Abstract
Functional imaging studies of single word production have consistently reported activation of the lateral prefrontal and cingulate cortex. Its contribution has been shown to be sensitive to task demands, which can be manipulated by the degree of response specification. Compared with classical verbal fluency, free word association relies less on response restrictions but to a greater extent on associative binding processes, usually subserved by the hippocampus. To elucidate the relevance of the frontal and medial-temporal areas during verbal retrieval tasks, we applied varying degrees of response specification. During fMRI data acquisition, 18 subjects performed a free verbal association (FVA), a semantic verbal fluency (SVF) task, and a phonological verbal fluency (PVF) task. Externally guided word production served as a baseline condition to control for basic articulatory and reading processes. As expected, increased brain activity was observed in the left lateral and bilateral medial frontal cortices for SVF and PVF. The anterior cingulate gyrus was the only structure common to both fluency tasks in direct comparison to the less restricted FVA task. The hippocampus was engaged during associative and semantic retrieval. Interestingly, hippocampal activity was selectively evident during FVA in direct comparison to SVF when it was controlled for stimulus–response relations. The current data confirm the role of the left prefrontal–cingulate network in constrained word production. Hippocampal activity during spontaneous word production is a novel finding and seems to be dependent on the retrieval process (free vs. constrained) rather than the variety of stimulus–response relationships that is involved.
INTRODUCTION
The production of words in response to an external stimulus (e.g., a letter, a word, or a picture) can be manipulated by the degree of voluntary or intrinsic processes involved. Intrinsic word generation (e.g., verbal fluency, noun or verb generation, picture naming) demands conscious selection among candidate verbal responses (Frith, Friston, Liddle, & Frackowiak, 1991; Levelt, 1989). As a consequence, word selection demands can be influenced by the constraints on the desired response, that is, its response specification. In contrast, no internally driven decision needs to be made during extrinsic production because its response is completely specified by an external cue (e.g., during reading or repetition). Studies investigating the impact of intrinsic compared with extrinsic word production are frequently reported in the literature (e.g., Basho, Palmer, Rubio, Wulfeck, & Mueller, 2007; Alario, Chainay, Lehericy, & Cohen, 2006; Blacker, Byrnes, Mastaglia, & Thickbroom, 2006; Tremblay & Gracco, 2006; Fu et al., 2002; Crosson et al., 2001; Blank, Scott, Murphy, Warburton, & Wise, 2000; Pihlajamäki et al., 2000; Schlösser et al., 1998; Pujol et al., 1996). These studies have shown that the inferior frontal cortex subserves executive aspects of semantic processing, including semantic search and selection among items, retrieving the word from semantic memory and keeping the retrieved information in verbal working memory for subsequent manipulation (for recent reviews, see Indefrey & Levelt, 2004; Bookheimer, 2002; Cabeza & Nyberg, 2000; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997). Increases in prefrontal activation for more difficult tasks are often interpreted as reflecting higher effort during language performance in healthy subjects (Fu et al., 2002; Roskies, Fiez, Balota, Raichle, & Petersen, 2001). Also, patients with language dysfunctions display enhanced prefrontal activation compared with healthy control groups (Bonner-Jackson, Haut, Csernansky, & Barch, 2005; Fu et al., 2005; Kubota et al., 2005; Heckers et al., 1998). Likewise the anterior cingulate gyrus shows attenuated activity as word generation demands are reduced (Fu et al., 2002; Crosson et al., 2001). This region is consistently associated with interrelated control functions, such as initiation, inhibition, attention, and selection during a variety of tasks, for example, semantic generation, working, or episodic memory (for an extensive review, see Cabeza & Nyberg, 2000). In general, the complexity of cognitive operations during single word production is likely to enhance BOLD-signal changes in lateral and medial frontal regions. Whether reduced task demands due to a relative lack of internal response restrictions (e.g., during free word association) result in attenuated prefrontal activity has not been investigated yet.
Unlike verbal fluency tasks, which pose a rather artificial demand on language production processes [e.g., “name an instance of the following category” (semantic fluency); “provide a word beginning with a specific letter” (phonological fluency)], the generation of spontaneous associations seems to be a more natural, ecologically valid task. Associated concepts are constantly and automatically activated while we read or speak (Levelt, 1989; Collins & Loftus, 1975), without being constrained to specific types of relations between the stimulus (a written, a spoken, or a heard word) and its response. Semantic priming tasks have been used to identify such implicit associative processes, and they have revealed that word retrieval is faster and less effortful for words that are highly related, independent of the kind of stimulus–response relationship (for reviews, see Hutchison, 2003; Neely, 1976, 1991). When the generation of associations demands explicit knowledge retrieval [e.g., during free verbal association (FVA) tasks], other work has also shown that associations are formed according to a variety of principles, which include taxonomic (hierarchical) or thematic (e.g., spatio-temporal cooccurrence) relations between words, phonological similarity (e.g., rhyming), or resemble individual experiences or memories being retrieved upon stimulation (Jung & Ricklin, 1906).
The brain region subserving the binding of items based on various aspects (e.g., spatial, temporal, or autobiographical relations) during explicit knowledge retrieval is the hippocampus. Most of the evidence for its involvement in associative processes is provided by mnemonic studies, investigating healthy volunteers (e.g., Whatmough & Chertkow, 2007; Heckers & Titone, 2005; Prince, Daselaar, & Cabeza, 2005; Henke et al., 2003; Simons & Spiers, 2003; Eichenbaum, Otto, & Cohen, 1994) or patients with hippocampal lesions who demonstrate a selective impairment of associative over item memory (e.g., Gold, Hopkins, & Squire, 2006; Mayes et al., 2004; Davachi & Wagner, 2002; Spiers, Maguire, & Burgess, 2001). Participation of the hippocampus during explicit word production (Whatmough & Chertkow, 2007; Pihlajamäki et al., 2000) and semantic tasks (Bartha et al., 2003; Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996) has also been reported. The relevance of the hippocampus during spontaneous word generation and the contribution of lateral prefrontal areas of the semantic network during this task have not been explored.
The aim of the current study was to isolate the impact of response specification onto fronto-hippocampal structures during intrinsic word production. Therefore, we manipulated the response constraints in three different intrinsic word generation tasks, with FVA posing less restrictions on word generation than semantic verbal fluency (SVF) and phonological verbal fluency (PVF). Consistent with the findings in the literature, we hypothesized prefrontal activity for both verbal fluency tasks as well as increased prefrontal activity during verbal fluency tasks compared with spontaneous association due to increased selection and control functions during word retrieval. Activation of the hippocampus was particularly expected during the generation of spontaneous verbal associates because of the task’s high demand on associative binding. To control for confounds of different stimulus–response relationships in the direct comparison of FVA and SVF, we also categorized responses during FVA into those describing superordinate–subordinate relations to match those responses produced during SVF.
METHODS
Participants
Twenty-one male subjects took part in the fMRI study. Due to motion-related image artifacts, the data from three subjects were discarded. Image data from the remaining 18 subjects were analyzed. All participants (M age = 27.89 years, SD = 7.61; M years of education = 14.06 years, SD = 2.26) were native German speakers, right-handed according to the Edinburgh Inventory of Handedness (Annett, 1970), and showed average or above-average verbal IQ as assessed by the Mehrfachwahlwortschatz test (Lehrl, Triebig, & Fischer, 1995) (M estimated verbal IQ = 120.83, SD = 15.55). Subjects were excluded on grounds of recent substance use, neurological disorders, or known medical disorders that affect cerebral metabolism or general MRI incompatibility. All participants had normal or corrected-to-normal vision, gave informed consent, and were paid €20 for participation in the study. The study was approved by the local ethics committee.
Tasks and Stimuli
In three separate scanning sessions, subjects overtly performed an FVA, an SVF, and a PVF task while BOLD-signal changes were measured with fMRI. In response to a visually presented German noun, subjects had to generate the first word that came to mind (FVA), generate a category member (SVF), or read a word aloud as a high-level baseline condition for both tasks, FVA and SVF. During PVF, participants had to produce a German word beginning with a certain letter. As a baseline task for PVF, subjects said the word “pause” (German translation: “Pause”) whenever the letter “X” appeared. Although no restrictions on the type of response were given during the association task, that is, participants were allowed to utter nouns, verbs, adjectives, or even complete phrases, the responses for both fluency tasks had to conform to certain rules. During PVF, word derivations and grammatical inflections were prohibited as well as repetitions and individual names or labels.
An initial word association study was performed as a pretest (n = 30 subjects), using a group of 140 German superordinate nouns for FVA and the respective baseline condition. Subjects had to say the first three words that came to mind in response to each of the 140 nouns, which were presented individually on a computer screen. From these nouns, items were excluded that evoked only category members (n = 14). Out of the remaining 126 items, stimuli sets for the fMRI version of the FVA task (80 items) and the control condition (40 items) were arranged such that between-group analyses revealed no significant differences according to word frequency, as assessed by the CELEX database (Baayen, Piepenbrock, & van Rijn, 1993) (M FVA = 65.78, SD = 143.55; M FVA_read = 83.50, SD = 195.55; t(118) = −0.56, p = .58), length in letters (M FVA = 6.41, SD = 2.10; M FVA_read = 6.85, SD = 2.19; t(118) = −1.06, p = .29), and length in syllables (M FVA = 2.0, SD = 0.84; M FVA_read = 2.0, SD = 0.82; t(118) < 0.001, p = 1.0). Additionally, concreteness, imageability, and emotional content were rated by a second group of volunteers (n = 10) on a 4-point scale. Again, no differences between the two stimuli sets were found (concreteness: M FVA = 2.07, SD = 0.72; M FVA_read = 2.0, SD = 0.45; t(118) = 0.58, p = .56; imageability: M FVA = 1.93, SD = 0.62; M FVA_read = 1.95, SD = 0.52; t(118) = −0.20, t(118) = −0.73, p = .84; emotion: M FVA = 2.93, SD = 0.61; M FVA_read = 3.02, SD = 0.69; p = .47). Following the same matching procedure, stimuli sets for SVF (40 items) and the respective control condition (40 items) were created. Importantly, the number of stimuli in the FVA (80 nouns) compared with the SVF (40 nouns) condition was doubled to gain a sufficient number of FVA responses denoting category members. This was essential to avoid confounds of stimulus–response relationships in the direct comparison of FVA and SVF (see response-dependent FVA analysis). No items were used for FVA, which were included into the SVF set and vice versa. For the phonological fluency task, eight high-frequent letters were chosen (S, W, D, B, H, E, A, and F). Each letter was repeated five times consecutively. Examples of stimuli used in the activation and baseline tasks and example responses for each of the three word generation tasks are shown in Table 1.
Table 1.
Examples of Stimuli and Appropriate Responses for FVA, SVF, and PVF and the Corresponding Baseline Conditions (FVA_read, SVF_read, PVF_repeat), Respectively
| Task | Stimulus | Response |
|---|---|---|
| FVA | Tree | “leaf,” “oak,” “garden,…” |
| FVA_read | Artist | “artist” |
| SVF | Vegetable | “tomato,” “carrot,” “potato, …” |
| SVF_read | Musician | “musician” |
| PVF | D | “dumb,” “donut,” “drain, …” |
| PVF_repeat | X | “pause” |
Only one response per cue was allowed.
In addition, recognition and recall of the stimuli and corresponding responses was tested in an independent sample of 10 naïve subjects. Each participant performed the FVA and the SVF tasks identically to the one presented in the fMRI study but without the interleaved control condition, that is, reading. Immediately afterwards, each participant was given a word list containing all previously presented words (targets) and the same number of novel items (foils). All foils and targets were German superordinates matched for word frequency, length in letters and syllables within (comparisons target vs. foils: frequency_FVA, p > .7; syllables_FVA, p > .1; letters_FVA, p > .1; frequency_SVF, p > .6; syllables_SVF, p > .07; letters_SVF, p > .3) and between conditions (comparisons FVA vs. SVF: frequency_targets, p > .5; syllables_targets, p = 1.0; letters_targets, p > .2; frequency_foils, p > .8; syllables_foils, p > .5; letters_foils, p > .3). To assess their recognition performance, we instructed subjects to indicate whether they had seen the word before on the computer screen (“old”) or not (“new”). If the word was recognized as “old,” subjects were instructed to write down the response they had given earlier during FVA or SVF. Participants were 89% correct overall for FVA [FVA: discriminability (d′) = 2.58; hit rate = 0.81; false alarm rate = 0.04] and 93% correct for SVF [SVF: discriminability (d′) = 2.79; hit rate = 0.91; false alarm rate = 0.05] in the recognition test and 85% correct overall for FVA [FVA: discriminability (d′) = 2.63; hit rate = 0.71; false alarm rate = 0.04] and 92% correct for SVF [SVF: discriminability (d′) = 2.65; hit rate = 0.87; false alarm rate = 0.08] in the recall test. Comparing FVA and SVF, participants differed only with respect to the hit rate of both recognition [t(18) = −2.71, p < .02] and recall [t(18) = −3.17, p < .01].
fMRI Procedure
An ABAB blocked design was used to present activation (FVA, SVF, and PVF) and baseline (reading and repetition) conditions alternately. The tasks were counterbalanced across subjects to avoid sequence effects. However, FVA was always performed prior to SVF because we wanted to avoid a bias toward generating category members during spontaneous association and, thereby, enhance different verbal retrieval processes during the two very similar tasks. At the beginning of each block, an instruction slide was shown for 2000 msec (FVA: “generate a word”; SVF: “generate a category member”; PVF: “generate a word to the letter”; FVA_read/SVF_read: “read the word”; PVF_repeat: “say pause”). Then a fixation cross appeared in the center of the screen for 500 msec, which was followed by the stimulus word for 3000 msec. The participant was required to respond within this time window. A fixation cross shown immediately after the stimulus for 500 msec indicated the appearance of the following stimulus. Each block consisted of 10 stimuli. Appearance of the # symbol for 6000 msec indicated the end of each block.
Presentation of stimuli was controlled by a computer using the Presentation 10.1 software package (Neurobehavioral Systems, http://www.neurobs.com/). MRI-compatible goggles (VisuaStim XGA, Resonance Technology, Inc., http://www.mrivideo.com/) were used for stimuli presentation. All overt verbal responses were recorded (Commander XG, Resonance Technology, http://www.mrivideo.com/), filtered (Digidesign Pro Tools | HD), and transcribed. Subjects were instructed to speak clearly and to respond as fast as possible. Errors and misses were excluded from later event-related analysis.
Before the fMRI experiment and at the beginning of each session, all subjects performed 10 practice trials and were given feedback. All items used for training differed from the experimental stimuli used in the fMRI version.
MRI Acquisition
Imaging was performed at 1.5 T (Gyroscan Intera, Philips Medical Systems, Best, The Netherlands) using standard gradients and a circularly polarized phase array head coil. For each subject, we acquired three series of functional volumes of T2*-weighted axial EPI scans parallel to the AC–PC line with the following parameters: number of slices (NS), 31; slice thickness (ST), 3.5 mm; inter-slice gap (IG), 0.35 mm; matrix size (MS), 64 × 64; field of view (FOV), 240 × 240 mm; echo time (TE), 30 msec; repetition time (TR), 2.8 sec. One run each was acquired for FVA, SVF, and PVF. One hundred eighty-five functional volumes were acquired in total for FVA and 126 functional volumes each for the fluency tasks (SVF, PVF).
fMRI Data Analysis
MR images were analyzed using Statistical Parametric Mapping software (SPM2; www.fil.ion.ucl.ac.uk) implemented in MATLAB 6.5 (Mathworks Inc., Sherborn, MA). After discarding the first three volumes, all images were realigned to the first image to correct for head movement. Unwarping was used to correct for the interaction of susceptibility artifacts and head movement. After realignment and unwarping, the signal measured in each slice was shifted relative to the acquisition time of the middle slice using a sinc interpolation in time to correct for their different acquisition times. Volumes were then normalized into standard stereotaxic anatomical MNI space by using the transformation matrix calculated from the first EPI scan of each subject and the EPI template. Afterwards, the normalized data with a resliced voxel size of 4 × 4 × 4 mm were smoothed with a 10-mm full-width at half-maximum isotropic Gaussian kernel to accommodate intersubject variation in brain anatomy. The time series data were high-pass filtered with a high-pass cutoff of 1/128 Hz. The autocorrelation of the data was estimated and corrected for.
The expected hemodynamic response at stimulus onset for each event type (FVA, SVF, PVF, and the respective control conditions) was modeled by two response functions, a canonical hemodynamic response function (HRF; Friston et al., 1998) and its temporal derivative. The temporal derivative was included in the model to account for the residual variance resulting from small temporal differences in the onset of the hemodynamic response, which is not explained by the canonical HRF alone. The functions were convolved with the event train of stimulus onsets to create covariates in a general linear model. The VOI was restricted to gray-matter voxels by use of an inclusive mask created from the segmentation of the standard brain template (SPM2). Subsequently, parameter estimates of the HRF regressor for each of the different conditions were calculated from the least mean squares fit of the model to the time series. Parameter estimates for the temporal derivative were not further considered in any contrast.
An SPM2 random-effects group analysis was performed by entering parameter estimates for all conditions (FVA, FVA_read, SVF, SVF_read, PVF, and PVF_repeat) into a within-subject one-way ANOVA.
Response-dependent FVA Analysis
To directly compare SVF and FVA and, therefore, identify retrieval (free vs. restricted) rather than response-type-dependent processes, we classified the responses produced during FVA into those denoting category members (FVA_cat) and others (FVA_other). For example, the association “bone” in response to the cue word “dog” was classified as FVA_other, whereas the response “oak” given the cue “tree” was classed as FVA_cat because the participant produced a subordinate of the category “tree” (like in SVF). We included only those individuals in the response-dependent FVA analysis who produced a sufficient number of category members (≥20) during the association task. Within that subgroup, the two response classes FVA_cat and FVA_other were modeled separately. As a consequence, the within-subject one-way ANOVA was performed with the association task being represented by three conditions (FVA_cat, FVA_other, and FVA_read).
All main effects of condition (FVA, SVF, and PVF) relative to the corresponding baseline task were corrected for multiple comparisons [familywise error (FWE)] at p < .05. The same threshold was applied for the conjunction analyses. More complex differential contrasts, describing interactions, were reported on an uncorrected level of p < .001. All reported brain activation exceeded a voxel cluster of k ≥ 10 contiguous voxels.
The reported voxel coordinates of activation peaks were transformed from MNI space to Talairach and Tournoux (1988) atlas space by nonlinear transformations (www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html).
Behavioral Analysis
Speech Onset Latency Analysis
Speech onsets were determined individually for each subject and each event by visual and auditory inspection of the filtered sound waves (Adobe Audition 1.5, http://www.adobe.com/). Speech onset latencies were defined over the difference between speech and stimulus presentation onset for each condition, activation, and control, separately. Errors (i.e., reading the word aloud during the intrinsic word generation tasks, violating the response criteria for verbal fluency), misses, and outliers (±2 SD in response latency away from the subject’s individual mean) were excluded from further analysis. A repeated measures ANOVA with task (FVA, SVF, and PVF) and control (control+ and control −) as within-subject factors was performed.
RESULTS
Behavioural Results
Speech Onset Latency
Due to errors, misses, and outliers, 4.4% of the FVA data, 5.3% of the SVF data, and 7.4% of the PVF data had to be discarded. The repeated measures ANOVA of the speech onset latencies revealed highly significant main effects of task [F(2, 34) = 77.78, p < .001] and control [F(1, 17) = 185.08, p < .001] and a Task × Control interaction [F(2, 34) = 14.313, p < .001]. Post hoc analysis (paired t tests) showed that subjects were fastest during PVF and showed comparable performance during FVA and SVF [t(17) = 1.730, p > .1]. With respect to the control tasks, response latencies increased from PVF_repeat to SVF_read to FVA_read [PVF vs. SVF: t(17) = 5.193, p < .001; SVF vs. FVA: t(17) = 2.419, p = .027; PVF vs. FVA: t(17) = 4.325, p < .001]. In general, participants responded more slowly in the activation task compared with the respective control condition [FVA: t(17) = 9.360, p < .001; SVF: t(17) = 11.891, p < .001; PVF: t(17) = 2.492, p = .023] (Table 2).
Table 2.
Speech Onset Latencies for FVA, SVF, and PVF and the Corresponding Baseline Conditions
| Task | Onset Latency (msec) | SD (msec) |
|---|---|---|
| FVA | 1682.83 | 248.37 |
| FVA_read | 985.77 | 207.88 |
| SVF | 1566.22 | 164.86 |
| SVF_read | 842.26 | 175.32 |
| PVF | 925.49 | 336.50 |
| PVF_repeat | 685.90 | 186.01 |
Imaging Data
FVA
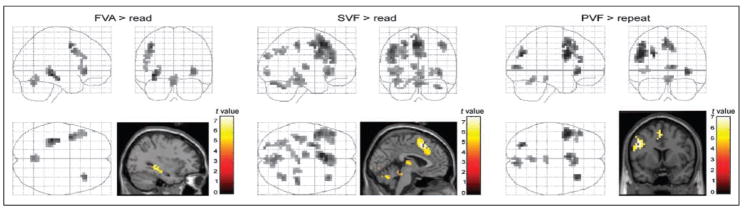
FVA compared with reading revealed peak activation in the left hippocampus, accompanied by frontal BOLD enhancements in the left ventral premotor area (vPMA), the left inferior frontal gyrus (IFG) (BA 44, 45), and the anterior portion of the right insula. Additional activation was found in the cerebellar vermis (see Table 3 and Figure 1).
Table 3.
Brain Activation for the Main Effects of FVA, SVF, and PVF Relative to the Corresponding Baseline Tasks in 18 Healthy Male Individuals
| FVA > Read |
SVF > Read |
PVF > Repeat |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral Area | BA | x | y | z | Z | Cl | BA | x | y | z | Z | Cla | BA | x | y | z | Z | Cla |
| Frontal | ||||||||||||||||||
| vPMA | 6/9 | −40 | 2 | 40 | 6.01 | 10 | 6/9 | −40 | 2 | 40 | 5.79 | 101 | 6 | −44 | 6 | 44 | 5.83 | 86 |
| IFG | 44 | −44 | 9 | 33 | 5.48 | 32 | 44 | −48 | 9 | 33 | 6.14 | 44 | −48 | 9 | 25 | 6.32 | ||
| 45 | −51 | 20 | 17 | 4.99 | 13 | 45 | −48 | 28 | 21 | 5.75 | 45 | −48 | 28 | 13 | 5.52 | |||
| – | – | – | – | – | – | 47 | −44 | 19 | −4 | 5.50 | 51 | 47 | −44 | 23 | −1 | 4.69 | 10 | |
| aINS (R) | 47 | 32 | 27 | −1 | 5.66 | 18 | 47 | 36 | 23 | −5 | 5.72 | 19 | 47 | 36 | 23 | −5 | 5.84 | 18 |
| MFG | – | – | – | – | – | – | 6 | −24 | −2 | 44 | 5.94 | 22 | – | – | – | – | – | – |
| mSFG | – | – | – | – | – | – | 6 | −8 | 14 | 44 | 6.68 | 130 | 6 | −4 | 10 | 47 | 5.78 | 53 |
| ACC | – | – | – | – | – | – | 32 | 0 | 29 | 28 | 5.73 | 32 | 8 | 21 | 39 | 5.60 | ||
| Temporal | ||||||||||||||||||
| ITG | – | – | – | – | – | – | 20 | −40 | −28 | −12 | 5.52 | 33 | – | – | – | – | – | – |
| HC | −36 | −31 | −5 | 6.63 | 32 | −36 | −31 | −5 | 5.19 | – | – | – | – | – | – | |||
| HC (R) | – | – | – | – | – | – | 24 | −27 | −5 | 4.92 | 11 | – | – | – | – | – | – | |
| Parietal | ||||||||||||||||||
| PocG (R) | – | – | – | – | – | – | 2 | 40 | −29 | 46 | 5.71 | 20 | – | – | – | – | – | – |
| mIPL | – | – | – | – | – | – | −28 | −56 | 38 | 5.25 | 25 | – | – | – | – | – | – | |
| mSPL | – | – | – | – | – | – | – | – | – | – | – | – | −24 | −68 | 29 | 6.04 | 18 | |
| Occipital | ||||||||||||||||||
| SOG | – | – | – | – | – | – | 19 | −20 | −84 | 30 | 5.28 | 10 | – | – | – | – | – | – |
| LG (R) | – | – | – | – | – | – | 18 | 4 | −82 | 1 | 5.17 | 18 | – | – | – | – | – | – |
| CS (R) | – | – | – | – | – | – | – | – | – | – | – | – | 17 | 8 | −81 | 11 | 5.57 | 24 |
| Cerebellum (R) | – | – | – | – | – | – | 36 | −56 | −24 | 5.54 | 12 | – | – | – | – | – | – | |
| Cerebellar Vermis | −4 | −63 | −17 | 5.31 | 32 | 4 | −75 | −16 | 5.54 | 30 | 0 | −55 | −14 | 5.20 | 19 | |||
| Thalamus | – | – | – | – | – | – | −8 | −15 | 8 | 5.34 | 18 | – | – | – | – | – | – | |
| Brain stem (R) | – | – | – | – | – | – | 8 | −28 | −9 | 5.18 | 33 | 8 | –28 | –9 | 4.93 | 10 | ||
Coordinates are listed in the Talairach and Tournoux (1988) atlas space. BA is the Brodmann’s area nearest to the coordinate and should be considered approximate. The significance level is given in Z values and cluster size (Cl) in number of voxels for p < .05 (FWE), extent threshold = 10 voxels. R = right. Abbreviated cerebral areas are explained under Table 4.
If a voxel cluster covers two distinct BAs, both are listed and assigned to the same cluster.
Figure 1.
Brain regions activated during FVA, SVF, and PVF compared with the respective baseline conditions in 18 male subjects. Brain activation is projected onto the standard SPM2 glass brain and selected brain sections showing voxel clusters of peak activation. During FVA compared with reading peak, activation was observed in the left hippocampus, whereas both verbal fluency tasks produced strongest BOLD-signal changes in medial and lateral structures of the frontal cortex relative to the baseline tasks [p < .05 (FWE), cluster extent = 10 voxels].
Restricted Word Production (SVF, PVF)
Stronger BOLD signals were observed for SVF compared with reading in the frontal cortex, including the left vPMA, the left IFG (BA 44, 45, 47), the right anterior insula, the left middle frontal gyrus (BA 6), the ACC (BA 32), and the adjoining medial structures of the superior frontal cortex. More posteriorly brain activation was found in the right postcentral gyrus (BA 2), the left inferior temporal gyrus and left hippocampus, the right hippocampus, right lingual gyrus, left superior occipital gyrus (BA 19), and the left inferior parietal lobe medially. Other activated brain structures included the right cerebellum, the cerebellar vermis, the right brain stem, and the left thalamus (see Table 3 and Figure 1).
Similar to SVF, the most prominent brain activation was observed during PVF compared with repetition in the frontal lobe, comprising the left vPMA, the left IFG (BA 44, 45, 47), the right anterior insula, the ACC (BA 32), and the adjoining medial structures of the superior frontal cortex (BA 6). Isolated BOLD enhancements were found medially in the left parietal and the right occipital lobe (calcarine sulcus) and also in the cerebellar vermis and the right brain stem (see Table 3 and Figure 1).
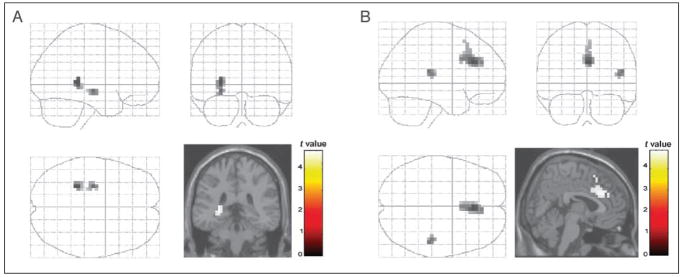
FVA versus Restricted Word Production
Contrasting SVF with FVA (relative to the respective baseline conditions), we found BOLD enhancements for SVF in medial frontal structures, including the ACC (BA 24, 32) and the medial part of the superior frontal gyrus (BA 9), and in the right superior temporal gyrus (BA 42) (see Table 4 and Figure 2).
Table 4.
Brain Activation Common to All Intrinsic Tasks (FVA, SVF, and PVF), Common to Restricted Response Tasks (SVF and PVF), and Specific to SVF and PVF Compared with FVA in 18 Healthy Male Individuals
| (FVA_read) ∩ (SVF_read) ∩ (PVF_repeat) |
(SVF_read) ∩ (PVF_repeat) |
(SVF_read) > (FVA_Read) |
(PVF_repeat) > (FVA_Read) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral Area |
BA | x | y | z | Z | Cla | BA | x | y | z | Z | Cla | BA | x | y | z | Z | Cla | BA | x | y | z | Z | Cla |
| Frontal | ||||||||||||||||||||||||
| vPMA | 6 | −44 | 6 | 37 | 5.48 | 25 | 6/9 | −40 | 2 | 40 | 5.61 | 70 | – | – | – | – | – | – | 6 | −55 | 6 | 40 | 4.32 | 42 |
| IFG | – | – | – | – | – | 44 | −48 | 9 | 33 | 6.10 | – | – | – | – | – | – | 44 | −55 | 5 | 18 | 4.81 | |||
| 45 | −48 | 28 | 21 | 4.98 | 45 | −48 | 28 | 21 | 5.35 | – | – | – | – | – | – | – | – | – | – | – | – | |||
| – | – | – | – | – | – | 47 | −44 | 23 | −1 | 4.69 | 10 | – | – | – | – | – | – | – | – | – | – | – | – | |
| aINS (R) | 47 | 36 | 23 | −1 | 5.46 | 10 | 47 | 36 | 23 | −5 | 5.72 | 14 | – | – | – | – | – | – | – | – | – | – | – | – |
| MFG (R) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 9 | 28 | 48 | 34 | 4.71 | 23 |
| SFG | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 8 | −24 | 37 | 42 | 4.46 | 45 |
| mSFG | – | – | – | – | – | – | 6 | −4 | 10 | 47 | 5.78 | 46 | 9 | 4 | 40 | 24 | 3.93 | 62 | – | – | – | – | – | – |
| ACC | – | – | – | – | – | – | 32 | 0 | 21 | 39 | 5.37 | 24/32 | 4 | 28 | 24 | 4.54 | 32 | 4 | 21 | 36 | 4.16 | 118 | ||
| Temporal | ||||||||||||||||||||||||
| STG (R) | – | – | – | – | – | – | – | – | – | – | – | – | 42 | 48 | −30 | 16 | 4.05 | 11 | – | – | – | – | – | – |
| ITG | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 37 | −55 | −55 | −14 | 4.05 | 23 |
| ITG (R) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 37 | 55 | −55 | −14 | 4.36 | 17 |
| FG (R) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 19 | 44 | −74 | −10 | 3.80 | 11 |
Coordinates are listed in the Talairach and Tournoux (1988) atlas space. BA is the Brodmann’s area nearest to the coordinate and should be considered approximate. The significance level is given in Z values and cluster size (Cl) in number of voxels for p < .05 (FWE), extent threshold = 10 voxels for the conjunction analyses, and p < .001 (uncorrected), extent threshold = 10 voxels for interactions. The reverse contrasts ([FVA_read] > [SVF_read]; [FVA_read] > [PVF_repeat]) did not yield any significant results. R = right; ACC = anterior cingulate cortex; CS = calcarine sulcus; FG = fusiform gyrus; HC = hippocampus; IFG = inferior frontal gyrus; aINS = anterior portion of the insula cortex; mIPL = medial part of the inferior parietal lobe; ITG = inferior temporal gyrus; LG = lingual gyrus; MFG = middle frontal gyrus; OFG = orbito-frontal gyrus; PocG = postcentral gyrus; (m)SFG = (medial aspect of) superior frontal gyrus; SOG = superior occipital gyrus; mSPL = medial part of the superior parietal lobe; STG = superior temporal gyrus; vPMA = ventral premotor area.
If a voxel cluster covers two distinct BAs, both are listed and assigned to the same cluster.
Figure 2.
Brain regions essential for spontaneous and restricted semantic verbal generation. (A) Brain activation during the generation of subordinates during free verbal association (FVA_cat > read) compared with semantic verbal fluency (SVF > read). (B) Brain activation during SVF as opposed to FVA relative to baseline. (A) The left hippocampus (x = −28, y = −35, z = 5) was engaged during the spontaneous association of category members (FVA_cat) but significantly less during the equivalent forced condition (SVF). (B) During the more restricted semantic task (SVF), the anterior cingulate gyrus (x = 4, y = 28, z = 24) was activated compared with FVA [p < .001 (uncorrected), cluster extent = 10 voxels].
Similarly, PVF compared with FVA (relative to the respective baseline conditions) revealed higher activation for PVF in ACC (BA 24/32) and the right middle frontal gyrus (BA 9). Increased BOLD signals were also observed in the left vPMA and the adjoining IFG (BA 44), the left superior frontal gyrus (BA 8), the right fusiform gyrus (BA 19), and the inferior temporal gyrus (BA 37) bilaterally (see Table 4). The reverse contrasts [FVA_read] > [SVF_read] and [FVA_read] > [PVF_repeat] did not reveal any significant brain activation.
Common Brain Activation
The results of the conjunction analysis of all three intrinsic conditions ([FVA_read] ∩ [SVF_read] ∩ [PVF_repeat]) revealed peak brain activity in the left vPMA, reaching into the IFG (BA 45) at its most ventral position. The anterior portion of the right insula was also activated during all intrinsic word generation tasks, irrespective of the degree of response specification (see Table 4).
Brain activation common to both restricted word production tasks ([SVF_read] ∩ [PVF_repeat]) comprised the same frontal brain structures (left vPMA, left BA 45, and right anterior insula). Additional brain activation was found in the left IFG (BA 44, 47), the ACC, and the adjacent medial parts of the superior frontal gyrus (BA 6) (see Table 4).
Response-dependent FVA Analysis
On average, subjects produced 26.66% (SD = 16.49) subordinates and 68.89% (SD = 17.64) other associations and committed 4.45% (SD = 5.95) errors during FVA. Ten of 18 subjects exceeded the set threshold of producing at least 25% (20 items) or more category members (M = 38.75%, SD = 11.16). Those 10 subjects were included in the response-dependent FVA analysis.
The response-dependent FVA analysis, differentiating between responses denoting category members (FVA_cat) and other associations (FVA_other) during FVA, was performed to avoid confounds of stimulus–response relationships in the direct comparison of FVA with SVF. Therefore, the contrasts of interest were composed of the conditions FVA_cat, FVA_other, SVF, and the corresponding baseline tasks and, in particular, the subset denoting FVA_cat and SVF. To confirm the reliability of the results, we also calculated the main effects of all conditions (FVA, SVF, and PVF) compared with the respective baseline (see Table 5).
Table 5.
Brain Activation Assessed by the Response-dependent FVA Analysis for FVA, SVF, and PVF Compared with Baseline in 10 Male Participants
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Cerebral Area | H | BA | x | y | z | Z | Cl |
| Main Effects | |||||||
| FVA_all > read | |||||||
| HC | L | −36 | −31 | −5 | 5.68 | 31 | |
| MFG | L | 8 | −44 | 13 | 32 | 4.86 | 12 |
| FVA_cat > read | |||||||
| HC | L | −28 | −35 | 5 | 5.66 | 35 | |
| HC | L | −36 | −31 | −5 | 5.61 | ||
| MFG | L | 8 | −44 | 13 | 32 | 5.09 | 12 |
| FVA_other > read | |||||||
| GH | L | 36 | −40 | −28 | −12 | 5.11 | 10 |
| HC | L | −36 | −31 | −5 | 5.07 | ||
| SVF > read | |||||||
| MFG | L | 8 | −44 | 13 | 32 | 6.20 | 68 |
| MFG | L | 6 | −24 | 2 | 44 | 5.41 | 12 |
| PocG | R | 2 | 44 | −25 | 45 | 5.30 | 17 |
| PVF > repeat | |||||||
| IFG | L | 9/44 | −44 | 13 | 29 | 6.36 | 94 |
| IFG | L | 46 | −48 | 32 | 13 | 5.83 | 21 |
| Selected Contrasts of Interest | |||||||
| (FVA_all) > (SVF_read) | |||||||
| HC | L | −28 | −35 | 9 | 4.12 | 15 | |
| (FVA_all) < (SVF_read) | |||||||
| No region of statistical significance | |||||||
| (FVA_cat-read) > (SVF_read) | |||||||
| HC | L | −28 | −35 | 5 | 4.38 | 15 | |
| HC | L | −28 | −16 | −6 | 4.07 | 11 | |
| (FVA_cat-read) < (SVF_read) | |||||||
| No region of statistical significance | |||||||
| (FVA_other-read) > (SVF_read) | |||||||
| No region of statistical significance | |||||||
| (FVA_other-read) < (SVF_read) | |||||||
| No region of statistical significance | |||||||
For the analysis, responses of the FVA were classified into those denoting category members (FVA_cat) and others (FVA_other) or merged (FVA_all).
Coordinates are listed in the Talairach and Tournoux (1988) atlas space. BA is the Brodmann’s area nearest to the coordinate and should be considered approximate. The significance level is given in Z values and cluster size (Cl) in number of voxels for p < .05 (FWE), extent threshold = 10 voxels for the main effects, and p < .001 (uncorrected), extent threshold = 10 voxels for interactions. H = hemisphere; L = left; R = right; GH = hippocampal gyrus; HC = hippocampus; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; PocG = postcentral gyrus.
The response-dependent analysis replicated the main effects observed in the whole group analysis (n = 18), in which all responses were considered irrespective of response class: merging both response categories (FVA_all, i.e., FVA_cat + FVA_other) compared with reading revealed BOLD enhancements in the left hippocampus and the left middle frontal gyrus (BA 8). Both fluency tasks compared with the respective baseline conditions engaged the left middle (BA 6, 8) or IFG (BA 44, 46), compatible with the results of the whole group analysis (cf. Tables 3 and 5). Activity of the left hippocampus and/or hippocampal gyrus was also evident when each response class (FVA_cat and FVA_other) was analyzed separately relative to reading (see Table 5). Considering the contrasts of interest, SVF compared with FVA_all (relative to the respective baseline tasks) revealed a stronger BOLD signal in the left hippocampus during FVA_all. Comparing each FVA response class (FVA_cat, FVA_other) separately with SVF produced increased hippocampal activity during FVA_cat but not during FVA_other. No increased brain activity was observed for SVF in any of the three contrasts with FVA (FVA_all, FVA_cat, and FVA_other).
DISCUSSION
In the current study, we investigated the impact of response specification onto lateral and medial prefrontal and hippocampal brain regions during intrinsic word production. Hippocampal activity was evident during spontaneous word association and SVF. Strong involvement of prefrontal brain regions, particularly the left inferior frontal and anterior cingulate gyrus, was observed in the restricted verbal generation tasks, such as semantic and phonological fluency. Direct comparison between spontaneous and restricted word generation showed that both fluency tasks evoked significantly stronger BOLD signals in the anterior cingulate gyrus compared with FVA. On the other hand, increased hippocampal activity for FVA compared with SVF was only evident in the response-dependent FVA analysis, in particular, when stimulus–response relationships between FVA and SVF were kept constant. These results are consistent with the function of strategic search and control commonly assigned to both inferior and medial frontal brain structures during controlled word production. They further allow novel insights into the role of the hippocampus during verbal generation.
The Role of the Hippocampus during Word Production
The results of the current investigation suggest that the hippocampus mediates associative and semantic binding between a stimulus and its verbal response during word production. More interestingly, hippocampal activity was modulated when subjects produced responses of the same stimulus–response relation (i.e., superordinate–subordinate relation) under different task instructions. Hereby, the generation of subordinates during FVA in direct comparison to SVF engaged the left hippocampus. Thus, hippocampal activity cannot be attributed to semantic/associative retrieval in general but might rather be dependent on processing differences, either encoding or retrieval related, which are evoked by different degrees of response specifications (free vs. constrained). However, it seems unlikely that the increase in hippocampal activity can be ascribed to advantages in automatic stimulus–response encoding during FVA (for confounds of incidental encoding during retrieval task, see Stark & Okado, 2003). The results of the memory performance on the cues and responses revealed an advantage of SVF over FVA, but no corresponding increase in hippocampal activity during the SVF compared with the FVA fMRI version was observed. Possibly, stimulus–response pairs were encoded more thoroughly during SVF because the task required specific taxonomic knowledge rather than any kind of associations, thus introducing the possibility of committing errors during this task (which was absent during FVA). This might have evoked higher individual attention levels and stronger cognitive control during the execution of this task, which in turn resulted in stronger encoding (see levels of processing effect; Craik & Lockhart, 1972). According to the literature, an increase in encoding performance is accompanied by hippocampal BOLD enhancements (e.g., Stark & Okado, 2003; Davachi & Wagner, 2002; Strange, Otten, Josephs, Rugg, & Dolan, 2002). We have found reduced activation and, therefore, ascribe the hippocampal activation differences during FVA and SVF to differences in retrieval rather than encoding processes.
More precisely, we propose that the retrieval of spontaneous verbal associates (FVA) compared with the selection of subordinates (SVF) allows for a higher degree of associative binding and less cognitive control processes. Although SVF and FVA stimulus–response pairs are both associated in some way, successful retrieval during SVF calls for a goal-directed access to semantic knowledge (a dog is an animal, a pumpkin is a kind of fruit), whereas FVA also allows for complementary access to episodic events (my dog is lazy and called Tom, pumpkins remind me of Halloween). To perform tasks like SVF accurately, one cannot solely rely on processes of linking the stimulus to any of its associates but have to take additional search, selection, and control processes into account. Jung and Ricklin (1906) have shown that superordinate–subordinate relations are produced in fewer than 20% of their trials during free word association (in our study: 27%), which strengthens the point that responses uttered during SVF require less spontaneous associative processes but a higher amount of supervision and monitoring functions.
Retrieval processes during word production tasks can be mediated by a hippocampal–cortical network (Whatmough & Chertkow, 2007). According to Braak et al. (1999), the information flow from sensory input (e.g., word reading) to motor output (e.g., overt speaking) can follow either a neocortical route to enter the pFC or passes by limbic structures. The latter pathway is favored when retrieval allows for emotional or mnemonic binding between the stimulus and its response. Whatmough and Chertkow (2007) assumed that the hippocampus, rather than cortical structures, is involved during easier tasks, whereas increased task difficulty is related to a higher involvement of association cortices. In this respect, the role of the hippocampus during word retrieval is not obligatory but rather seen as auxiliary (Whatmough & Chertkow, 2007). This might explain the lack of language dysfunctions in patients with hippocampal amnesia (for a review, see Spiers et al., 2001) and the inconsistent findings regarding hippocampal activity during language production (Awad, Warren, Scott, Turkheimer, & Wise, 2007; Whatmough & Chertkow, 2007; Pihlajamäki et al., 2000).
Finally, we suggest that the reduced frontal activation during word association compared with the characteristic, widespread frontal activation during verbal fluency can be related to the relatively spontaneous and unrestricted nature of this task (FVA). Less executive effort is demanded during FVA compared with SVF. This might involve the suppression of inappropriate but highly associative and competitive responses during SVF, which results in enhanced cognitive control during verbal retrieval (e.g., Badre & Wagner, 2002). A similar explanation was offered by Martin and Cheng (2006) who proposed an impact of association strength on inferior frontal activity. Following their interpretation, the observed attenuation of BOLD-signal changes in the frontal cortex during FVA compared with verbal fluency was due to higher associative linking between cue and response words, making it easier to retrieve the item.
Brain Regions Essential for Restricted, Intrinsic Word Production
Independent of the retrieval process (semantically or phonologically guided), verbal fluency produced brain activation in the lateral (IFG and vPMA) and medial (ACC) frontal cortices, predominantly in the left hemisphere (see conjunction analysis). However, the only modulation of brain activation common to both contrasts of verbal fluency with FVA (i.e., SVF > FVA, PVF > FVA) was observed in the anterior cingulate gyrus.
According to Levelt (1989), word production relies on lexical selection, which is a competitive process between a set of appropriate candidates. This procedure is inherent to all three tasks we investigated (FVA, SVF, and PVF) but to varying degrees. Thus, we argue that activity in the anterior cingulate gyrus does not represent a word selection process per se. Rather, the anterior cingulate gyrus is seen as a modulatory system that interacts with executive brain regions in the pre-frontal (e.g., inferior frontal) cortex and adapts to the current task demands (for a review, see Paus, 2001; Gazzaniga, Ivry, & Mangun, 1998). Several reciprocal connections to lateral prefrontal areas support this network (Gazzaniga et al., 1998). This interpretation was supported, for example, by Fu et al. (2002) who reported enhanced recruitment of the anterior cingulate gyrus during the generation of words in response to “difficult” as opposed to “easy” letters. Also, manipulating high- and low-selection demands during a semantic retrieval task, Thompson-Schill et al. (1997) observed higher involvement of inferior frontal and anterior cingulate regions (among others) during the more complex (i.e., high-selection) condition.
Brain Regions Independent of Response Specification
The strongest BOLD-signal enhancement during all three intrinsic tasks compared with baseline was observed in the vPMA, indicating that this structure seems to be engaged in core processes of internal response generation, that is, verbal selection (for contrary views, see, e.g., Crosson et al., 2001; Goldberg, 1985). The activated area occupied the junction of the ventral precentral sulcus and the most dorsal edge of Broca’s area. The ventral PMA has been consistently cited in PET studies of silent verbalization (i.e., producing a verb to a noun) (for a review, see Grèzes & Decety, 2001) or during traditional verbal fluency using fMRI (Fu et al., 2002; Pihlajamäki et al., 2000; Pujol et al., 1996; Warburton et al., 1996). Our results are also compatible with the findings of Tremblay and Gracco (2006) who compared word reading, the generation of words from a category (e.g., name flower) or a subcategory (e.g., name a red flower) with each other. Both intrinsic word generation tasks recruited the left PMA significantly more than the external task (reading) (Tremblay & Gracco, 2006).
The other brain region involved in all three intrinsic tasks was the anterior portion of the right insula. Activation of the right anterior insula was also reliably found during word generation tasks (for an overview, see Indefrey & Levelt, 2004). Within the language domain, the anterior portion of the insula cortex has been associated with phonological processing, motor planning, and articulation, although most of the studies reported left-lateralized brain activation or data of patients with lesions in the left anterior insula. In contrast, the function attributed to its right homologue seems to be more heterogeneous and less language specific. It has also been implicated in sensory, motor, and mental control, particularly in response selection and suppression mechanisms. Therefore, the right anterior insula might support the selection processes subserved by the vPMA and additionally coordinates motor/articulatory aspects of overt verbal generation.
Concluding Comments
Our results provide evidence of an involvement of medial-temporal brain regions in free, associative verbal retrieval. The contrast between restricted and free semantic word generation revealed that hippocampal activity was dependent on the manipulation of response specification rather than variations in stimulus–response relationship. Task-dependent modulations of the retrieval process also had an impact on the left inferior frontal and anterior cingulate cortices, which seem to be specific for constrained retrieval. The results of our study, therefore, provide novel insights into the organization of the language production network, which includes brain regions outside the classic perisylvian areas.
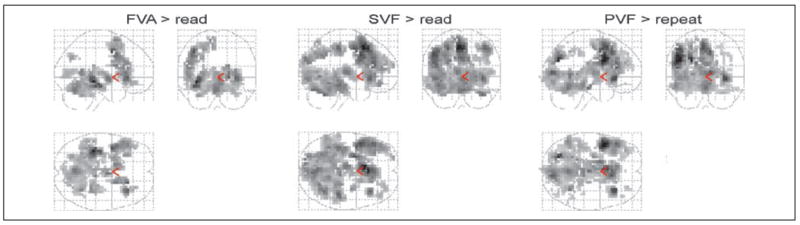
Figure A1.
Brain activation for the main effects (FVA > read, SVF > read, PVF > read) of the whole-group analysis (n = 18) (p < .001, Monte Carlo, extent = 12).
Table A1.
Brain Activation for the Main Contrasts (FVA > Read, SVF > Read, and PVF > Repeat) of the Whole-group Analysis (n = 18) Corrected at p < .05 (FWE) with an Extent Threshold = 0 Voxels (Matlab Printout)
| Cluster Level |
Voxel Level |
|||||||
|---|---|---|---|---|---|---|---|---|
| p(cor) | kE | p(unc) | p(FWE) | p(FDR) | T | Z | p(unc) | x, y, z (mm) |
| FVA > Read | ||||||||
| 0.000 | 32 | 0.000 | 0.000 | 0.000 | 7.62 | 6.63 | 0.000 | −36 −32 −8 |
| 0.000 | 0.000 | 6.61 | 5.92 | 0.000 | −36 −24 −12 | |||
| 0.012 | 2 | 0.229 | 0.000 | 0.000 | 7.15 | 6.31 | 0.000 | 20 −16 28 |
| 0.001 | 10 | 0.013 | 0.000 | 0.000 | 6.73 | 6.01 | 0.000 | −40 0 44 |
| 0.005 | 4 | 0.096 | 0.000 | 0.000 | 6.66 | 5.95 | 0.000 | −40 −28 −16 |
| 0.001 | 8 | 0.024 | 0.000 | 0.000 | 6.50 | 5.84 | 0.000 | 20 28 0 |
| 0.001 | 0.000 | 5.72 | 5.25 | 0.000 | 20 24 8 | |||
| 0.000 | 18 | 0.002 | 0.000 | 0.000 | 6.26 | 5.66 | 0.000 | 32 28 0 |
| 0.000 | 32 | 0.000 | 0.000 | 0.000 | 6.02 | 5.48 | 0.000 | −44 8 36 |
| 0.004 | 0.000 | 5.39 | 4.98 | 0.000 | −48 28 24 | |||
| 0.005 | 0.000 | 5.31 | 4.92 | 0.000 | −48 20 28 | |||
| 0.001 | 8 | 0.024 | 0.001 | 0.000 | 5.84 | 5.34 | 0.000 | 4 −8 0 |
| 0.000 | 32 | 0.000 | 0.001 | 0.000 | 5.81 | 5.31 | 0.000 | −4 −64 −24 |
| 0.005 | 0.000 | 5.33 | 4.93 | 0.000 | 4 −60 −12 | |||
| 0.001 | 8 | 0.024 | 0.001 | 0.000 | 5.67 | 5.21 | 0.000 | 20 8 20 |
| 0.014 | 0.000 | 5.04 | 4.70 | 0.000 | 20 16 16 | |||
| 0.007 | 3 | 0.145 | 0.002 | 0.000 | 5.57 | 5.13 | 0.000 | −48 28 16 |
| 0.012 | 2 | 0.229 | 0.002 | 0.000 | 5.52 | 5.09 | 0.000 | 8 −28 −12 |
| 0.020 | 1 | 0.395 | 0.002 | 0.000 | 5.49 | 5.07 | 0.000 | 0 −8 24 |
| 0.005 | 4 | 0.096 | 0.003 | 0.000 | 5.44 | 5.03 | 0.000 | 32 −52 8 |
| 0.007 | 3 | 0.145 | 0.003 | 0.000 | 5.44 | 5.03 | 0.000 | −12 4 64 |
| 0.000 | 13 | 0.006 | 0.003 | 0.000 | 5.40 | 4.99 | 0.000 | −52 20 20 |
| 0.006 | 0.000 | 5.28 | 4.89 | 0.000 | −52 20 8 | |||
| 0.006 | 0.000 | 5.26 | 4.88 | 0.000 | −48 20 0 | |||
| 0.020 | 1 | 0.395 | 0.005 | 0.000 | 5.30 | 4.91 | 0.000 | 36 −44 0 |
| 0.002 | 7 | 0.033 | 0.007 | 0.000 | 5.23 | 4.86 | 0.000 | 32 −36 4 |
| 0.017 | 0.000 | 4.98 | 4.65 | 0.000 | 36 −32 −8 | |||
| 0.020 | 1 | 0.395 | 0.008 | 0.000 | 5.19 | 4.83 | 0.000 | −8 20 16 |
| 0.020 | 1 | 0.395 | 0.010 | 0.000 | 5.13 | 4.77 | 0.000 | −16 20 16 |
| 0.005 | 4 | 0.096 | 0.014 | 0.000 | 5.04 | 4.70 | 0.000 | −24 4 48 |
| 0.007 | 3 | 0.145 | 0.017 | 0.000 | 4.99 | 4.66 | 0.000 | −28 28 −4 |
| 0.020 | 1 | 0.395 | 0.022 | 0.000 | 4.92 | 4.60 | 0.000 | 12 −8 28 |
| 0.020 | 1 | 0.395 | 0.023 | 0.000 | 4.90 | 4.59 | 0.000 | −40 −44 −12 |
| 0.012 | 2 | 0.229 | 0.027 | 0.000 | 4.86 | 4.56 | 0.000 | −4 −28 −12 |
| 0.020 | 1 | 0.395 | 0.030 | 0.000 | 4.83 | 4.52 | 0.000 | −32 −52 −32 |
| 0.020 | 1 | 0.395 | 0.031 | 0.000 | 4.82 | 4.52 | 0.000 | −8 16 52 |
| 0.012 | 2 | 0.229 | 0.032 | 0.000 | 4.82 | 4.52 | 0.000 | −40 0 28 |
| 0.020 | 1 | 0.395 | 0.035 | 0.000 | 4.79 | 4.49 | 0.000 | −40 32 −8 |
| 0.020 | 1 | 0.395 | 0.046 | 0.000 | 4.71 | 4.43 | 0.000 | −16 4 24 |
| 0.020 | 1 | 0.395 | 0.048 | 0.000 | 4.69 | 4.41 | 0.000 | −24 −68 36 |
| 0.020 | 1 | 0.395 | 0.049 | 0.000 | 4.69 | 4.41 | 0.000 | −16 −4 64 |
| SVF > Read | ||||||||
| 0.000 | 130 | 0.000 | 0.000 | 0.000 | 7.69 | 6.68 | 0.000 | −8 12 48 |
| 0.000 | 0.000 | 7.40 | 6.49 | 0.000 | 0 20 48 | |||
| 0.000 | 0.000 | 6.35 | 5.73 | 0.000 | 0 28 32 | |||
| 0.000 | 101 | 0.000 | 0.000 | 0.000 | 6.91 | 6.14 | 0.000 | −48 8 36 |
| 0.000 | 0.000 | 6.43 | 5.79 | 0.000 | −40 0 44 | |||
| 0.000 | 0.000 | 6.37 | 5.75 | 0.000 | −48 28 24 | |||
| 0.000 | 22 | 0.001 | 0.000 | 0.000 | 6.64 | 5.94 | 0.000 | −24 −4 48 |
| 0.000 | 19 | 0.001 | 0.000 | 0.000 | 6.34 | 5.72 | 0.000 | 36 24 −4 |
| 0.000 | 20 | 0.001 | 0.000 | 0.000 | 6.33 | 5.71 | 0.000 | 40 −32 48 |
| 0.000 | 30 | 0.000 | 0.000 | 0.000 | 6.11 | 5.54 | 0.000 | 4 −76 −24 |
| 0.000 | 0.000 | 6.08 | 5.52 | 0.000 | 0 −68 −24 | |||
| 0.000 | 12 | 0.008 | 0.000 | 0.000 | 6.10 | 5.54 | 0.000 | 36 −56 −32 |
| 0.000 | 33 | 0.000 | 0.000 | 0.000 | 6.08 | 5.52 | 0.000 | −40 −28 −16 |
| 0.001 | 0.000 | 5.65 | 5.19 | 0.000 | −36 −32 −8 | |||
| 0.002 | 0.000 | 5.52 | 5.09 | 0.000 | −36 −20 −16 | |||
| 0.000 | 51 | 0.000 | 0.000 | 0.000 | 6.05 | 5.50 | 0.000 | −44 20 −4 |
| 0.003 | 0.000 | 5.45 | 5.03 | 0.000 | −32 32 4 | |||
| 0.008 | 0.000 | 5.18 | 4.82 | 0.000 | −36 24 8 | |||
| 0.000 | 18 | 0.002 | 0.001 | 0.000 | 5.84 | 5.34 | 0.000 | −8 −16 8 |
| 0.001 | 10 | 0.013 | 0.001 | 0.000 | 5.76 | 5.28 | 0.000 | −20 −88 28 |
| 0.003 | 5 | 0.066 | 0.001 | 0.000 | 5.74 | 5.26 | 0.000 | −12 −88 32 |
| 0.009 | 0.000 | 5.17 | 4.81 | 0.000 | −4 −88 28 | |||
| 0.000 | 25 | 0.000 | 0.001 | 0.000 | 5.72 | 5.25 | 0.000 | −28 −60 36 |
| 0.003 | 0.000 | 5.42 | 5.01 | 0.000 | −32 −52 36 | |||
| 0.011 | 0.000 | 5.12 | 4.77 | 0.000 | −28 −68 32 | |||
| 0.012 | 2 | 0.229 | 0.001 | 0.000 | 5.71 | 5.24 | 0.000 | 24 −64 0 |
| 0.000 | 33 | 0.000 | 0.001 | 0.000 | 5.63 | 5.18 | 0.000 | 8 −28 −12 |
| 0.004 | 0.000 | 5.37 | 4.97 | 0.000 | −4 −32 −20 | |||
| 0.009 | 0.000 | 5.16 | 4.80 | 0.000 | −16 −44 −20 | |||
| 0.000 | 18 | 0.002 | 0.001 | 0.000 | 5.62 | 5.17 | 0.000 | 4 −84 −4 |
| 0.034 | 0.000 | 4.79 | 4.49 | 0.000 | 8 −84 8 | |||
| 0.005 | 4 | 0.096 | 0.002 | 0.000 | 5.60 | 5.15 | 0.000 | −40 −48 −12 |
| 0.001 | 8 | 0.024 | 0.002 | 0.000 | 5.53 | 5.10 | 0.000 | 12 −48 −24 |
| 0.032 | 0.000 | 4.81 | 4.51 | 0.000 | 16 −56 −8 | |||
| 0.001 | 11 | 0.010 | 0.005 | 0.000 | 5.30 | 4.92 | 0.000 | 24 −28 −8 |
| 0.007 | 0.000 | 5.24 | 4.87 | 0.000 | 24 −36 0 | |||
| 0.001 | 9 | 0.018 | 0.006 | 0.000 | 5.29 | 4.90 | 0.000 | −40 44 16 |
| 0.005 | 4 | 0.096 | 0.006 | 0.000 | 5.28 | 4.90 | 0.000 | −44 40 −12 |
| 0.005 | 4 | 0.096 | 0.007 | 0.000 | 5.22 | 4.85 | 0.000 | −24 −40 48 |
| 0.002 | 7 | 0.033 | 0.009 | 0.000 | 5.17 | 4.80 | 0.000 | 20 28 0 |
| 0.020 | 1 | 0.395 | 0.010 | 0.000 | 5.12 | 4.77 | 0.000 | −36 −68 24 |
| 0.012 | 2 | 0.229 | 0.011 | 0.000 | 5.12 | 4.77 | 0.000 | 32 −8 48 |
| 0.020 | 1 | 0.395 | 0.017 | 0.000 | 4.98 | 4.65 | 0.000 | −16 −68 60 |
| 0.012 | 2 | 0.229 | 0.019 | 0.000 | 4.96 | 4.64 | 0.000 | 48 −36 28 |
| 0.020 | 1 | 0.395 | 0.027 | 0.000 | 4.86 | 4.55 | 0.000 | 20 −68 8 |
| 0.012 | 2 | 0.229 | 0.030 | 0.000 | 4.83 | 4.53 | 0.000 | −40 −8 48 |
| 0.020 | 1 | 0.395 | 0.030 | 0.000 | 4.83 | 4.52 | 0.000 | −24 64 20 |
| 0.020 | 1 | 0.395 | 0.034 | 0.000 | 4.79 | 4.50 | 0.000 | −20 −40 −4 |
| 0.020 | 1 | 0.395 | 0.036 | 0.000 | 4.78 | 4.49 | 0.000 | 0 −52 −20 |
| 0.020 | 1 | 0.395 | 0.039 | 0.000 | 4.75 | 4.46 | 0.000 | 0 8 28 |
| 0.012 | 2 | 0.229 | 0.041 | 0.000 | 4.74 | 4.45 | 0.000 | −24 −64 −28 |
| 0.020 | 1 | 0.395 | 0.042 | 0.000 | 4.73 | 4.45 | 0.000 | 16 64 −4 |
| 0.020 | 1 | 0.395 | 0.045 | 0.000 | 4.72 | 4.43 | 0.000 | −4 16 24 |
| 0.020 | 1 | 0.395 | 0.045 | 0.000 | 4.71 | 4.43 | 0.000 | 0 −20 −20 |
| 0.020 | 1 | 0.395 | 0.046 | 0.000 | 4.71 | 4.43 | 0.000 | −56 −52 −16 |
| 0.020 | 1 | 0.395 | 0.047 | 0.000 | 4.70 | 4.42 | 0.000 | −12 −84 4 |
| 0.020 | 1 | 0.395 | 0.048 | 0.000 | 4.69 | 4.41 | 0.000 | −20 −56 −12 |
| PVF > Repeat | ||||||||
| 0.000 | 86 | 0.000 | 0.000 | 0.000 | 7.17 | 6.32 | 0.000 | −48 8 28 |
| 0.000 | 0.000 | 6.49 | 5.83 | 0.000 | −44 4 48 | |||
| 0.000 | 0.000 | 6.08 | 5.52 | 0.000 | −48 28 16 | |||
| 0.000 | 18 | 0.002 | 0.000 | 0.000 | 6.77 | 6.04 | 0.000 | −24 −72 28 |
| 0.000 | 18 | 0.002 | 0.000 | 0.000 | 6.50 | 5.84 | 0.000 | 36 24 −4 |
| 0.000 | 53 | 0.000 | 0.000 | 0.000 | 6.42 | 5.78 | 0.000 | −4 8 52 |
| 0.000 | 0.000 | 6.18 | 5.60 | 0.000 | 8 20 44 | |||
| 0.000 | 24 | 0.000 | 0.000 | 0.000 | 6.14 | 5.57 | 0.000 | 8 −84 8 |
| 0.002 | 6 | 0.047 | 0.001 | 0.000 | 5.67 | 5.21 | 0.000 | −16 0 24 |
| 0.012 | 2 | 0.229 | 0.001 | 0.000 | 5.66 | 5.20 | 0.000 | 0 −20 −20 |
| 0.000 | 19 | 0.001 | 0.001 | 0.000 | 5.66 | 5.20 | 0.000 | 0 −56 −20 |
| 0.005 | 4 | 0.096 | 0.002 | 0.000 | 5.50 | 5.07 | 0.000 | 32 −52 4 |
| 0.002 | 6 | 0.047 | 0.005 | 0.000 | 5.33 | 4.93 | 0.000 | −8 28 28 |
| 0.001 | 10 | 0.013 | 0.005 | 0.000 | 5.33 | 4.93 | 0.000 | 8 −28 −12 |
| 0.006 | 0.000 | 5.29 | 4.90 | 0.000 | 0 −28 −16 | |||
| 0.001 | 9 | 0.018 | 0.005 | 0.000 | 5.30 | 4.91 | 0.000 | 4 20 20 |
| 0.008 | 0.000 | 5.19 | 4.83 | 0.000 | 0 12 24 | |||
| 0.003 | 5 | 0.066 | 0.008 | 0.000 | 5.21 | 4.84 | 0.000 | −52 −56 −16 |
| 0.020 | 1 | 0.395 | 0.009 | 0.000 | 5.17 | 4.81 | 0.000 | −8 20 16 |
| 0.012 | 2 | 0.229 | 0.014 | 0.000 | 5.05 | 4.71 | 0.000 | −24 −12 52 |
| 0.001 | 10 | 0.013 | 0.015 | 0.000 | 5.03 | 4.69 | 0.000 | −44 24 0 |
| 0.028 | 0.000 | 4.85 | 4.54 | 0.000 | −36 24 8 | |||
| 0.003 | 5 | 0.066 | 0.016 | 0.000 | 5.01 | 4.68 | 0.000 | −8 −12 8 |
| 0.020 | 1 | 0.395 | 0.016 | 0.000 | 5.01 | 4.68 | 0.000 | −24 −48 4 |
| 0.020 | 1 | 0.395 | 0.017 | 0.000 | 5.00 | 4.66 | 0.000 | −4 −4 32 |
| 0.020 | 1 | 0.395 | 0.019 | 0.000 | 4.96 | 4.63 | 0.000 | −20 −40 4 |
| 0.012 | 2 | 0.229 | 0.021 | 0.000 | 4.93 | 4.61 | 0.000 | −52 20 20 |
| 0.005 | 4 | 0.096 | 0.027 | 0.000 | 4.86 | 4.55 | 0.000 | 0 −84 −8 |
| 0.020 | 1 | 0.395 | 0.035 | 0.000 | 4.79 | 4.49 | 0.000 | 4 −40 −16 |
| 0.020 | 1 | 0.395 | 0.036 | 0.000 | 4.78 | 4.48 | 0.000 | −48 0 56 |
| 0.007 | 3 | 0.145 | 0.037 | 0.000 | 4.77 | 4.48 | 0.000 | −52 32 8 |
| 0.020 | 1 | 0.395 | 0.049 | 0.000 | 4.69 | 4.41 | 0.000 | −48 −52 −4 |
Acknowledgments
The study was supported by a grant from the Interdisciplinary Center for Clinical Research “BIOMAT” within the Faculty of Medicine at the RWTH Aachen University (IZKF VV N68) and the International Research Training Group 1328 supported by the German Research Foundation. The authors thank Olga Sachs for helpful comments and stimulating discussions.
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Research. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Annett M. Classification of hand preference by association analysis. British Journal of Psychiatry. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJS. A common system for the comprehension and production of narrative speech. Journal of Neuroscience. 2007;27:11455–11464. doi: 10.1523/JNEUROSCI.5257-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX Lexical Database (CD-ROM) Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1993. [Google Scholar]
- Badre D, Wagner AD. Semantic retrieval, mnemonic control, and prefrontal cortex. Behavioral Cognitive Neuroscience Review. 2002;1:206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- Bartha L, Brenneis C, Schocke M, Trinka E, Köylü B, Trieb T. Medial temporal lobe activation during semantic language processing: fMRI findings in healthy left-and right handers. Cognitive Brain Research. 2003;17:339–346. doi: 10.1016/s0926-6410(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, Mueller RA. Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and overt speech. Neuropsychologia. 2007;45:1697–1706. doi: 10.1016/j.neuropsychologia.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker D, Byrnes ML, Mastaglia FL, Thickbroom GW. Differential activation of frontal lobe areas by phonical and semantic language tasks: A functional magnetic resonance imaging study. Journal of Clinical Neuroscience. 2006;13:91–95. doi: 10.1016/j.jocn.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJS. Speech production: Wernicke, Broca and beyond. Brain. 2000;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding cortical organization of semantic processing. Annual Reviews of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer’s disease: What is new since A. Alzheimer? European Archives of Psychiatry and Clinical Neuroscience. 1999;249:S14–S22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychological Review. 1975;82:407–428. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gökcay D, Mohr CM, Auerbach EJ, et al. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. Journal of Cognitive Neuroscience. 2001;13:272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behavioral Brain Science. 1994;17:449–518. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RSJ. A PET study of word finding. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Morgan K, Suckling J, Williams SCR, Andrew C, Vythelingum GN, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: Greater anterior cingulate activation with increased task demand. Neuroimage. 2002;17:871–879. [PubMed] [Google Scholar]
- Fu CHY, Suckling J, Williams SCR, Andrew CM, Vythelingum GN, McGuire PK. Effects of psychotic state and task demand on prefrontal function in schizophrenia: An fMRI study of overt verbal fluency. American Journal of Psychiatry. 2005;162:485–494. doi: 10.1176/appi.ajp.162.3.485. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR. Cognitive neuroscience: The biology of the mind. New York: Norton & Company; 1998. [Google Scholar]
- Gold JJ, Hopkins RO, Squire LR. Single-item memory, associative memory, and the human hippocampus. Learning and Memory. 2006;13:644–649. doi: 10.1101/lm.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: Review and hypothesis. Behavioral Brain Science. 1985;8:567–616. [Google Scholar]
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapping. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heckers S, Titone D. Hippocampus, IV: Relational memory. American Journal of Psychiatry. 2005;162:663. doi: 10.1176/appi.ajp.162.4.663. [DOI] [PubMed] [Google Scholar]
- Henke K, Mondadori CRA, Treyer V, Nitsch RM, Buck A, Hock C. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia. 2003;41:863–876. doi: 10.1016/s0028-3932(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Hutchison K. Is semantic priming due to association strength or feature overlap? A microanalytic review. Psychonomic Bulletin & Review. 2003;10:785–813. doi: 10.3758/bf03196544. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jung CG, Ricklin F. Experimentelle Untersuchungen über Assoziationen Gesunder. In: Jung CG, editor. Diagnostische Assoziationsstudien. Leipzig: Barth; 1906. pp. 7–145. [Google Scholar]
- Kubota Y, Toichi M, Shimizu M, Mason RA, Coconcea CM, Findling RL, et al. Prefrontal activation during verbal fluency tests in schizophrenia—A near-infrared spectroscopy (NIRS) study. Schizophrenia Research. 2005;77:65–73. doi: 10.1016/j.schres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Lehrl S, Triebig G, Fischer B. Multiple-choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurologica Scandinavica. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Speaking: From intention to articulation. Cambridge: MIT Press; 1989. [Google Scholar]
- Martin CM, Cheng Y. Selection demands versus association strength in the verb generation task. Psychonomic Bulletin & Review. 2006;13:396–401. doi: 10.3758/bf03193859. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, Gummer A, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14:763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming and retrieval from lexical memory: Evidence for facilitatory and inhibitory processes. Memory & Cognition. 1976;4:648–654. doi: 10.3758/BF03213230. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: A selective review of current findings and theories. In: Besner D, Humphreys GW, editors. Basic processes in reading. Hillsdale: Erlbaum; 1991. pp. 264–336. [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, Tanila H, Hänninen T, Könönen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: A functional magnetic resonance imaging study. Annals of Neurology. 2000;47:470–476. [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Deus J, Kulisevsky J, Marti-Vilalta JL, Garcia C, et al. Frontal lobe activation during word generation studied by functional MRI. Acta Neurologica Scandinavica. 1996;93:403–410. doi: 10.1111/j.1600-0404.1996.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, et al. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Journal of Neurology, Neurosurgery and Psychiatry. 1998;64:492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–382. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. Journal of Neuroscience. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal and parahippocampal roles during verbal encoding. Journal of Neuroscience. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of the left prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences; U.S.A. 1997. pp. 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage. 2006;33:947–957. doi: 10.1016/j.neuroimage.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects—Studies with PET. Brain. 1996;119:159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H. rCBF to the hippocampal complex covaries with superior semantic memory retrieval. Behavioural Brain Research. 2007;181:262–269. doi: 10.1016/j.bbr.2007.04.017. [DOI] [PubMed] [Google Scholar]