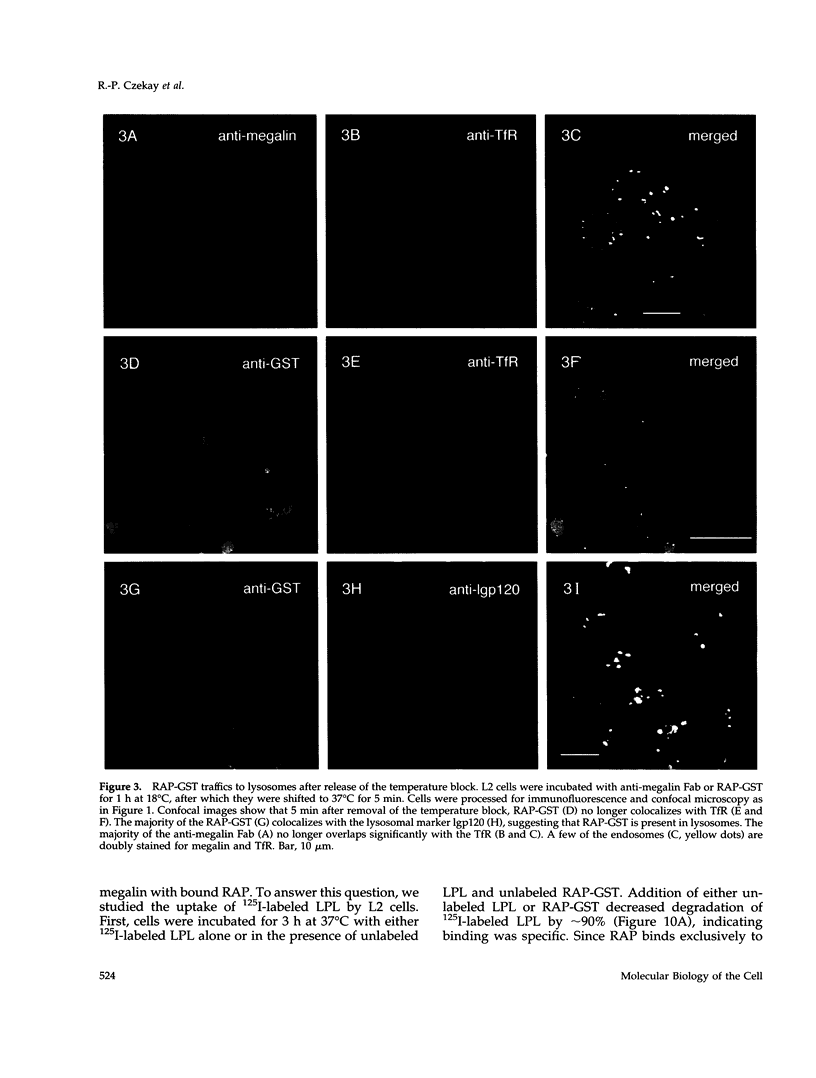
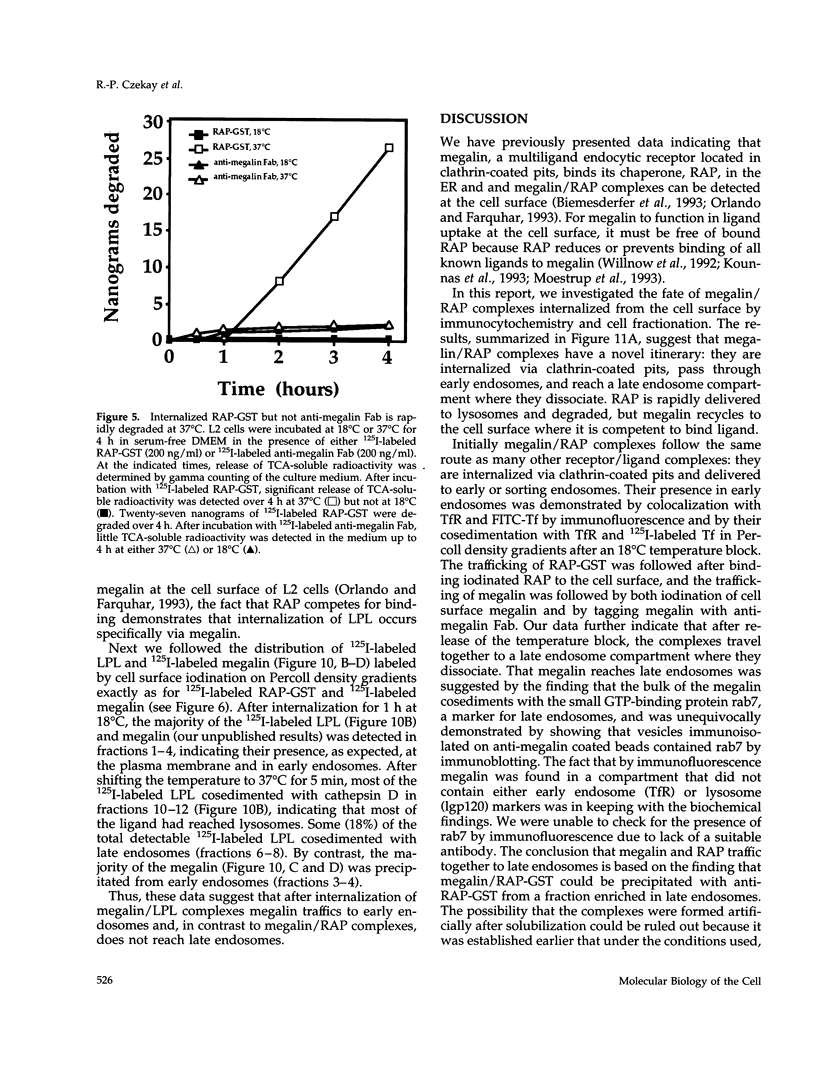
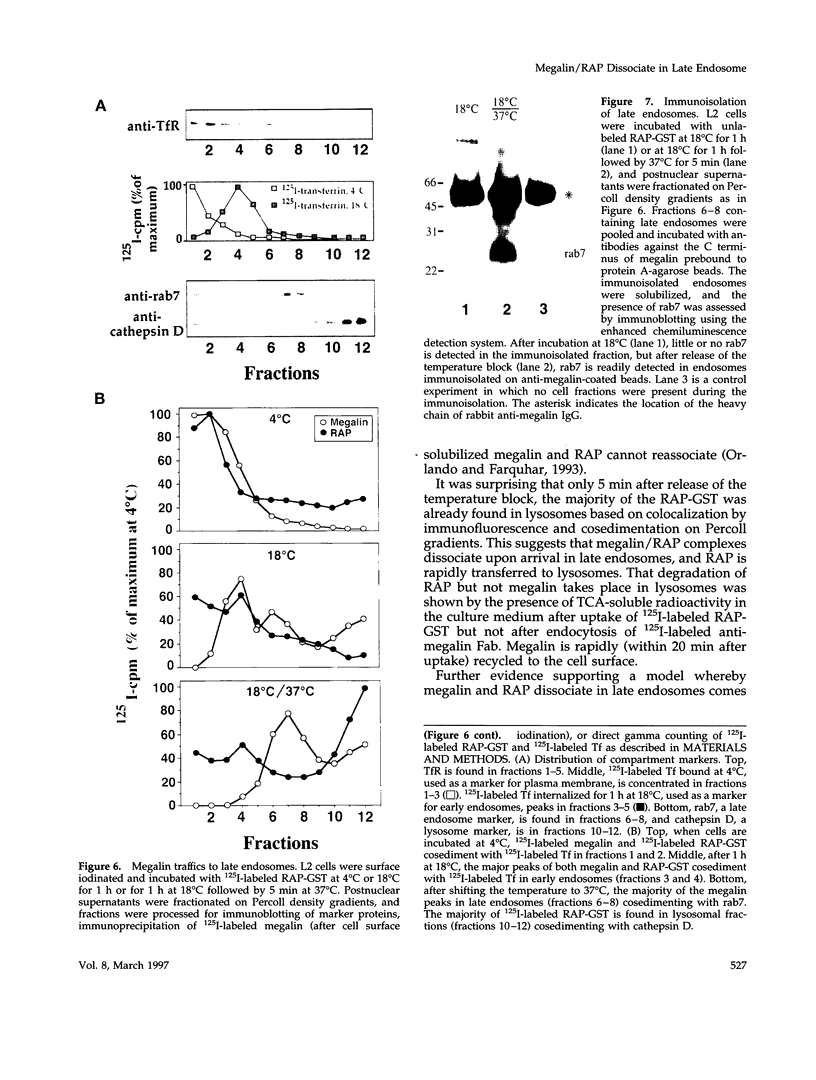
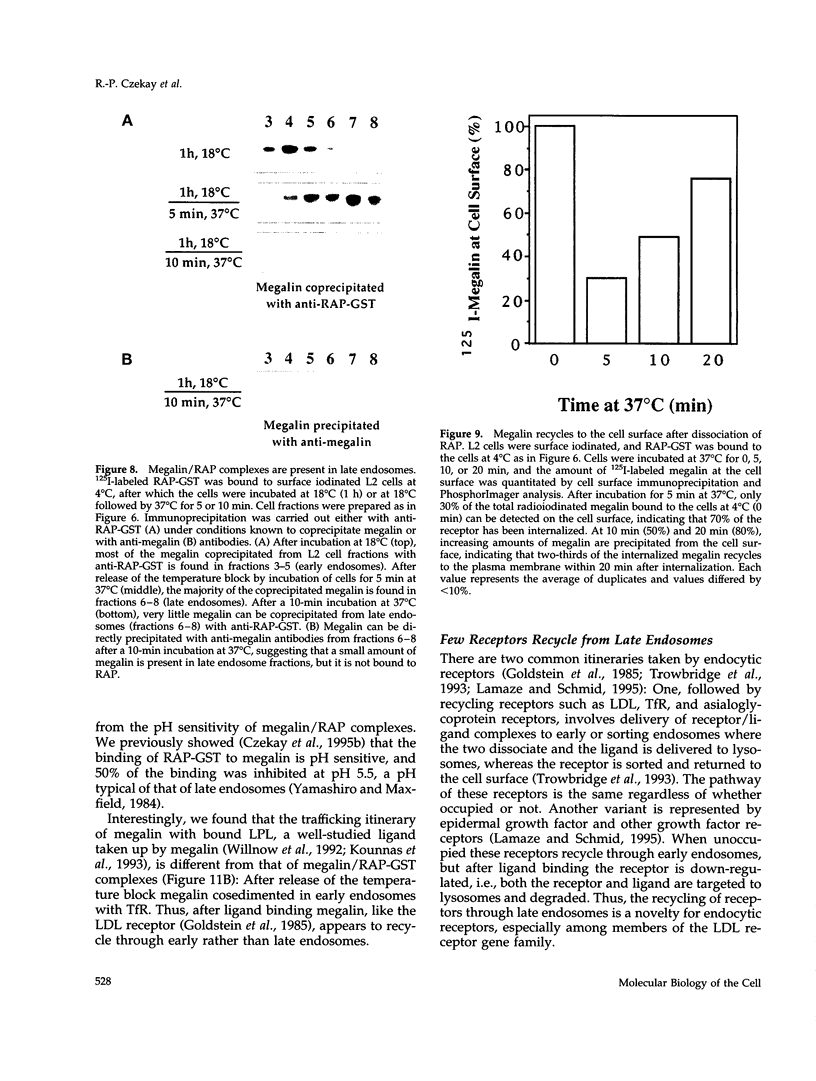
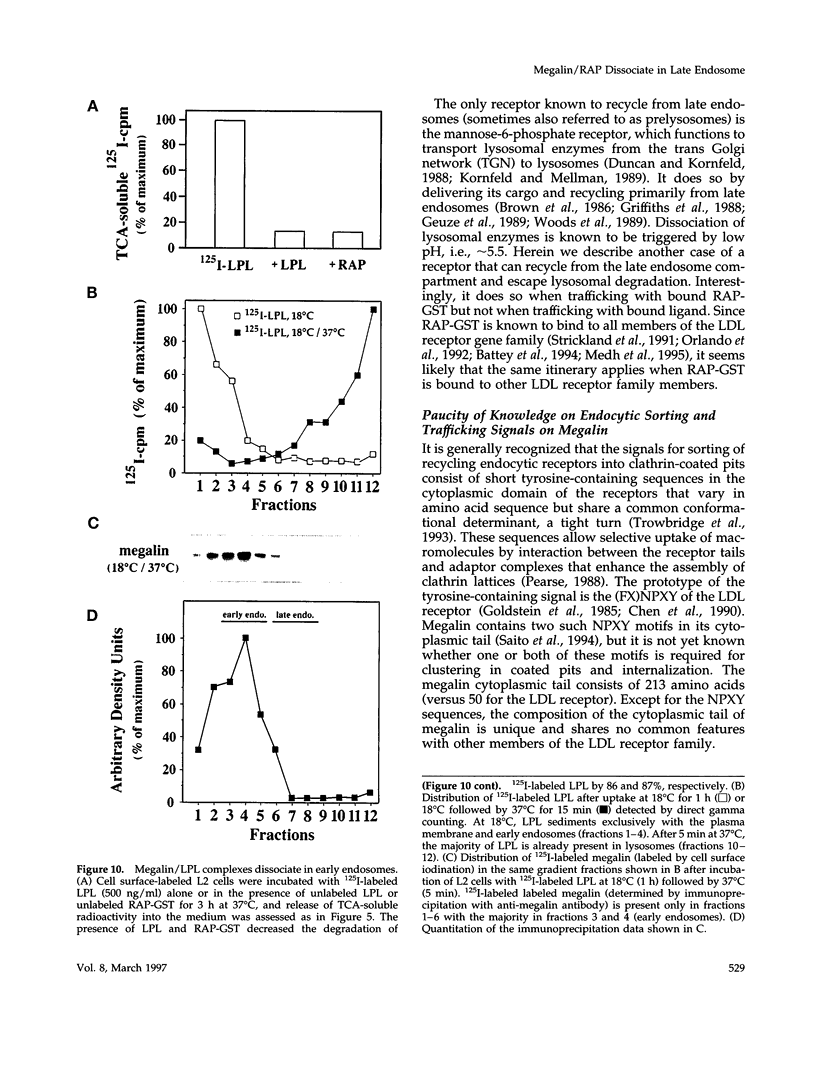
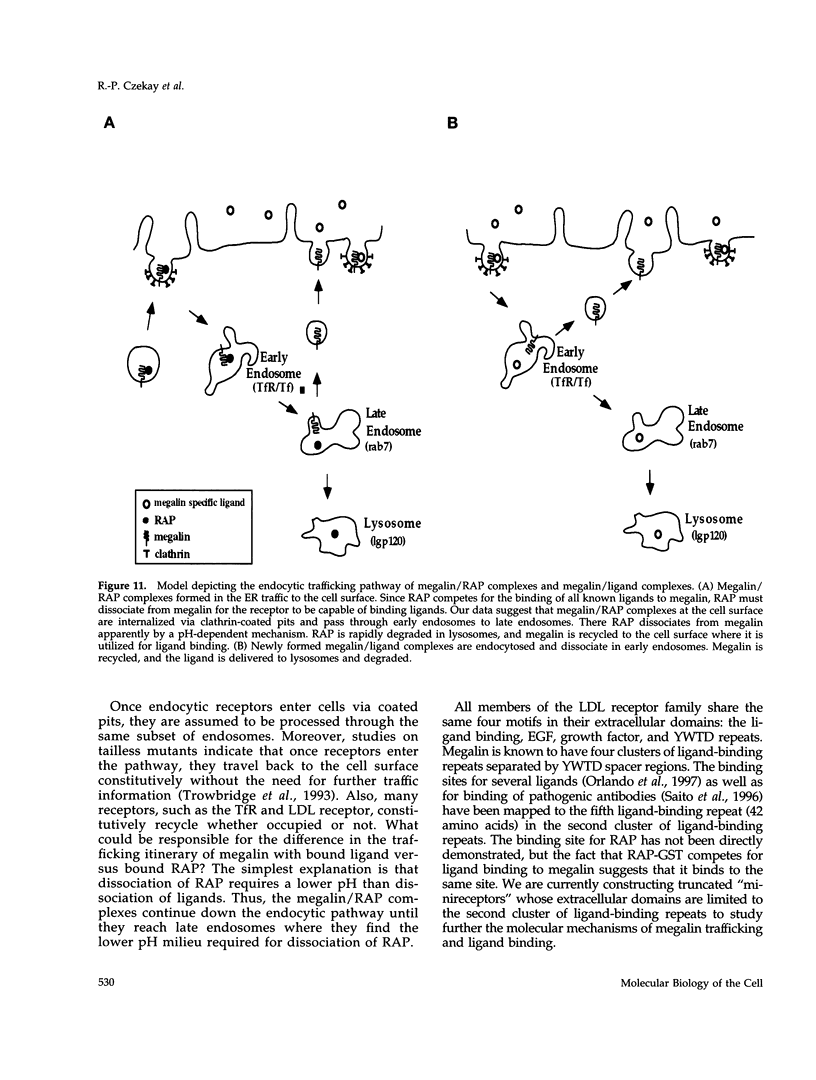
Megalin (gp330) is a member of the low-density lipoprotein receptor gene family. Like other members of the family, it is an endocytic receptor that binds a number of specific ligands. Megalin also binds the receptor-associated protein (RAP) that serves as an exocytic traffic chaperone and inhibits ligand binding to the receptor. To investigate the fate of megalin/RAP complexes, we bound RAP glutathione-S-transferase fusion protein (RAP-GST) to megalin at the surface of L2 yolk sac carcinoma cells and followed the trafficking of the complexes by immunofluorescence and immunogold labeling and by their distribution on Percoll gradients. We show that megalin/RAP-GST complexes, which are internalized via clathrin-coated pits, are delivered to early endosomes where they accumulate during an 18 degrees C temperature block and colocalize with transferrin and transferrin receptor. Upon release from the temperature block, the complexes travel to late endosomes where they colocalize with rab7 and can be coprecipitated with anti-RAP-GST antibodies. Dissociation of the complex occurs in late endosomes and is most likely triggered by the low pH (approximately 5.5) of this compartment. RAP is then rapidly delivered to lysosomes and degraded whereas megalin is recycled to the cell surface. When the ligand, lipoprotein lipase, was bound to megalin, the receptor was found to recycle through early endosomes. We conclude that in contrast to receptor/ligand complexes, megalin/RAP complexes traffic through late endosomes, which is a novelty for members of the low-density lipoprotein receptor gene family.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey F. D., Gåfvels M. E., FitzGerald D. J., Argraves W. S., Chappell D. A., Strauss J. F., 3rd, Strickland D. K. The 39-kDa receptor-associated protein regulates ligand binding by the very low density lipoprotein receptor. J Biol Chem. 1994 Sep 16;269(37):23268–23273. [PubMed] [Google Scholar]
- Biemesderfer D., Dekan G., Aronson P. S., Farquhar M. G. Biosynthesis of the gp330/44-kDa Heymann nephritis antigenic complex: assembly takes place in the ER. Am J Physiol. 1993 Jun;264(6 Pt 2):F1011–F1020. doi: 10.1152/ajprenal.1993.264.6.F1011. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Goodhouse J., Farquhar M. G. Mannose-6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol. 1986 Oct;103(4):1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G., Maksymovitch E. A., Geuze H., Schwartz A. L. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J Biol Chem. 1994 Nov 25;269(47):29874–29882. [PubMed] [Google Scholar]
- Bu G., Rennke S. Receptor-associated protein is a folding chaperone for low density lipoprotein receptor-related protein. J Biol Chem. 1996 Sep 6;271(36):22218–22224. doi: 10.1074/jbc.271.36.22218. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Christensen E. I., Gliemann J., Moestrup S. K. Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein. J Histochem Cytochem. 1992 Oct;40(10):1481–1490. doi: 10.1177/40.10.1382088. [DOI] [PubMed] [Google Scholar]
- Czekay R. P., Orlando R. A., Woodward L., Adamson E. D., Farquhar M. G. The expression of megalin (gp330) and LRP diverges during F9 cell differentiation. J Cell Sci. 1995 Apr;108(Pt 4):1433–1441. doi: 10.1242/jcs.108.4.1433. [DOI] [PubMed] [Google Scholar]
- Duncan J. R., Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988 Mar;106(3):617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., McGraw T. E., Maxfield F. R. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989 Dec;109(6 Pt 2):3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Kerjaschki D., Lundstrom M., Orlando R. A. gp330 and RAP: the Heymann nephritis antigenic complex. Ann N Y Acad Sci. 1994 Sep 10;737:96–113. doi: 10.1111/j.1749-6632.1994.tb44304.x. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Saito A., Kerjaschki D., Orlando R. A. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol. 1995 Jul;6(1):35–47. doi: 10.1681/ASN.V6135. [DOI] [PubMed] [Google Scholar]
- Feng Y., Press B., Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995 Dec;131(6 Pt 1):1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Stoorvogel W., Strous G. J., Slot J. W., Bleekemolen J. E., Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988 Dec;107(6 Pt 2):2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Hobman T. C., Woodward L., Farquhar M. G. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-Golgi compartment. J Cell Biol. 1992 Aug;118(4):795–811. doi: 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Ullrich R., Exner M., Orlando R. A., Farquhar M. G. Induction of passive Heymann nephritis with antibodies specific for a synthetic peptide derived from the receptor-associated protein. J Exp Med. 1996 May 1;183(5):2007–2015. doi: 10.1084/jem.183.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kounnas M. Z., Argraves W. S., Strickland D. K. The 39-kDa receptor-associated protein interacts with two members of the low density lipoprotein receptor family, alpha 2-macroglobulin receptor and glycoprotein 330. J Biol Chem. 1992 Oct 15;267(29):21162–21166. [PubMed] [Google Scholar]
- Kounnas M. Z., Chappell D. A., Strickland D. K., Argraves W. S. Glycoprotein 330, a member of the low density lipoprotein receptor family, binds lipoprotein lipase in vitro. J Biol Chem. 1993 Jul 5;268(19):14176–14181. [PubMed] [Google Scholar]
- Kounnas M. Z., Stefansson S., Loukinova E., Argraves K. M., Strickland D. K., Argraves W. S. An overview of the structure and function of glycoprotein 330, a receptor related to the alpha 2-macroglobulin receptor. Ann N Y Acad Sci. 1994 Sep 10;737:114–123. doi: 10.1111/j.1749-6632.1994.tb44305.x. [DOI] [PubMed] [Google Scholar]
- Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Schmid S. L. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995 Aug;7(4):573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Lundstrom M., Orlando R. A., Saedi M. S., Woodward L., Kurihara H., Farquhar M. G. Immunocytochemical and biochemical characterization of the Heymann nephritis antigenic complex in rat L2 yolk sac cells. Am J Pathol. 1993 Nov;143(5):1423–1435. [PMC free article] [PubMed] [Google Scholar]
- McCaffery J. M., Farquhar M. G. Localization of GTPases by indirect immunofluorescence and immunoelectron microscopy. Methods Enzymol. 1995;257:259–279. doi: 10.1016/s0076-6879(95)57031-4. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Medh J. D., Fry G. L., Bowen S. L., Pladet M. W., Strickland D. K., Chappell D. A. The 39-kDa receptor-associated protein modulates lipoprotein catabolism by binding to LDL receptors. J Biol Chem. 1995 Jan 13;270(2):536–540. doi: 10.1074/jbc.270.2.536. [DOI] [PubMed] [Google Scholar]
- Moestrup S. K., Cui S., Vorum H., Bregengård C., Bjørn S. E., Norris K., Gliemann J., Christensen E. I. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest. 1995 Sep;96(3):1404–1413. doi: 10.1172/JCI118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup S. K., Nielsen S., Andreasen P., Jørgensen K. E., Nykjaer A., Røigaard H., Gliemann J., Christensen E. I. Epithelial glycoprotein-330 mediates endocytosis of plasminogen activator-plasminogen activator inhibitor type-1 complexes. J Biol Chem. 1993 Aug 5;268(22):16564–16570. [PubMed] [Google Scholar]
- Orlando R. A., Farquhar M. G. Functional domains of the receptor-associated protein (RAP). Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3161–3165. doi: 10.1073/pnas.91.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando R. A., Farquhar M. G. Identification of a cell line that expresses a cell surface and a soluble form of the gp330/receptor-associated protein (RAP) Heymann nephritis antigenic complex. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4082–4086. doi: 10.1073/pnas.90.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando R. A., Kerjaschki D., Kurihara H., Biemesderfer D., Farquhar M. G. gp330 associates with a 44-kDa protein in the rat kidney to form the Heymann nephritis antigenic complex. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6698–6702. doi: 10.1073/pnas.89.15.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988 Nov;7(11):3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychowdhury R., Niles J. L., McCluskey R. T., Smith J. A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989 Jun 9;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- Saito A., Pietromonaco S., Loo A. K., Farquhar M. G. Complete cloning and sequencing of rat gp330/"megalin," a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Yamazaki H., Rader K., Nakatani A., Ullrich R., Kerjaschki D., Orlando R. A., Farquhar M. G. Mapping rat megalin: the second cluster of ligand binding repeats contains a 46-amino acid pathogenic epitope involved in the formation of immune deposits in Heymann nephritis. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8601–8605. doi: 10.1073/pnas.93.16.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Battey F., Behre E., McTigue K., Battey J. F., Argraves W. S. Primary structure of alpha 2-macroglobulin receptor-associated protein. Human homologue of a Heymann nephritis antigen. J Biol Chem. 1991 Jul 15;266(20):13364–13369. [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Williams S. E., Ashcom J. D., Argraves W. S., Strickland D. K. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992 May 5;267(13):9035–9040. [PubMed] [Google Scholar]
- Willnow T. E., Goldstein J. L., Orth K., Brown M. S., Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem. 1992 Dec 25;267(36):26172–26180. [PubMed] [Google Scholar]
- Willnow T. E., Rohlmann A., Horton J., Otani H., Braun J. R., Hammer R. E., Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996 Jun 3;15(11):2632–2639. [PMC free article] [PubMed] [Google Scholar]
- Woods J. W., Goodhouse J., Farquhar M. G. Transferrin receptors and cation-independent mannose-6-phosphate receptors deliver their ligands to two distinct subpopulations of multivesicular endosomes. Eur J Cell Biol. 1989 Oct;50(1):132–143. [PubMed] [Google Scholar]
- Yamamoto K., Katsuda N., Himeno M., Kato K. Cathepsin D of rat spleen. Affinity purification and properties of two types of cathepsin D. Eur J Biochem. 1979 Apr;95(3):459–467. doi: 10.1111/j.1432-1033.1979.tb12985.x. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J Cell Biochem. 1984;26(4):231–246. doi: 10.1002/jcb.240260404. [DOI] [PubMed] [Google Scholar]