Abstract
Objective
The purpose of this study was to determine the effects of an atherogenic diet on immune function in LDLr−/−, ApoA-I−/− mice.
Methods and Results
When LDLr−/−, ApoA-I−/− (DKO) and LDLr−/− (SKO) mice were fed an atherogenic diet DKO had larger peripheral lymph nodes (LNs) and spleens compared to SKO mice. LNs were enriched in cholesterol and contain expanded populations of T, B, dendritic cells and macrophages. Expansion of all classes of LN cells was accompanied by a ∼1.5-fold increase in T cell proliferation and activation. Plasma antibodies to dsDNA, β2-glycoprotein I, and oxidized LDL were increased in DKO, similar to levels in diet-fed Faslpr/lpr mice suggesting the development of an autoimmune phenotype. Both LN enlargement and cellular cholesterol expansion were “prevented” when diet-fed DKO mice were treated with helper dependent adenovirus expressing apoA-I. Independent of the amount of dietary cholesterol, DKO mice consistently showed lower plasma cholesterol than SKO mice, yet greater aortic cholesterol deposition and inflammation.
Conclusions
ApoA-I prevented cholesterol associated lymphocyte activation and proliferation in peripheral LN of diet-fed DKO mice. A ∼1.5-fold increase in T cell activation and proliferation was associated with a ∼3-fold increase in concentrations of circulating autoantibodies and ∼2-fold increase in the severity of atherosclerosis suggesting a common link between plasma apoA-I, inflammation and atherosclerosis.
Keywords: ApoA-I, cholesterol, lymphocytes, lymph node, atherosclerosis
Introduction
High concentrations of plasma high density lipoproteins (HDL) are a well established negative risk factor for coronary heart disease (CHD)1. Apolipoprotein A-I (apoA-I) which makes up approximately 70% of HDL protein is secreted by the liver and intestine and is essential for HDL formation and function2. The formation of HDL depends on the ATP binding cassette transporter A1 (ABCA1) which effluxes cholesterol onto apoA-I3, while ABCG1, another member of the ABC transporter family, primarily effluxes cholesterol to HDL particles4. Recent studies demonstrate that HDL apoA-I is an anti-inflammatory mediator modulating the progression of atherosclerosis through immune cell function.
A direct link between immune cell function and lipoprotein metabolism was recently demonstrated when lymphotoxin and LIGHT produced by T cells were found to regulate plasma triglyceride levels5. Moreover, monocyte populations may take on dendritic cell (DC)-like characteristics, migrate into an atherosclerotic lesion, become cholesterol enriched and migrate out, contributing to the stability of the plaque6, 7. Interestingly, hyperlipidemic apoE−/− and LDLr−/− mice show reduced migration of DCs between skin and LNs, but when HDL and/or apoA-I was administered normal migration was restored8.
Based on these associations, it has been speculated that HDL apoA-I and autoimmunity are linked. Humans with autoimmune disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA)9 and mouse models of these disorders are associated with increased atherosclerosis10, 11, with SLE and RA patients showing decreased HDL levels when compared to control subjects12. The correlation among autoimmune disease, atherosclerosis and plasma HDL apoA-I raises the possibility of a common link involving all three factors. We, therefore, undertook the current studies to investigate this link and found that apoA-I can modulate immune cell function by regulating cellular cholesterol balance, which in turn prevents LN cell expansion, activation and the progression of atherosclerosis.
Materials and Methods
LDLr−/−,ApoA-I−/− (DKO), LDLr−/− (SKO) and B6.MRL-Faslpr/J (Faslpr/lpr) mice were fully backcrossed into the C57BL/6 background13, 14. All mice were housed at the Wake Forest University Medical Center. The Wake Forest University Medical Center Committee for Animal Care and Use approved all procedures. For a more detailed description of “Materials and Methods” see Supplemental material at http://atvb.ahajournals.org.
Results
Cholesterol Accumulation and Expansion in Peripheral Lymph Nodes
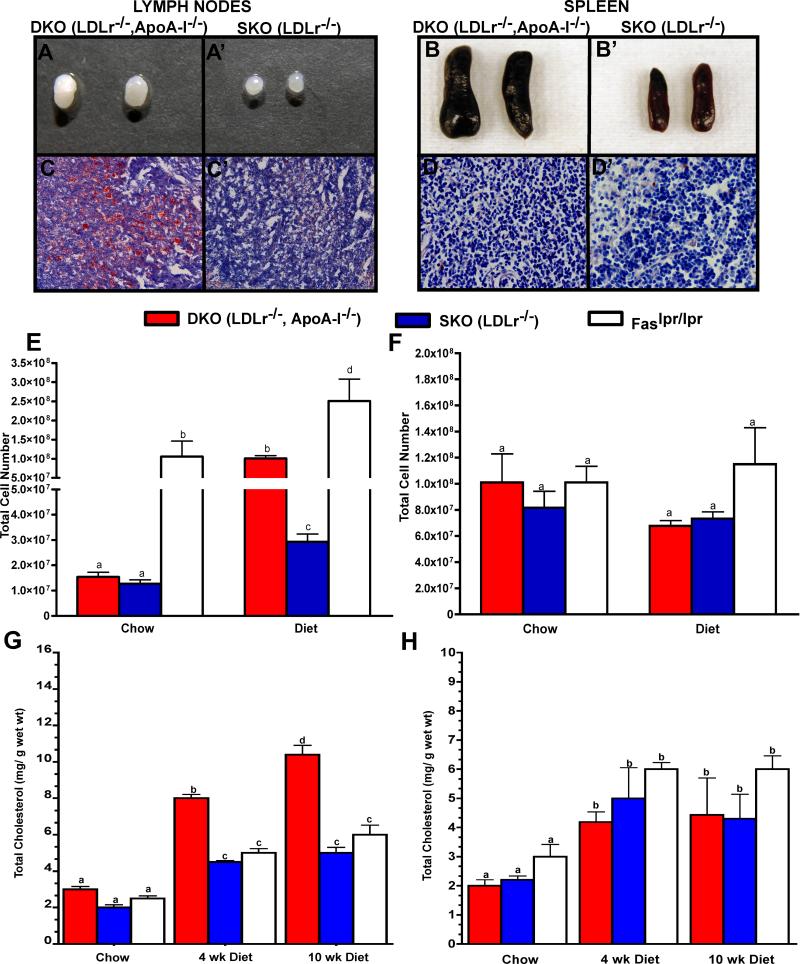
After 10 wks on an atherogenic diet, DKO mice acquired enlarged peripheral LNs, compared to SKO mice, shown in Figure 1, Panels A and A’, respectively. The enlargement of the DKO LNs was associated with increased Oil Red O staining, Figure 1, Panels C and C’, not seen in SKO mice. When fed an atherogenic diet for longer than 10 wks DKO mice develop skin lesions due to excessive scratching13, 14. To reduce the contribution of “open lesions” to the current studies, mice were fed diet for 10 wk, at which time external skin abnormalities were not present as shown in Supplemental Figure I. Although Figure 1 shows only brachial LNs, inguinal, axillary and superficial cervical LNs were also enlarged in diet-fed DKO mice. LNs from DKO weighed an average of 9.6±0.8 mg, while SKO LNs weighed 2.2±0.5 mg (n=5−10 mice per genotype, p<0.05).
Figure 1. Cholesterol accumulation in the lymph nodes of LDLr−/−, ApoA-I−/− mice.
Gross, histological and chemical analysis of 10 wk diet-fed DKO and SKO LNs, Panels A,A’- and C, C’, and spleens Panels B, B’ and D-D’, respectively. Oil Red O stained sections of LNs are shown in Panels C and C’ and of spleens in Panels D and D’. The mean ± SD of total lymphocytes and spenocytes from chow and diet-fed mice are plotted in Panels E and F, and represent 3 independent experiments with 4 to 5 mice per genotype. Panels G and H show the mean ± SD for LN and splenic cholesterol mass, respectively, measured by GC for 4 and 10 wk diet-fed mice and for 10 wk chow-fed mice with 8 to 10 mice per genotype. Unlike letters indicate statistical significance at p<0.05.
Autoimmune disorders typically show enlargement of peripheral LN accompanied by immune cell expansion and the production of autoantibodies. In a mouse model of autoimmunity, Faslpr/lpr, enlargement of both LNs and spleen15, 16 with age has been docummented17, 18. In the current studies we chose to compare DKO and SKO LN immune cell expansion with that in the Faslpr/lpr mouse17, 18. After 10 wk of chow, Faslpr/lpr mice had enlarged LN and spleens (data not shown) that contained a large number of immune cells compared to chow-fed DKO and SKO mice, shown in Figure 1, Panel E. However, DKO mice consuming the atherogenic diet for 10 wks had as many LN cells as 10 wk chow-fed Faslpr/lpr and contained ∼10-fold more LN cells than SKO mice. The atherogenic diet initiated a 2.5-fold increase in the total number of LN cells in Faslpr/lpr mice compared to their chow-fed counterparts, suggesting a cholesterol driven LN cell expansion even in the Faslpr/lpr genetic environment.
Figure 1, Panel G shows that LN enlargement and intense Oil Red O staining was associated with the increase in the mass of LN cholesterol as measured by GC. The increase in DKO LN cholesterol content occurred as early as 4 wk after initiating the diet, while both SKO and Faslpr/lpr mice did not show significant LN cholesterol increase in response to diet.
DKO mice also had enlarged spleens compared to SKO mice, as shown in Figure 1, Panels B and B’. DKO spleens weighed an average of 159.6±69.8 mg, while SKO spleens weighed 91.8±13.7 mg. Despite the enlargement, Oil Red O staining showed minimal lipid accumulation as shown in Figure 1, Panels D and D’. Neither splenocyte number, Figure 1, Panel F, nor GC derived cholesterol content, Figure 1, Panel H, was different between DKO, SKO and Faslpr/lpr mice.
Diet-Induced Expansion of Lymph Nodes Immune Cells
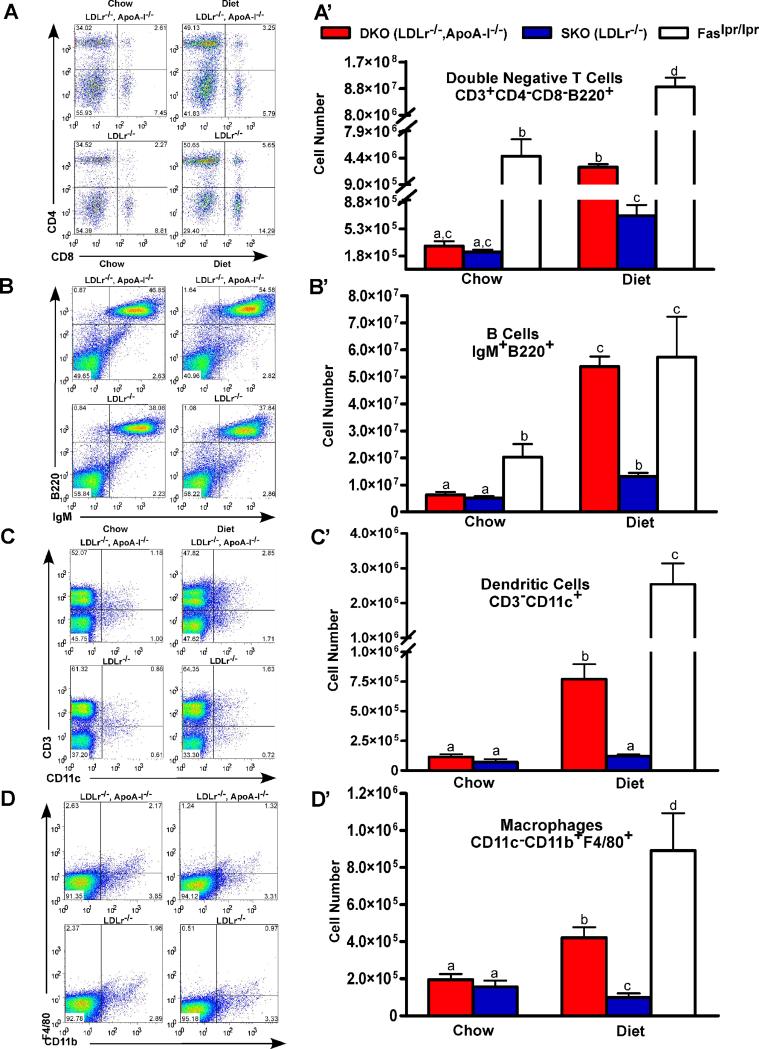
Studies of the effect of the atherogenic diet on specific LN cell populations, Figure 2, dot plots (Panels A-D) and total cell number (Panels A’-D’), are shown for double negative T (DNT) cells (CD3+CD4−CD8−B220+) Panels A and A’; naïve B cells (IgM+B220+), Panels B and B’; DCs (CD3−CD11c+), Panels C and C’; and macrophages (CD11c−CD11b+F4/80+), Panels D and D’. For each cell type, diet-fed DKO LN cell expansion was ∼8-fold higher than chow-fed DKO mice. SKO mice showed a less dramatic ∼2.5-fold increase in response to diet. Although a large number of specific T cell subsets were investigated, including regulatory T cells (data not shown), only DNT cells are shown, since all subsets increased in diet-fed DKO mice. Increased numbers of DNT cells, CD3+CD4−CD8−B220+, are characteristic of the loss of self-tolerance in Faslpr/lpr mice19, 20. Dietary cholesterol consumption increased specific LN cell numbers in both DKO and Faslpr/lpr mice, although Faslpr/lpr mice apparently did not need cholesterol feeding to stimulate expansion.
Figure 2. Expansion of lymph node cell populations.
Panels A to D show dot plots for 4 lymphocyte populations while Panels A’ to D’ show the corresponding total cell numbers, mean ± SD, for 10 wk chow and diet-fed mice. Panels A and A’ show DNT cells (CD3+CD4−CD8−B220+); Panels B and B’ show naïve B cells (IgM+B220+); Panels C and C’ show DCs (CD3−CD11c+); and Panels D and D’ show macrophages (CD11c−CD11b+F4/80+). Faslpr/lpr mice, a model of SLE, were studied for comparison. All dot plots were gated on viable cells with additional gating for the CD3+B220+ cell population in Panel A; and for the CD11c− population in Panel D. Results represent 3 independent experiments with 4 to 5 mice per genotype. Unlike letters indicate statistical significance at p<0.05.
In contrast to the LN cells, splenocyte populations were not different between DKO and SKO mice, as shown in Supplemental Figure II for DNT cells, Panels A and A’; naïve B cells, Panels B and B’; and DCs, Panels C and C’. Only the macrophage population, Panels D and D’, showed significant increases for chow versus diet-fed DKO mice. A simplification of the data contained in Figure 2 and Supplemental Figure II are shown in Supplemental Figure III, Panels A-C for spleen and in Panels D-F for the LN, showing changes in DKO relative to SKO mice for 10wk chow, and 4 and 10 wk atherogenic diet.
LN T and B cells Accumulate Cholesterol Ester in Response to Diet
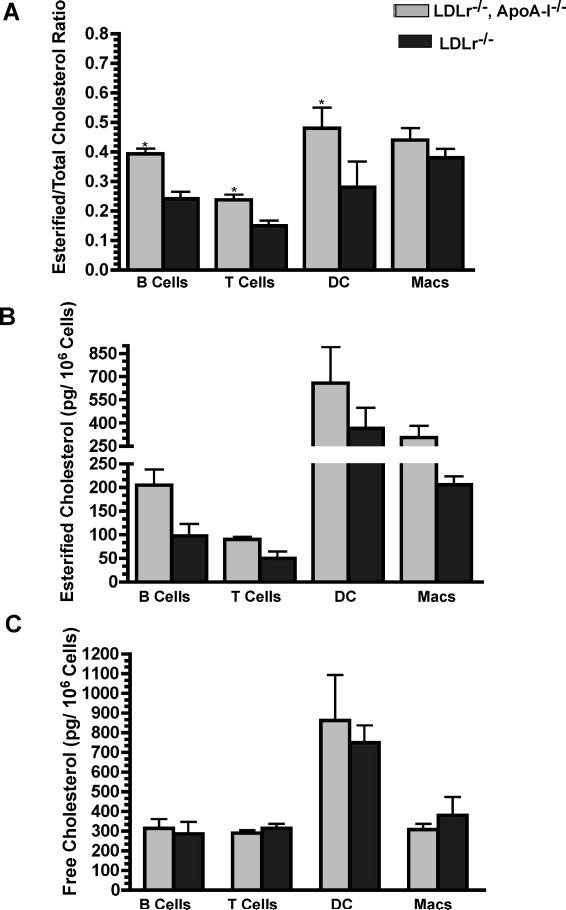
All cell types except macrophages showed a ∼2-fold increase in their esterified cholesterol (EC) content after 10 wks on the atherogenic diet. Remarkably, DC contained the largest mass of TC compared to all other LN cell types. Figure 3, Panel A shows a statistically significant increase in the EC/TC ratio for B, T and dendritic cells in DKO versus SKO mice. Although not statically significant (p<0.08), DKO macrophages also appeared to follow this trend. The increase in the EC/TC ratio was due to an increase in the total mass of EC, shown in Figure 3, Panel B, and not due to a decrease in the mass of free cholesterol, shown in Figure 3, Panel C.
Figure 3. Lymphocyte specific cholesterol and cholesterol ester mass.
Panel A shows the ratio of esterified cholesterol to total cholesterol (EC/TC). The mean ± SD for LN cell populations are plotted in Panels B and C. The masses were determined by GC/MS of sorted LN cells from 10 wk diet-fed DKO and SKO mice. The results are based on 4 independent experiments with 5 mice per genotype. Asterisks indicate statistical significance differences at p<0.05.
T Cell Activation
The activation state and functionality of T cells, CD4+CD69high and CD8+CD69high populations are shown in Supplemental Figure IV, Panels A and B for LNs and Panels C and D for spleen. These data show that the percent of CD69high cells was significantly higher in 10 wk chow-fed Faslpr/lpr mice LNs compared to chow-fed DKO or SKO mice. However, in response to 10 wks of the atherogenic diet both Faslpr/lpr and DKO mice showed ∼1.5-fold increase in the percent of CD69high cells suggesting a heightened activation state in response to dietary cholesterol.
Proliferation of CD4+CD44high Cells
Proliferation of effector CD4+CD44high and CD8+CD44high T cells was studied by measuring BrdU incorporation into LNs and spleens of 10 wk diet-fed DKO and SKO mice. Supplemental Figure V, Top and Bottom Panels A-C, shows the results for LN and spleen for DKO mice which had ∼1.5-fold more CD4+CD44high cells in their LNs, averaging 52.5%, versus 35.0% in SKO mice, while mice not receiving BrdU showed a background of ∼0.1%. BrdU incorporation. For CD8+CD44 high cells, shown in Supplemental Figure V, Top and Bottom Panels D-F, for LN and spleen, respectively, incorporation was similar between genotypes. These data suggest that CD4+CD44high effector T cells in diet-fed DKO mice undergo selective proliferation.
Circulating Autoantibodies in Response to Diet
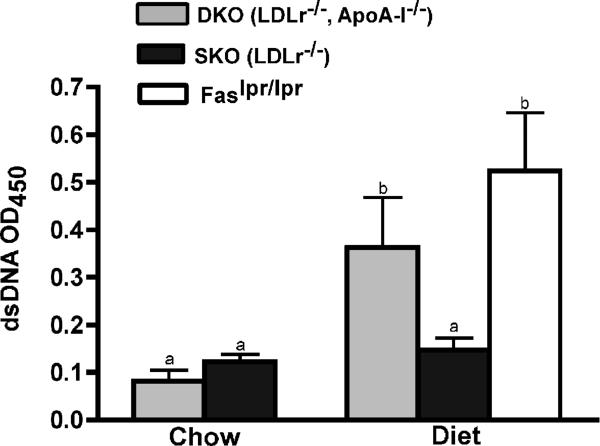
In light of the increased numbers of activated and DNT cells in10 wk diet-fed DKO LNs the development of an autoimmune disorder was considered. Plasma autoantibodies for dsDNA shown in Figure 4, indicates that DKO, like Faslpr/lpr mice, had a 2-fold increase in autoantibodies levels. The relative levels for β2-glycoprotein (β2-GPI) and oxidized LDL (oxLDL) were measured in 10 wk diet-fed DKO, SKO and Faslpr/lpr mice and are shown in Supplemental Figure VI Panels A and B, respectively. These autoantibodies also showed increases similar to that seen in Faslpr/lpr mice.
Figure 4. Dietary cholesterol induction of plasma autoantibodies.
Relative levels of anti-dsDNA in response to an atherogenic diet. Autoantibodies were measured in the plasma from 10 wk chow and diet-fed DKO, SKO and Faslpr/lpr mice. The values represent the mean ± SD of 4 to 12 mice per genotype. Unlike letters indicate statistical significance at p<0.05.
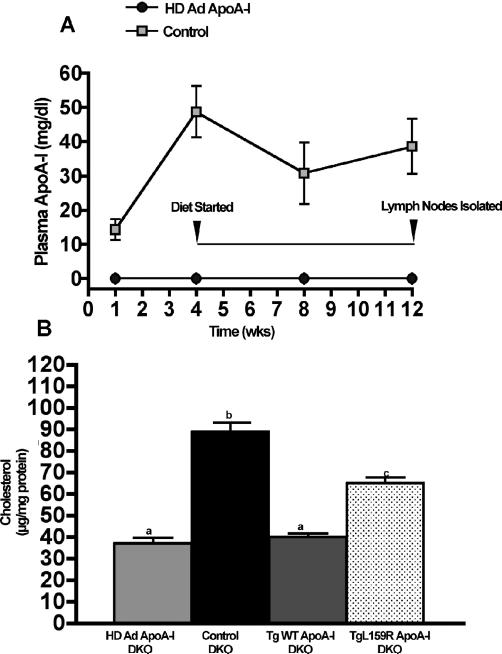
LN Cholesterol Accumulation is Prevented by Plasma ApoA-I
We next tested whether the presence of plasma apoA-I would prevent LN cholesterol accumulation. Four wks prior to starting the atherogenic diet DKO mice were injected with helper-dependent adenovirus expressing human apoA-I (HD Ad ApoA-I)21, 22 while “Control” mice were injected with an equal amount of HD-Ad that did not carry the apoA-I gene. Figure 5, Panel A, shows that the plasma apoA-I levels plateaued by 4 wks and remained constant at ∼40 mg/dl to the end of the study. LN cholesterol content after 8 wks on diet is shown in Figure 5, Panel B. Compared to control vector treated mice, diet-fed DKO mice expressing apoA-I had normal levels of LN cholesterol, similar to TgWT ApoA-I mice that have plasma apoA-I concentrations of ∼110 mg/dl. DKO mice expressing the mutant apoA-I, Tg L159R, have plasma levels of a mutant form of human apoA-I at ∼10 mg/dl23. In these mice LN cholesterol levels were intermediate between control and HD Ad ApoA-I mice, but this low level was sufficient to reduce LN cell expansion. Plasma autoantibodies to dsDNA (data not shown) in diet-fed HD Ad and TgWT ApoA-I mice were similar to those measured in diet-fed SKO mice, while diet-fed control (no ApoA-I) DKO dsDNA autoantibody levels were similar to diet-fed DKO (Figure 4).
Figure 5. Plasma apoA-I prevents cholesterol accumulation in lymph nodes.
Panel A shows the increase in plasma apoA-I concentrations following injection of HD Ad ApoA-I or Control (empty HD vector) 21, 22 into DKO (LDLr−/−, ApoA-I−/−) mice. Mice began the atherogenic diet 4 wk after injection to allow for maximum expression of plasma apoA-I and continued on diet for a total of 8 wks. Panel B shows the LN cholesterol content for the DKO mice, for 8 wk diet-fed DKO mice expressing wild-type human apoA-I (TgWT ApoA-I) and for 8 wk diet-fed DKO mice expressing Tg L159R ApoA-I, a mutant apoA-I that impairs reverse cholesterol transport. LN cholesterol from GC analysis is expressed as the mean ± SD of μg total cholesterol per mg LN protein with 3 to 6 mice per genotype. Unlike letters indicate statistical significance at p<0.05. TgWT ApoA-I in DKO mice have apoA-I plasma concentrations ∼110 mg/dl while DKO mice expressing L159R ApoA-I have plasma apoA-I concentrations ∼10 mg/dl23.
Atherosclerosis Development Is Independent of Plasma Cholesterol
Previous studies have shown that DKO and SKO mice fed diets containing the same amount of dietary cholesterol (0.1% cholesterol) have different total plasma cholesterol (TPC) levels13, 14. SKO mice have a 2.5 fold higher TPC than DKO mice, and yet, the two genotypes had similar levels of aortic cholesterol accumulation13, 14. To quantify atherosclerosis when TPC were similar, the amount of dietary cholesterol fed to each genotype was adjusted based on their differential responses. DKO mice were fed 0.5% cholesterol, 5-fold more than the standard 0.1% diet, while SKO mice were fed the same base diet containing 0.05% cholesterol. Even with these manipulations after 10 wks DKO mice still had a lower TPC, 736±58 mg/dl, than did SKO mice, 1,048±55 mg/dl. Both the VLDL and HDL particle diameters were significantly larger in diet-fed DKO compared to SKO mice, with no significant difference in LDL diameter between genotypes, see Supplemental Table I. The plasma triglyceride level was not statistically different between DKO and SKO mice, 206±85 mg/dl versus 215±55 mg/dl (n=7 mice per genotype). Although HDL particles were larger in size in DKO mice they made up less than 5−10% of the d >1.21 g/ml mass compared to SKO mice. The increased HDL particle diameter likely indicates the presence of LDL/HDL transition particles enriched in apoE13, 14 and devoid of apoA-I.
The extent of atherosclerosis was quantified by both morphometric evaluation of Oil Red O stained aortic root and by measuring the total aortic cholesterol mass. Supplemental Figure VII, Panel A, shows representative Oil Red O sections from the aortic root for each genotype. The percent of lesion for DKO and SKO mice was 42.7±3.8% versus 33.4±3.4%, respectively (mean ± SD of n=5, with p<0.05). These measurements were supported by total aortic cholesterol mass, shown in Supplemental Figure VII, Panel B. Both male and female DKO mice had a statistically significant increase in total aortic cholesterol mass compared to male and female SKO mice. The aortas from DKO mice had a 3-fold increase in IL-1β mRNA after 8 and 16 wks on an atherogenic diet when compared to matched SKO mice and shown in Supplemental Figure VII, Panel C, suggesting a heightened inflammatory state in the DKO aortic environment.
Discussion
Our studies demonstrate that in response to an atherogenic diet DKO mice develop enlarged skin draining LNs that contain expanded populations of CE enriched lymphocytes. The cellular expansion and cholesterol loading affected the major classes of LN cells, including T, B cells, DCs, and to a lesser extent macrophages. Associated with these morphological changes was a 1.5-fold increase in the proliferation of CD4+CD44high T cells, but not CD8+CD44high T cells. Changes in T cell proliferation and activation occurred in conjunction with the production of plasma autoantibodies and inflammatory cell infiltrates in the dermis. In contrast, diet-fed SKO mice did not show an increase in LN size or cellularity even after consuming the atherogenic diet for 24 wks (M.Zabalawi, unpublished observations). However, after long term diet-feeding ∼50% of SKO mice presented skin lesions that were not associated with LN enlargement, cholesterol accumulation, or cellular expansion as seen in diet-fed DKO mice. These studies demonstrate that plasma apoA-I prevented the phenotype by preventing LN enlargement, cholesterol loading, as well as accumulation of skin cholesterol in DKO mice13, 14. Taken together these results suggest a direct link between apoA-I, cholesterol metabolism, inflammation and autoimmunity.
Based on these results we speculate that cholesterol accumulates in skin draining LNs under hypercholesterolemic conditions because of inefficient or nonexistent cellular cholesterol efflux. As cholesterol accumulates in lymphocytes, the lack of apoA-I slows or prevents cholesterol efflux from these cells and eventually leads to disruption in cellular function. Lymphocyte proliferation and activation, as well as cytokine production exacerbates atherosclerosis and eventually leads to loss of self-tolerance and skin pathogenesis. LN immune cells play a vital role in atherosclerosis progression and regression from the formation of foam cells24 to the migration of DCs from atherosclerotic plaques to LNs6, 7, although, the precise role apoA-I plays in these processes remain undefined. T and B cells, as well as DCs, are believed to migrate between atherosclerotic lesions and regional LNs25 and contribute to disease progression and/or regression. In one study, the migration of DCs in diet-fed apoE−/− and LDLr−/− mice showed impaired migration from the skin to the LNs that was reversed by administering apoA-I8.
In autoimmune disorders CD4+T cells are primarily responsible for the pathogenic anti-DNA autoantibody production in Faslpr/lpr mice18. In these studies proliferation of CD4+CD44high T cells, but not CD8+CD44high cells, was similar to that seen in a mouse model of SLE18. In addition, CD69 an early T cell activation marker, involved in signal transduction, cell proliferation and cytokine secretion was higher in SLE patients than in controls 26 and it is also higher in our model. Another significant similarity characteristic of autoimmune disorders in mouse models of SLE was the increase in DNT cells20. The defect in lpr mice is caused by insertion of a retroviral transposon into the second intron of fas that interferes with its ability to bind to the FAS ligand and mediate cell death through apoptosis. LNs in lpr mice enlarge in part from a massive expansion of DNT cells. As more T cells are produced the autoimmune process attacks tissues and organs including the skin, kidney and joints. As in human SLE patients one mechanism of tissue damage involves autoantibody and immune complex deposition, as well as increased infiltration of select tissues by lymphocytes19.
Both human and animal studies have shown a convincing link between autoimmune disorders and atherosclerosis with a number of autoimmune “mouse models” exhibiting advanced atherosclerosis10, 11, 27. Stanic et al. showed that in a mouse model of autoimmunity, atherosclerotic progression was worsened by the lack of self-tolerance11, while Gu et al., using quantitative trait analysis of the MRL/lpr autoimmune mouse, provided further evidence for a link between autoimmunity and HDL metabolism28. In human patients suffering from autoimmune disorders HDL levels are significantly decreased12, 29, 30.
In light of these connections we compared the extent of atherosclerosis between SKO and DKO mice. In a previous study, DKO and SKO mice were fed 0.1% cholesterol and 10% palm oil for 16 wks14. This diet produced a 2.5-fold higher TPC in SKO mice compared to DKO mice, however, despite the large difference in TPC, both genotypes developed similar atherosclerosis13, 14. In an attempt to adjust TPC levels DKO mice were fed 10-fold more dietary cholesterol than SKO mice. Under these conditions SKO mice still had ∼ 1.5 fold higher plasma cholesterol, but the DKO mice developed ∼2-fold greater atherosclerosis clearly demonstrating the protective effects of HDL apoA-I.
Although DKO mice share several common characteristics with autoimmune mouse models including skin lesions31 and advanced atherosclerosis, one aspect which remains unusual was the enormous accumulation of cholesterol in the skin. After 12−16 wks on diet13, 14 diet-fed DKO mice die of inflammation induced by the massive skin cholesterol accumulation and its resulting pathogenesis as opposed to the renal failure and glomerulonephritis seen in 28−30 wk chow-fed Faslpr/lpr mice. The presence of activated T cells in DKO LN and skin (A. Wilhelm, unpublished observations) suggests that skin is the primary site of pathogenesis in DKO mice.
DKO mice accumulate 2.5-fold more whole body cholesterol compared to SKO mice13 fed the same diet, with skin being the primary site of cholesterol accumulation13. However, the accumulation of cholesterol in the skin of diet-fed DKO mice is not unique, but similar in magnitude to that reported for diet-fed LXRα−/−,apoE−/− mice that had a 2.5-fold increase in whole body cholesterol compared to apoE−/− only mice with lipid accumulation occurring predominately in the skin32. This unusual phenotype has been previously described in other mouse models that carry disruptions in cholesterol homeostatic genes including, e.g., ACAT1−/−,LDLr−/−33 and ABCA1−/−,LDLr−/−3, 34, suggesting that disruption in immune cell cellular cholesterol mobilization may be a common mechanism leading to the skin cholesterol accumulation phenotype. This is in contrast to apoC-I transgenic mice, where apoC-I modulates plasma triglyceride metabolism and was associated with severe skin lesions, infiltration of inflammatory cells, but no skin cholesterol accumulation35. Other cholesterol homeostatic models, such as in ABCG1−/− mice show severe cholesterol accumulation, but predominately in the lungs4, 36, 37. In ABCG1−/− mice this was accompanied by an increase in inflammatory cells and cytokines in the lung that were largely absent from the plasma compartment36. Despite this, the skin is a common tissue affected in autoimmune disorders such as SLE31, 38, 39, but these lesions are not usually associated with cholesterol accumulation. Thus, it remains to be determined if cholesterol loaded inflammatory cells enter the skin in response to a specific stimulus or if a constant infiltration of cholesterol in the skin via LDL causes lymphocyte migration and activation, thereby triggering the resulting phenotype.
In conclusion, we have demonstrated that apoA-I prevents lymphocyte cholesterol accumulation, activation and proliferation in skin draining LNs of diet-fed DKO mice. An increase in T cell activation and proliferation was linked to increased levels of circulating autoantibodies and a ∼2-fold increase in aortic cholesterol accumulation. These results strongly suggest a common link between plasma apoA-I, inflammation and atherosclerosis.
Acknowledgments
Sources of Funding
M.S.T. was supported by NIH HL-49373 and HL-64163; A.S.M. was supported by NIH HL-089310 and The Lupus Research Institute, New York, NY; J.M.G. was supported by an American Cancer Society Research Scholar Grant #RSG-04-066-01 MBC and NIH A1-068952. A.J.W received an NIH T32 Kirschtein NRSA HL091797-01. We grateful acknowledge the Texas AgriLife Research project #8738 to R.L.W. The generation of HD Ad ApoAI was supported by grants HL-51586 to L.C. and HL-73144 to K.O. The Trace MS was purchased with funds from the NC Biotechnology Shared Instrumentation Program and the Winston-Salem Foundation.
Footnotes
Disclosures
None
References
- 1.Gordon T, Castelli W, Hjortland M, Kannel W, Dawber T. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Zannis V, Chroni A, Krieger M. Role of apoA-I, ABCA-I, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 3.Aiello RJ, Brees D, Bourassa P-A, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased Atherosclerosis in Hyperlipidemic Mice With Inactivation of ABCA1 in Macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 4.Baldan A, Tarr P, Vales CS, Frank J, Shimotake TK, Hawgood S, Edwards PA. Deletion of the Transmembrane Transporter ABCG1 Results in Progressive Pulmonary Lipidosis. J. Biol. Chem. 2006;281:29401–29410. doi: 10.1074/jbc.M606597200. [DOI] [PubMed] [Google Scholar]
- 5.Lo JC, Wang Y, Tumanov AV, Bamji M, Yao Z, Reardon CA, Getz GS, Fu YX. Lymphotoxin {beta} Receptor-Dependent Control of Lipid Homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 6.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. PNAS. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 8.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia Associated with Atherosclerotic Disease Systemically Alters Dendritic Cell Mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121:S3–8. doi: 10.1016/j.amjmed.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X, Li H, Rumbin AA, Wang X, La Cava A, Brechtelsbauer K, Castellani LW, Witztum JL, Lusis AJ, Tsao BP. ApoE−/−Fas−/− C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J. Lipid Res. 2007;48:794–805. doi: 10.1194/jlr.M600512-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Stanic AK, Stein CM, Morgan AC, Fazio S, Linton MF, Wakeland EK, Olsen NJ, Major AS. Immune dysregulation accelerates atherosclerosis and modulates plaque composition in systemic lupus erythematosus. PNAS. 2006;103:7018–7023. doi: 10.1073/pnas.0602311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bresnihan B, Gogarty M, FitzGerald O, Dayer J- M, Burger D. Apolipoprotein A-I infiltration in rheumatoid arthritis synovial tissue: a control mechanism of cytokine production? Arthritis Res Ther. 2004;6:R563–R566. doi: 10.1186/ar1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabalawi M, Bharadwaj M, Horton H, Cline M, Willingham M, Thomas MJ, Sorci-Thomas MG. Inflammation and skin cholesterol in LDLr−/−, apoA-I−/− mice: link between cholesterol homeostasis and self-tolerance? J. Lipid Res. 2007;48:52–65. doi: 10.1194/jlr.M600370-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Zabalawi M, Bhat S, Loughlin T, Thomas MJ, Alexander E, Cline M, Bullock B, Willingham M, Sorci-Thomas MG. Induction of Fatal Inflammation in LDL Receptor and ApoA-I Double-Knockout Mice Fed Dietary Fat and Cholesterol. Am J Pathol. 2003;163:1201–1213. doi: 10.1016/S0002-9440(10)63480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis Regulators Bim and Fas Function Concurrently to Control Autoimmunity and CD8+ T Cell Contraction. Immunity. 2008 doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Katagiri T, Cohen P, Eisenberg R. The lpr gene causes an intrinsic T cell abnormality that is required for hyperproliferation. J. Exp. Med. 1988;167:741–751. doi: 10.1084/jem.167.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabs DA, Kuppers RC, Saboori AM, Burek CL, Enger C, Lee B, Prendergast RA. Effects of early and late treatment with anti-CD4 monoclonal antibody on autoimmune disease in MRL/MP-lpr/lpr mice. Cell Immunol. 1994;154:66–76. doi: 10.1006/cimm.1994.1057. [DOI] [PubMed] [Google Scholar]
- 18.Giese T, Davidson WF. Evidence for early onset, polyclonal activation of T cell subsets in mice homozygous for lpr. J Immunol. 1992;149:3097–3106. [PubMed] [Google Scholar]
- 19.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford MS, Young KJ, Zhang Z, Ohashi PS, Zhang L. The Immune Regulatory Function of Lymphoproliferative Double Negative T Cells In Vitro and In Vivo. J. Exp. Med. 2002;196:261–267. doi: 10.1084/jem.20020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastore L, Belalcazar LM, Oka K, Cela R, Lee B, Chan L, Beaudet AL. Helper-dependent adenoviral vector-mediated long-term expression of human apolipoprotein A-I reduces atherosclerosis in apo E-deficient mice. Gene. 2004;327:153–160. doi: 10.1016/j.gene.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Belalcazar LM, Merched A, Carr B, Oka K, Chen K- H, Pastore L, Beaudet A, Chan L. Long-Term Stable Expression of Human Apolipoprotein A-I Mediated by Helper-Dependent Adenovirus Gene Transfer Inhibits Atherosclerosis Progression and Remodels Atherosclerotic Plaques in a Mouse Model of Familial Hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 23.Owen JS, Bharadwaj MS, Thomas MJ, Bhat S, Samuel MP, Sorci-Thomas MG. Ratio determination of plasma wild-type and L159R apoA-I using mass spectrometry: tools for studying apoA-IFin. J. Lipid Res. 2007;48:226–234. doi: 10.1194/jlr.D600031-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Ludewig B, Laman JD. The in and out of monocytes in atherosclerotic plaques: Balancing inflammation through migration. PNAS. 2004;101:11529–11530. doi: 10.1073/pnas.0404612101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 26.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 27.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ, Jr., Walsh K. Impaired Clearance of Apoptotic Cells Promotes Synergy between Atherogenesis and Autoimmune Disease. J. Exp. Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu L, Johnson MW, Lusis AJ. Quantitative Trait Locus Analysis of Plasma Lipoprotein Levels in an Autoimmune Mouse Model : Interactions Between Lipoprotein Metabolism, Autoimmune Disease, and Atherogenesis. Arterioscler Thromb Vasc Biol. 1999;19:442–453. doi: 10.1161/01.atv.19.2.442. [DOI] [PubMed] [Google Scholar]
- 29.Frostegard J. Autoimmunity, oxidized LDL and cardiovascular disease. Autoimmunity Reviews. 2002;1:233–237. doi: 10.1016/s1568-9972(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 30.Hyka N, Dayer J- M, Modoux C, Kohno T, Edwards CK., III Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1{beta} and tumor necrosis factor-{alpha} by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 31.Umeuchi H, Kawashima Y, Aoki CA, Kurokawa T, Nakao K, Itoh M, Kikuchi K, Kato T, Okano K, Gershwin ME, Miyakawa H. Spontaneous scratching behavior in MRL/lpr mice, a possible model for pruritus in autoimmune diseases, and antipruritic activity of a novel [kappa]-opioid receptor agonist nalfurafine hydrochloride. Eur J Pharmacol. 2005;518:133–139. doi: 10.1016/j.ejphar.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR-beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR-alpha and apoE. J Clin Invest. 2007;118:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Accad M, Smith SJ, Newland DL, Sanan DA, King LE, Jr., Linton MF, Fazio S, Farese RVJ. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J Clin Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiello RJ, Brees D, Francone OL. ABCA1-Deficient Mice: Insights Into the Role of Monocyte Lipid Efflux in HDL Formation and Inflammation. Arterioscler Thromb Vasc Biol. 2003;23:972–980. doi: 10.1161/01.ATV.0000054661.21499.FB. [DOI] [PubMed] [Google Scholar]
- 35.Jong MC, Gijbels MJJ, Dahlmans VEH, van Gorp PJJ, Koopman S- J, Ponec M, Hofker MH, Havekes LM. Hyperlipidemia and Cutaneous Abnormalities in Transgenic Mice Overexpressing Human Apolipoprotein C1. J Clin Invest. 1998;101:145–152. doi: 10.1172/JCI791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A Critical Role for ABCG1 in Macrophage Inflammation and Lung Homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 37.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 Results in Chronic Pulmonary Inflammation. J Immunol. 2008;180:3560–3568. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa M, Fujimoto M, Takehara K, Sato S. Pathogenesis of systemic sclerosis: Altered B cell function is the key linking systemic autoimmunity and tissue fibrosis. Journal of Dermatological Science. 2005;39:1–7. doi: 10.1016/j.jdermsci.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Zoller M, Gupta P, Marhaba R, Vitacolonna M, Freyschmidt-Paul P. Anti-CD44-mediated blockade of leukocyte migration in skin-associated immune diseases. J Leukoc Biol. 2007;82:57–71. doi: 10.1189/jlb.0107063. [DOI] [PubMed] [Google Scholar]