Abstract
Background
Cultural factors and biomarkers are emerging emphases in social epidemiology that readily ally with human biology and anthropology. Persistent health challenges and disparities have established biocultural roots, and environment plays an integral role in physical development and function that form the bases of population health. Biomarkers have proven to be valuable tools for investigating biocultural bases of health disparities.
Aims
We apply recent insights from biology to consider how culture gets under the skin and evaluate the construct of embodiment. We analyze contrasting biomarker models and applications, and propose an integrated model for biomarkers. Three examples from the Great Smoky Mountains Study (GSMS) illustrate these points.
Subjects and methods
The longitudinal developmental epidemiological GSMS comprises a population-based sample of 1420 children with repeated measures including mental and physical health, life events, household conditions, and biomarkers for pubertal development and allostatic load.
Results
Analyses using biomarkers resolved competing explanations for links between puberty and depression, identified gender differences in stress at puberty, and revealed interactive effects of birthweight and postnatal adversity on risk for depression at puberty in girls.
Conclusion
An integrated biomarker model can both enrich epidemiology and illuminate biocultural pathways in population health.
Keywords: biomarkers, embodiment, culture, epidemiology
Introduction
Persistent disparities and global challenges in health combined with advances in the social and biological sciences have renewed epidemiology's attention to social factors in population health. Reciprocally, globalization and social transformations have combined with biocultural models and critical theory to propel anthropological efforts to link the global and the local in human welfare. These efforts have involved conceptual and empirical reintegration of culture and biology that provides a rich resource for social epidemiology. Complementarily, the scales of inquiry, empirical reach, and direct linkages to policy and practice that epidemiology commands provide compelling and useful models for both human biologists and anthropologists.
Insights from the biological sciences—particularly developmental neuroscience, immunology, and epigenetics—have expanded the role of context in biology and therefore in population health (Worthman and Kohrt 2005), and secondarily validated established anthropological approaches in human biology and social ecology. The latter tradition has used biomarkers systematically to document the impact of life circumstances on human growth and development (Ulijaszek et al. 1998), adult function (Ellison 2001), and population health (Cameron and Demerath 2002). During the last three decades, a biocultural approach has emerged from this tradition and taken up the body as a lens to reveal patterns and pathways in differential physical and mental health (Panter-Brick and Worthman 1999). In contrast to epidemiology, biocultural anthropology contextualizes health within a wider set of human concerns (meaning, social relationships, moral and social status, subsistence, reproduction) and situates biology within them (Armelagos et al. 2005; Goodman and Leatherman 1999).
Systematic use of biomarkers by other social sciences and social epidemiology is more recent. Intractable unexplained health disparities and their apparent linkages to social inequality (Krieger and Davey Smith 2004) have prompted epidemiology to turn not only to the social sciences (Krieger 2000) but also to use of biomarkers for tracing pathways to health and disease (Finch et al. 2000). Biomarkers can be used to probe the causal matrix generated by cumulative socially determined circumstances, lifetime experience and exposures, and biodynamics that shape health over the life course (Marmot et al. 1991). Human biology and anthropology's long engagement with cultural factors in differential well being and its experience with use of biomarkers in the field under ambient conditions offer theoretical, methodological, and empirical resources to advance current goals in epidemiology.
Here, we explore these issues in the context of our use of biomarkers for the Great Smoky Mountains Study (GSMS), an ongoing developmental epidemiological study in western North Carolina. The collaboration emerged from a shared interest in biosocial pathways in the well being of youth, and complementary expertise in psychiatry, social psychology, and anthropology. We first consider the expanded view of biology by asking how culture gets under the skin and considering how embodiment relates to that process. We then analyze contrasting conceptualizations and use of biomarkers in social science and social epidemiology versus biomedicine, and suggest a constructive resolution of the differences. Finally, we illustrate the use and value of biomarkers with a series of examples from the GSMS, and discuss how multifactorial designs can both enrich epidemiology and illuminate the roles of biocultural processes in population health.
Embodiment
Social epidemiology leads health sciences in adopting an expanded biocultural perspective for explaining and addressing health disparities (Krieger 2004). The call for “bringing the body back, in context” ((Krieger and Davey Smith 2004), p. 94) draws upon convergent insights from many lines of inquiry including epi/genetics, psychiatry, and developmental biology. The call also comes from new understandings of culture and society that revise how we understand human welfare. Such revision has flowed from shifts to bottom-up processual approaches that counterbalance established top-down structural views of society and individual. These shifts set the stage for cultural analyses of public health practices and politics that thwarted efficacy during a 1992-3 cholera epidemic in Venezuela (Briggs and Mantini-Briggs 2003), or of perceptions and poverty that underlie maternal neglect and infant mortality in a squatter settlement of northeast Brazil (Scheper-Hughes 1992).
But such cultural analysis stops short of specifying the linkages of culture and biology that forge associations of inequality and health (Wilkinson 2005). What are the means by which “culture gets under the skin” and shapes health at its foundations (Lupien et al. 2001)? Embodiment-defined as the impact of ongoing bio-contextual dynamics on physical form, functions, and capacities-may provide a construct for investigating this question and tracking dynamics among differential experience, function, and well-being (Worthman 1999a). The term initially was introduced in anthropology to address the body as a cultural phenomenon and as the phenomenological basis of experience, setting aside their biological dimensions (Csordas 1990). Embodiment then was applied in social epidemiology as a bridging concept invoking the interplay of society and the body (Krieger 1994), and has been elaborated as a core construct for tracing the linkages between social inequalities and health disparities (Krieger and Davey Smith 2004).
This expanded view of embodiment intersects directly with established lines of human biology and biocultural inquiry that relate cultural ecology to human development, function, and health (Adair et al. 2001; DeCaro and Worthman 2008; McGarvey 2007). The apparent universality in fundamentals of human biology is moderated by widespread evidence of variation in particulars of regulation and function related to localized, cultural ecological conditions (Worthman 1999b). Embodiment is a direct corollary of the body's incorporation of and adaptive response to the circumstances under which it develops and functions. Prime examples include the nervous and the immune systems, whose immense structural and functional complexity is driven by inputs or exposures that operate not only intensely through development but also persistently across adulthood (Changeux 1985; McDade and Worthman 1999). Only context carries sufficient information to tell such systems how to develop and function appropriately in the specific conditions under which they operate. On a larger scale, the impact of environmental quality on child development with enduring consequences for health also exemplifies how life circumstance are embodied in children, then adults (Grantham-McGregor et al. 2007; Stein et al. 2008).
Therefore, embodiment results from systematic obligate interactions of cultural and physical ecology with physiology across the life course. By shaping both objective and subjective conditions of living, culture informs biology through development (Super and Harkness 1999), across the life course (Dressler et al. 2005), and even across generations (Weaver 2007; Worthman and Kuzara 2005). Place-specific morphology and function emerge not simply from the objective conditions of life, but also from the experience of and responses to those conditions. The meanings of an event mediate its cognitive-emotional impact, influence biological responses, and condition future responses. Thus, for instance, social trauma provokes acute and enduring affective-physiological responses with consequences for health (Flinn 2006; Heim and Nemeroff 2001), but ambient cultural factors (practices, norms, hierarchies) can condition whether an experience is interpreted as being traumatic (Anderson-Fye 2003). Even the ability to enact cultural norms and goals influences mental and physical health (Dressler et al. 1998). Framed in epidemiological terms, culture conditions vulnerability as well as exposure to the social and material ecologies relevant to health at the level of the individual and the population.
Biomarkers: definitions and applications
Recognition that interactions with culture, context, and experience are embodied has fueled demand for biological measures to track the effects of culture on health and welfare. But much that would be important to know about bodily states and functions cannot be accessed by visual inspection or interview. In epidemiology, the use of biomarkers has surged as attention has shifted from identification of risk factors, to explanations of differential outcomes (why some at-risk individuals are affected and others are not) on the one hand, and integration of health risks into more comprehensive explanations of health disparities on the other (Berkman and Kawachi 2000). Biomarkers offer a means to track differential exposure as well as impact of exposure. As such, they reflect individual vulnerability, ongoing person-environment interaction, and unmeasured environmental factors that mediate the effect of exposures. For anthropologists and human biologists, biomarkers comprise a vital tool for research into biocultural dynamics that shape differential well-being. Thus, biomarkers can complement sociocultural analysis by probing biocultural pathways that shape health but lie outside of awareness, everyday observation, or cultural discourse (Panter-Brick and Worthman 1999).
Expanding use across diverse disciplines has increased the need for more precise yet complete conceptualizations of biomarkers. Divergent usages of the construct have emerged and reflect disparate disciplinary goals and logic. To maintain momentum across a diverse research front and avert unproductive confusion, now is the time for a conceptually grounded, empirically driven honing process toward definition, validation, and interpretation of biomarkers. Accordingly, in the following sections we discuss and compare schemas for biomarkers and their informing logics, propose a more comprehensive and broadly applicable model for biomarkers, and briefly present three exemplars from our collaborative research on developmental epidemiology of mental health.
Definitions of a “Biomarker”
Convergent trends drive the burgeoning interest in biomarkers. In 2000, an influential report from the Biodemography group in the National Academies of Medicine highlighted the potential for integration of biomarkers in the study of “environmental factors that influence human health” ((Finch et al. 2000), p. 5). Shortly thereafter, the NIH Roadmap for health was initiated and emphasized new discovery tools alongside translation to treatment and prevention, from “bench to bedside” (Zerhouni 2003). These two influential events also signal distinctive approaches to biomarkers that loosely characterize social science and social epidemiological perspectives on the one hand, and biological science and biomedical viewpoints on the other.
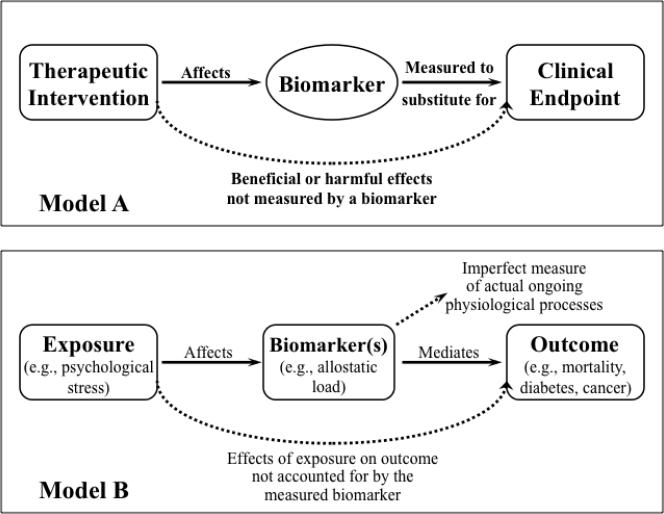
Essentially, a biomarker is a measurable feature that taps into the pathways linking a health outcome to the factors that influence it, and therefore opens a window onto the impact of such factors on that outcome (Figure 1). From this common understanding, biomarker usage has diverged in the relative weight given to either side of the equation. On one side, usage common in social sciences and social epidemiology emphasizes the use of biomarkers for detecting the effects of context or risk exposure on health (Steptoe et al. 2007; Taylor et al. 2006). On the other side, usage prevalent in treatment-oriented biomedical literatures focuses on the value of biomarkers for predicting health outcomes (Weir and Walley 2006). The diagrams in Figure 1 exemplify these contrasting approaches.
Figure 1.
Model A, upper panel, delineates the role of biomarkers as mediators for effects of therapeutic interventions on clinical outcomes (in this case, specifically for clinical trials). Redrawn from(Biomarkers Definitions Working 2001), p. 93. Model B, lower panel, emphasizes the place of biomarkers in mediating pathways from exposure to outcome, but also highlights complex exposures (e.g., stressors), integrative biomarkers (e.g., allostatic load), chronic (e.g., diabetes) or multifactorial (e.g., mortality) outcomes, and measurement uncertainty. Redrawn with minor modification from (Loucks et al. 2008), p. 526.
Biomedical orientations
In Figure 1, Model A depicts the scheme advanced by the Biomarkers Definitions Working Group, which defined a biomarker as: “A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working 2001), p. 91). Although the definition includes the full logic behind biomarkers, the report and the model concentrate on the right side of the equation, on their role in detection of disease and progression, and especially in the development and use of novel treatments. In this therapeutic orientation, a valid biomarker must accurately predict the impact of an intervention on the target clinical outcome (Weir and Walley 2006). Examples include blood pressure for monitoring cardiovascular disease (Psaty et al. 1999), blood glucose for diagnosis of diabetes mellitus (Biomarkers Definitions Working 2001), and CD4 for HIV progression (Deyton 1996).
By definition, a biomarker predicts an outcome (Lassere et al. 2007). For purposes of clinical care and biomedical research, an ideal biomarker lies directly on the functional pathway that mediates pathology, such as a gene expression marker for a key enzyme. It predicts likelihood of the outcome with complete certainty and specificity, and therefore provides early warning of the endpoint (e.g., stroke, death) (Lesko and Atkinson 2001). Such an ideal biomarker can act as a surrogate endpoint to mobilize efforts to avert impending events through appropriate treatment (Lassere et al. 2007). Thus, strongly predictive biomarkers can accelerate early detection, increase diagnostic precision, tailor choice of drug treatment, monitor treatment efficacy, and help navigate co-morbidity (Weir and Walley 2006). These advantages have placed biomarkers-identified through advanced technologies such as genomics, proteomics, or imaging-at the center of efforts toward individualized prevention, treatment, and clinical care in predictive medicine (Woodcock and Woosley 2008). Such high stakes have raised the pressure to establish criteria for valid biomarkers that meet clinical, industrial, and ethical needs.
But biomarkers are necessarily imperfect, for two sorts of reasons (dotted lines, Figure 1). First, virtually any significant health outcome is mediated and modulated by multiple pathways, so a final common pathway is unlikely and a “perfect” biomarker will be elusive. Similarly, an outcome may be affected by an exposure (in this case, a treatment) via multiple pathways. Hence, a path-specific marker may not represent other pathways and give incomplete information about effects of treatment on outcome. Therefore, what a nearly ideal biomarker may gain in precision to pinpoint a specific outcome may be offset by its lack of breadth for tapping into sources of comorbidity and broad-spectrum threats to health. Second, any biomarker represents an estimate of actual physical states or physiological processes. Sources of imprecision include not simply methodological limitations, but also sampling constraints including invasiveness, affordability, timing/frequency, and limited time depth. Such limitations suggest the need for more broadly conceived definitions of a biomarker. Psychiatry, for instance (Kraemer et al. 2002), has emphasized a necessary distinction between the biomarker and the event or processes that it marks, and the absence of an obligate causal connection between them. Consequently, treatments that affect the biomarker may not produce significant change in the target outcome.
Social science and epidemiologic orientations
Health researchers who focus on the contextual determinants of health approach the biomarker equation rather differently (Model B, Figure 1). Often, they seek to understand the impact of daily experience and social conditions. Those goals are best served by biomarkers that tap cumulative, broad-spectrum effects on function and well-being. Such biomarkers should stand downstream of the dynamic interplay of context and bodily states (including cognition, emotion, and physiology), and represent the aggregate burden or benefit of life as it is lived and experienced. A prominent example, stress, illustrates these points. Psychosocial stress has been linked to myriad mental and physical health sequelae (Sapolsky 1998), but is difficult to measure and for that reason, biomarkers play a crucial role in its assessment. Because a key feature of stress is its distinction as reflecting impact rather than exposure to stressors, assessments of stress must tap internal states rather than mere exposure. But the value of self-reported stress is limited because respondents may modulate, recast, fail to report, or actually be unaware of their levels of stress. Time course also presents difficulties, depending on whether the goal is to track acute responses to stressors or cumulative impact of stressors for prediction of health outcomes.
Physiologic measures surmount these challenges: some index acute responsivity and ongoing states, while others reflect the cumulative burden of hardship and act as bioassays for aggregate stress. Both acute and chronic stressors have been linked to altered regulation of the HPA (hypothalamo-pituitary-adrenal) axis that in turn intersects with changes in immune profiles and metabolic regulation. Together, this suite of effects increases vulnerability to psychosocial stress and psychopathology (Heim and Nemeroff 2001; Simeon et al. 2007), to physical morbidities and infections (Christian et al. 2006; Goodkin and Visser 2000), and to chronic metabolic and cardiovascular disease (Gruenewald et al. 2006; Taylor et al. 2006). Clearly, identification of a single biomarker to represent either stress levels or the burden of stress would be misguided. The widespread effects of stress have prompted the search for an aggregate measure of the physical burdens of coping with stress by creating an index representing key functions or systems affected by stress, termed allostatic load (McEwen and Wingfield 2003; Seeman et al. 2001). Constructs of allostatic load have met with varied success for predicting health outcomes (Gersten 2008; Singer et al. 2004), and their predictive capacity clearly relates to the measures used to contruct allostatic load (Worthman and Panter-Brick 2008) as well as their aggregate relationship to a specific outcome (Loucks et al. 2008).
Selection of biomarkers
In sum, identification of an ideal biomarker must depend on the purposes for which it will be used. If the goal is to gauge the impact of multiple contextual, experiential, and behavioral factors (such as poverty or social conditions), then biomarkers are needed that stand downstream from and essentially represent bioassays for the combined effects of those dynamics. Such biomarkers also are selected on the basis of their known linkages to health outcomes, but those linkages may be multiple and remote. For example, stunted height has been linked to reduced life expectancy, but is used largely as a sensitive index for the quality of early environments rather than as a predictor of mortality (WHO 1995). The emphasis in using height is not on prediction of mortality risk, but first on gauging quality of the environment for child development to guide practices and early interventions, and second on its known relationships to a wide spectrum of outcomes. Many public health interventions seek to optimize such multiplier effects across multiple systems and capacities.
On the other hand, if the goal is to predict a specific outcome and then to gauge the effect of treatment against that outcome, then selection of an ideal biomarker will be guided by the specificity, predictive power, and extent of advance warning it provides. The reliance of clinical care on a biomarker places a premium on these criteria, for misidentification of a marker could lead to misdirected treatment and increased rather than decreased morbidity and mortality. The use of premature ventricular contractions in the 1970s and 1980s as surrogate outcomes for effective treatment against sudden cardiac arrest is a case in point (Kraemer et al. 2002): use of prophylactics against such contractions proved to have no effect on outcome and many died from ineffective treatment. The emphasis in selecting a biomarker for an outcome such as cardiac arrest necessarily focuses on power to predict the outcome and track the impact of interventions, pharmacologic or otherwise, on the outcome. Clinical care emphasizes ability to predict specific outcomes reliably.
Biomarkers at the intersection of cultural ecology and epidemiology
For those interested in effects of culture and social ecology on health, biomarkers offer a valuable means to track relationships between general social conditions or more specific beliefs, values, or practices with capacities, function, or load. Thus, in a study of health effects of social transformation in Nepal, biomarkers revealed that widespread urban migration and the rising numbers of street children were rooted in poor rural conditions and high health burden: even street or squatter children in Kathmandu were physically better off than those on rural farms (Worthman and Panter-Brick 2008). Furthermore, the importance of maintaining maternal ethnobotanical knowledge for child welfare in a resource-poor Bolivian context was identified from biomarkers of child growth, nutrition, and immune function (McDade et al. 2007). Another study of orphaned and institutionalized Nepali boys demonstrated a relationship of low cortisol with aggression that confirmed these associations for the first time in non-western children (Hruschka et al. 2005) and informs debates over treatment practices and care for traumatized children such as orphans (Cohen et al. 2008; De Bellis et al. 1999).
Here we illustrate our use of biomarkers for teasing out interactions of culture and social ecology with developmental processes to influence the mental health risks for children growing up under less materially challenging conditions, in the U.S. We draw upon three examples from our 16-year experience of collaboration on the Great Smoky Mountains Study, which was launched in 1992 as a population-based longitudinal study of the developmental epidemiology of psychiatric disorder in rural and urban youth (Costello et al. 1996). A representative sample of 1420 children ages 9, 11, and 13 years at intake was recruited from an 11-county area of western North Carolina; the study is ongoing and includes assessment of mental health status using the Child and Adolescent Psychiatric Assessment (CAPA) (Angold and Costello 2000), household demographics, function, and health histories, and biomarkers for pubertal development and allostatic load (anthropometrics, morphometrics, and blood spots).
A generalized model for biomarkers
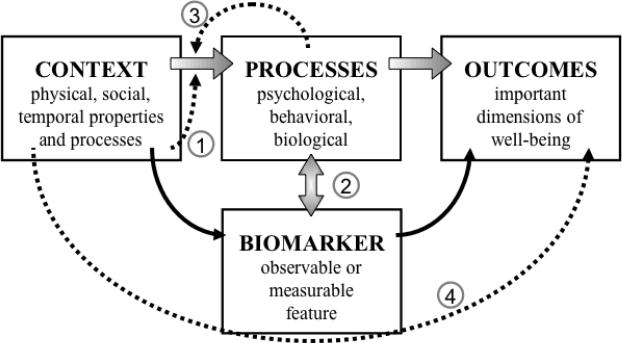
The bioanthropological side of this research has been guided by a working model for conceptualization, use, and interpretation of biomarkers, presented in Figure 2. We seek to understand relationships between context (poverty or family dysfunction) and outcomes (mental or physical health). Such relationships are mediated by processes that are difficult to measure fully and therefore require use of a biomarker that indexes processes mediating target outcomes. Statistical criteria for a mediator/marker are that it must: 1. be significantly associated with both risk and outcome variables; and 2. reduce or eliminate the main effect of the risk variable on the outcome when it is included in the model (Nurnberger 1992; Seeman et al. 2001). The open shaded arrows denote “real” but partly unmeasurable relationships; the solid arrows indicate measurable relationships required for validity of a biomarker; and the dotted lines indicate pathways that moderate predictiveness of the focal context-process-outcome pathway.
Figure 2.
Generalized model showing relationship of a biomarker to the target pathway from context through processes to outcomes. Features distinct from the target pathway are: dotted line 1: unmeasured effects of context on the relationship between context and processes; vertical double arrow 2: a range in strength of relationship between the biomarker and the target, from tight mediation to indirect indicator; dotted line 3: potential moderation of the relationship of context to processes by the impact of context on processes; and dotted line 4: unmeasured effects of context on outcome.
Our working model is quite similar to schema depicted in Figure 1. We highlight differences regarding components that, in our experience, make our model more flexible and effective across the diverse performance demands to which biomarkers are subject. Starting from the input or exposure side at the left in Figure 2, exposure conditions are glossed as context. The choice of such a generic term forces precise thinking about the exposure factor in question-why it is selected, how it is defined, and how it is measured. As discussed above, the exposure of concern may be a global condition such as poverty for some investigations, or a specific condition such as pharmacological intervention for others. For the former, reliance on a single biomarker may overlook the multi-systemic effects of poverty and fail to identify its active ingredients in health outcomes. Biomarkers can be deployed to parse out those active ingredients by helping identify the conditions and processes that have greatest impact on them. For the latter, use of a narrowly focused biomarker for treatment effects may miss other important contextual moderators of treatment efficacy, such as poverty or social support (dotted arrow numbered “1”, Figure 2), as well as side effects on other systems not indexed by the marker (Loucks et al. 2008). Biomarkers can be used in a systematic search for confounding conditions and significantly enrich the understanding of contextual determinants of health, while a wider biomarker array can facilitate the search for side effects.
Turning to the center of the model, we distinguish between mediating processes and biomarkers to emphasize that the two are not necessarily isomorphic, as indicated by a bidirectional arrow between them (numbered “2”, Figure 2). A biomarker may strongly predict an outcome yet not be a central agent in the pathway to it; the strength of correlation denoted by the arrow may be well below one. Valuable biomarkers may index cumulative effects of mediating processes over time or across multiple pathways, rather than be prime mediators themselves. For example, the dominant acute phase inflammatory protein, C-reactive protein (CRP), indexes systemic burden and predicts risk for cardiovascular disease at least 15 years into the future by reflecting ongoing inflammatory response to cardiovascular wear and tear (Ridker 2008; Sakkinen et al. 2002), although it does play an atherogenic role in plaque formation from childhood onwards (Jarvisalo et al. 2002). CRP also indexes risk for diabetes and even all-cause mortality (Ridker 2008), less because it is the principal agent in pathogenesis of many disorders but because it reflects the inflammatory responses that attend and may exacerbate pathogenic conditions.
A common feature of adaptation, but especially of pathways for psychobehavioral outcomes, is that the impact of previous exposures to contextual challenge (life events, social marginalization) on mediating processes (vigilance, physiologic reactivity to stressors) alters the context-process relationship itself (dotted line 3, Figure 2). Thus, the information value of a biomarker may vary by prior exposures that interact with endogenous vulnerability to yield wide variation in context-process relations (bidirectional arrow 2, Figure 2). Post-traumatic stress disorder (PTSD) is a case in point. The definition of PTSD as a stress-related condition has prompted investigation of the mediating role of the principal neuro-endocrine agent in stress responses, the hypothalamo-pituitary-adrenal axis (HPA), as reflected in circulating cortisol. But the relationship of cortisol as a biomarker of PTSD risk or status remains uncertain, due apparently to the effects of endemic (gender, genetic constitution) and circumstantial (history and nature of exposures, social support, time course) factors that moderate the relationship between exposure and response (Meewisse et al. 2007).
Finally, with regard to the right hand side of Figure 2, “outcomes” is plural because a biomarker may be selected for specificity to one outcome or high representation of overall health effects. Recall the previous discussion regarding the differing selection criteria for biomarkers that maximize specificity for a given outcome such as heart attack, versus those that represent cumulative, broad-spectrum effects from a type of exposure such as inequity. Such global markers also may reduce the risk of overlooking effects of exposure on outcomes that are unmeasured by the biomarker (dotted line 4, Figure 4). Turning again to the example of CRP, its value as a biomarker for cardiovascular risk derives from indexing inflammatory responses to systemic load. But its capacity to predict many other health outcomes has made it a far more valuable biomarker of lifestyle health risk than more specific markers of cardiovascular condition such as the treadmill stress test (Vasan 2006).
Overall, our more generic configuration of the biomarker model accommodates the need for critical empirical evaluation in conceptualization of each element comprising the biomarker equation, from contexts through processes to outcomes.
Puberty and depression
An initial goal for inclusion of biomarkers in the GSMS was to test competing hypotheses about the role of puberty in development of a major disparity in mental health, namely the emergence between ages 10 and 15 of a roughly 2:1 difference in rates of depression in girls and boys, respectively (Angold and Rutter 1992; Angold and Worthman 1993). Debate had centered on the role of psychosocial and cultural factors (maturity-graded treatment, self perception, peer selection and pressures, gendered expectations of behavior, appearance, and opportunity) versus that of biological ones (puberty-related neuro-endocrine changes, associated brain maturation and cognitive shifts)(Angold and Worthman 1993). We used annual assessments of central and peripheral endocrine markers (gonadotropins, and gonadal and adrenal steroids) as well as morphological changes of puberty along with interviews regarding peer relations, stressors, self perceived maturation, and mental health status to test four hypothesized pathways to gender differences in rates of depression—social mediation, maturational cuing, central nervous system mediation, and endocrine mediation (Angold et al. 2003). Contrastive analyses revealed that circulating hormones (gonadal and adrenal steroids) manifested a threshold effect and accounted more strongly for depression in girls than did the competing hypotheses—relationships and exposure to stressors (life events), morphologic changes (visible cues), timing of puberty (peer effects and social cuing), or central nervous system shifts (gonadotropins) (Angold et al. 1999; Angold et al. 1998; Angold et al. 2003).
These findings are based on our relatively comprehensive but still incomplete assessments of the social and physical factors involved in the pathways we tested, and as such cannot be viewed as a final account. At the least, endocrine status must interact with precipitating factors, for not all girls become depressed. Nevertheless, the results exemplify the value of integrating biomarkers with interview measures to first set up an array of competing hypotheses about biological, cultural, social, and psychological sources of a major health disparity, and then systematically test them.
Gender, stress, and puberty
The onset of depression at adolescence in girls is much better understood than in boys. The gap is troubling because, although boys have lower rates of depression, the correlates and comorbidities related to depression can be more lethal for them (Gould et al. 2003). One could go beyond asking why girls become depressed and consider why boys do not. We found that the early stages of pubertal progression are accompanied by reduction in prevalence of depression in boys (Angold et al. 1998). We also found suggestive evidence that the relationship between stressful life circumstances and depressive symptoms was nearly extinguished in early-mid puberty for boys, while it increased sharply in girls. To test this possibility, we measured an immunologic biomarker of stress in a GSMS subsample of 256 boys and girls ages 9-13, and hypothesized that its relationship with stressful life circumstance would follow a corresponding trajectory of gender difference during puberty. The biomarker, Epstein Barr Virus (EBV), reflects the effect of ongoing psychosocial stress on cell-mediated immune function: the higher the titer, the greater the level of ongoing stress-related immunocompromise (Glaser and Kiecolt-Glaser 1994).
We identified gender differences in the associations of stressors with this immunologic marker of stress that suggest genders differ in the impact of exposure to stressors (McDade et al. 2000). Traumatic life events were associated with increased EBV in girls, not boys. Moreover, the relationship of traumatic events to EBV was present in girls under high but not low life strain (strain was summed from 32 ecological stressors), and absent in boys at either level of strain. Such gender differences and interactive effects may be due to differences in perception of, reactivity to, or coping with stressors. Whatever the etiology, results suggest that the burden of depression from exposure to stressful life circumstances will fall more heavily on girls than boys. The findings underscore two points about the relationship of culture to health. This relationship extends well beyond determining momentary exposures to risk or advantage insofar as culture shapes person x environment interactions in developmental ecology as well as in meanings of and responses to life experiences. Consequently, individuals within a society can reside in the same place, but inhabit different cultural and epidemiological spaces. Gender and gender-differential health risks exemplify this phenomenon.
Birthweight and depression
Intensive investigation of the concept of fetal programming has revealed effects of fetal conditions on subsequent function and health (Barker et al. 2002). These discoveries have led to recognition that early environments are important contexts for health, and that birthweight represents not only a gestational outcome but also a biomarker for health risk. Furthermore, such risk operates contingent on postnatal conditions: the long-term health effects of fetal adjustments to gestational conditions depend on match or mismatch with conditions under which the individual must function after birth (Worthman and Kuzara 2005).
The effects of early environment may extend to mental health. With data from the GSMS, we tested whether low birth weight (LBW) acted as one of many cumulative risk factors, or via altered sensitivity to postnatal conditions (Costello et al. 2007). We found that LBW indeed was associated with depression at puberty in girls, not boys, and that LBW girls showed a sensitization effect to adversity. Specifically, adversity is linked to onset of depression in our sample and others (Agid et al. 2000; Brown and Harris 1979; Copeland et al. 2007); in the absence of adversities, LBW and normal birth weight girls had virtually no depression. Moreover, with each additional exposure to adversity, risk for depression increased more rapidly in low than in normal birth weight girls.
Therefore, both the adverse maternal-gestational conditions that led to low birth weight and the social-structural circumstances that increased postnatal exposure to adversities were associated with depression in girls at puberty. Such findings indicate synergistic routes of embodiment by which social conditions and practices influence health, both by shaping the course of physical development, and by determining individual life circumstances. Maternal condition and behavior-including psychosocial stress, nutrition, workload, smoking and drug use-influence gestational outcomes including birthweight. The case for fetal outcomes suggests that the condition and treatment of women will have long-term effects on their children's welfare (Adair et al. 2001), and directly implicates cultural values, attitudes and practices toward women and girls in determining population health. Reciprocally, the embodied outcomes of earlier circumstances interact with subsequent stressors and supports to produce pathways to health disparities. Hence, a biomarker such as birthweight both exemplifies processes of embodiment and aids in tracking pathways to health disparity.
Discussion
The concepts, models, and findings discussed here illustrate how epidemiology and biocultural anthropology are natural allies in forging new approaches to population health. First, the population-based epidemiologic framework provides representativeness and sample sizes required for complex biosocial research designs. Large samples that oversample for risk, as did GSMS, also capture sufficient case numbers to support investigation of outcomes with relatively low base rates, such as depression. Second, the study is longitudinal, an uncommon feature in human biology and biocultural anthropology. Longitudinal data are needed to track mediators and moderators in pathways to health outcomes. Third, the study was grounded in a thoroughly developmental ecological framework (Costello and Angold 2006) that attended to developmental processes and mandated measures of contexts, experiences, physical development and function, along with mental and physical health outcomes. Fourth, biomarkers were integrated into the study from the outset. As our findings indicate, we have used marker arrays rather than select a single biomarker, first as a means to track the multidimensional process of puberty, and increasingly to probe the impact of stressors and differential stress with markers of acute (cortisol), short range cumulative (EBV), and long range cumulative (CRP) stress burden. Identification of predictive markers of health risk, particularly genotype- or phenotype-environment interactions that contribute to the differential impact of stressors, must rely on systematic use of both biomarkers and assessment of context and experience. Thus, identification of an interaction of birthweight and adversity in risk for depression in girls required not only the biomeasure, but also assessment of adversities as well as other known risk factors. Finally, the mental health focus of this work addresses a widely recognized global need to identify and address the bases of mental health: depression presently constitutes the second largest contributor to the global burden of disease among those aged 15-44 years, and is projected to attain that rank for humans of all ages by 2020 (Murray and Lopez 1996).
Our generalized model of biomarkers offers a flexible but rigorous framework for their use by both social and biomedical sciences. We highlight that the biomarker model also demands more rigorous conceptualization of “context” than is usual. Findings from the GSMS illustrate points raised at the outset regarding embodiment and the role of biomarkers for tracking how culture gets under the skin to influence population health and health disparities. Note that these findings engage indirect effects of culture that determine life experience and conditions such as exposure and impact of material and social adversity, rather than our direct measures of cultural models (see Brown et al., this issue). We have found that biomarkers can be effective for distinguishing the roles of biological, cognitive, and sociocultural factors in differential health, in this case the emergence of sex differences in depression at puberty in the GSMS. Embodiment was manifest in the relationship of low birth weight to depression at puberty in girls: poor gestational conditions translated into phenotypic vulnerability. But the effect was indirect, operating through altered sensitivity to adversity for development of depression. Here, then, we observed a phenotype x environment interaction in depression risk that exemplifies why social address alone commonly explains a fraction of variance in health, the unique value of biomarkers for tapping unexplained variance, and the need for a generalized model of biomarkers such as we propose.
The findings also illustrate a role for recurrent bio-environment interactions thought to characterize pathways to health disparity. Gene-environment interactions have been reported in longitudinal studies linking allelic variants with differential impact of early rearing conditions on later outcomes such as conduct disorder (Caspi and Moffitt 2006). In the case of our data regarding low birth weight, early effects of poor gestational context manifest in birth outcome establish a heightened sensitivity to postnatal hardship that conduces to depression. We have not yet identified the physiologic correlates of low birth weight that may mediate such sensitivity, but depression initiates pathophysiology that, once initiated, sets the stage for chronic disorder (Fava and Kendler 2000). Such evidence suggests a potential pathway from maternal conditions, to differences in offspring vulnerability to adversity, to persistent mental health disparities among daughters related to stressful experience. Our findings also are consistent with preclinical studies documenting contextual mediation of epigenetic transmission of psychobehavioral phenotypes (Weaver 2007)
In sum, revised understandings of both biology and culture have energized a new generation of integrated research into previously intractable problems such as the bases of health disparities or the impact of poverty on well-being. Apparently, the body often can tell us what we need to know about psychological, sociological, or cultural factors previously approached solely through other means, such as questionnaires or population statistics. This discovery has spurred interest in biomarkers as a powerful tool for tracking health disparities, and for understanding the role of culture in mental and physical health. Important as social address (poverty, class, ethnicity, etc.) appears to be, established measures of social factors only explain a portion of the variation in health outcomes and fail to identify mediating pathways. The work reviewed here demonstrates the boost to explanatory power that biomarkers bring to the ambitious multifactorial research designs that social and biomedical sciences recognize as necessary for tackling heretofore intractable questions about population health and health disparities. It also illustrates effective collaboration of epidemiology and biocultural anthropology in this enterprise.
Acknowledgments
We thank participants in the Great Smoky Mountains Study whose sustained participation makes the research possible. Support for the study includes grants from National Institutes of Mental Health MH57761 and the W.T. Grant Foundation 94148992.
Footnotes
Declaration of interest: The authors identify no conflicts of interest, and affirm sole responsibility for the content and writing of the paper.
References
- Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation. 2001;104:1034–1039. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother. 2000;54:135–141. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- Anderson-Fye EP. Never leave yourself: Ethnopsychology as mediator of psychological globalization among belizean schoolgirls. Ethos. 2003;31:77–112. [Google Scholar]
- Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello E, Worthman C. Puberty and depression: The roles of age, pubertal status, and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. The child and adolescent psychiatric assessment (capa) J Am Acad Child Adol Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Angold A, Rutter M. Effects of age and pubertal status on depression in a large clinical sample. Dev Psychopathol. 1992;4:5–28. [Google Scholar]
- Angold A, Worthman CM. Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman CM, Costello EJ. Puberty and depression. In: Hayward C, editor. Gender differences at puberty. Cambridge University Press; New York: 2003. pp. 137–164. [Google Scholar]
- Armelagos GJ, Brown PJ, Turner B. Evolutionary, historical and political economic perspectives on health and disease. Soc Sci Med. 2005;61:755–765. doi: 10.1016/j.socscimed.2004.08.066. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: Strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Berkman L, Kawachi I, editors. Social epidemiology. Oxford University Press; Oxford: 2000. [Google Scholar]
- Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Briggs CL, Mantini-Briggs C. Stories in the time of cholera. University of California Press; Berkeley: 2003. [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. Free Press; New York: 1979. [Google Scholar]
- Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Yearbook Phys Anthropol. 2002;45:159–184. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Changeux J-P. Neuronal man. Pantheon; New York: 1985. [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Kelleher KJ, Mannarino AP. Identifying, treating, and referring traumatized children: The role of pediatric providers. Arch Pediatr Adolesc Med. 2008;162:447–452. doi: 10.1001/archpedi.162.5.447. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry. 2007;64:577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Angold A. Developmental epidemiology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, vol 1: Theory and method. 2nd Ed. John Wiley & Sons Inc.; Hoboken, NJ: 2006. pp. 41–75. [Google Scholar]
- Costello EJ, Angold A, Burns B, Stangl, Tweed D, Erkanli A, Worthman CM. The Great Smoky Mountains Study of youth: Goals, design, methods, and the prevalence of DSMIII-R disorders. Arch Gen Psychiatry. 1996;53:1129–1136. doi: 10.1001/archpsyc.1996.01830120067012. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Worthman CM, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression - a test of competing hypotheses. Arch Gen Psychiatry. 2007;64:338–344. doi: 10.1001/archpsyc.64.3.338. [DOI] [PubMed] [Google Scholar]
- Csordas TJ. Embodiment as a paradigm for anthropology. Ethos. 1990;18:5–47. [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- DeCaro JA, Worthman CM. Culture and the socialization of child cardiovascular regulation at school entry in the U.S. Am J Hum Biol. 2008;20:572–583. doi: 10.1002/ajhb.20782. [DOI] [PubMed] [Google Scholar]
- Deyton L. Importance of surrogate markers in evaluation of antiviral therapy for HIV infection. JAMA. 1996;276:159–160. [PubMed] [Google Scholar]
- Dressler WW, Balieiro MC, dos Santos JE. Culture, socioeconomic status, and physical and mental health in Brazil. Med Anthropol Q. 1998;12:424–446. doi: 10.1525/maq.1998.12.4.424. [DOI] [PubMed] [Google Scholar]
- Dressler WW, Oths KS, Gravlee CC. Race and ethnicity in public health research: Models to explain health disparities. Annu Rev Anthropol. 2005;34:231–252. [Google Scholar]
- Ellison PT, editor. Reproductive ecology and human behavior. Aldine de Gruyter; New York: 2001. [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Finch CE, Vaupel JW, Kinsella K, editors. Cells and surveys: Should biological measures be included in social science research? National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Flinn MV. Evolution and ontogeny of stress response to social challenges in the human child. Dev Rev. 2006;26:138–174. [Google Scholar]
- Gersten O. Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in taiwan. Soc Sci Med. 2008;66:507–519. doi: 10.1016/j.socscimed.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation and its implications for reactivation of latent herpes-viruses. In: Glaser R, Jones J, editors. Human herpesvirus infections. Marcel Dekker; New York: 1994. pp. 245–270. [Google Scholar]
- Goodkin K, Visser AP, editors. Psychoneuroimmunology: Stress, mental disorders, and health. American Psychiatric Publishing; Washington, DC: 2000. [Google Scholar]
- Goodman AH, Leatherman TL. Building a new biocultural synthesis: Political-economic perspectives on human biology. University of Michigan Press; Ann Arbor: 1999. [Google Scholar]
- Gould MS, Greenberg T, Velting DM, Shaffer D. Youth suicide risk and preventive interventions: A review of the past 10 years. J Am Acad Child Adol Psychiatry. 2003;42:386–405. doi: 10.1097/01.CHI.0000046821.95464.CF. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. The Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. PNAS. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jarvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, Lehtimaki mT, WSimell O, Simell O, Raitakari OT. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Schultz SK, Arndt S. Biomarkers in psychiatry: Methodological issues. Am J Geriatr Psychiatry. 2002;10:653–659. [PubMed] [Google Scholar]
- Krieger N. Epidemiology and the web of causation: Has anyone seen the spider? Soc Sci Med. 1994;39:887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Krieger N. Epidemiology and social sciences: Towards a critical reengagement in the 21st Century. Epidemiol Rev. 2000;22:155–163. doi: 10.1093/oxfordjournals.epirev.a018014. [DOI] [PubMed] [Google Scholar]
- Krieger N, editor. Embodying inequality: Epidemiologic perspectives. Baywood Publishing; 2004. [Google Scholar]
- Krieger N, Davey Smith G. “Bodies count,” and body counts: Social epidemiology and embodying inequality. Epidemiol Rev. 2004;26:92–103. doi: 10.1093/epirev/mxh009. [DOI] [PubMed] [Google Scholar]
- Lassere MN, Johnson KR, Boers M, Tugwell P, Brooks P, Simon L, Strand V, Conaghan PG, Ostergaard M, Maksymowych WP, et al. Definitions and validation criteria for biomarkers and surrogate endpoints: Development and testing of a quantitative hierarchical levels of evidence schema. J Rheumatol. 2007;34:607–615. [PubMed] [Google Scholar]
- Lesko LJ, Atkinson AJ., Jr Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: Criteria, validation, strategies. Annu Rev Pharmacol Toxicol. 2001;41:347–366. doi: 10.1146/annurev.pharmtox.41.1.347. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Juster RP, Pruessner JC. Neuroendocrine biomarkers, allostatic load, and the challenge of measurement: A commentary on Gersten. Soc Sci Med. 2008;66:525–530. [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Davey Smith G, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: The whitehall ii study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- McDade TW, Reyes-Garcia V, Blackinton P, Tanner S, Huanca T, Leonard WR. Ethnobotanical knowledge is associated with indices of child health in the Bolivian Amazon. Proc Natl Acad Sci U S A. 2007;104:6134–6139. doi: 10.1073/pnas.0609123104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Angold A, Costello EJ, Burleson M, Cacioppo JT, Glaser R, Worthman CM. Epstein-Barr virus antibodies in whole blood spots: A minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000;62:560–568. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- McDade TW, Worthman CM. Evolutionary process and the ecology of human immune function. Am J Hum Biol. 1999;11:705–717. doi: 10.1002/(SICI)1520-6300(199911/12)11:6<705::AID-AJHB1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McGarvey ST. Population health. Ann Hum Biol. 2007;34:393–396. doi: 10.1080/03014460701517157. [DOI] [PubMed] [Google Scholar]
- Meewisse M-L, Reitsma JB, de Vries G-J, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The global burden of disease. Harvard University Press/WHO; Cambridge, MA: 1996. [Google Scholar]
- Nurnberger JI. Should a biologic marker be sensitive and specific? Acta Psychiatr Scand. 1992;86:1–4. doi: 10.1111/j.1600-0447.1992.tb03217.x. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C, Worthman CM, editors. Hormones, health, and behavior: A socioecological and lifespan perspective. Cambridge University Press; New York, NY: 1999. [Google Scholar]
- Psaty BM, Weiss NS, Furberg CD, Koepsell TD, Siscovick DS, Rosendaal FR, Smith NL, Heckbert SR, Kaplan RC, Lin D, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282:786–790. doi: 10.1001/jama.282.8.786. [DOI] [PubMed] [Google Scholar]
- Ridker PM. High-sensitivity C-reactive protein as a predictor of all-cause mortality: Implications for research and patient care. Clin Chem. 2008;54:234–237. doi: 10.1373/clinchem.2007.099465. [DOI] [PubMed] [Google Scholar]
- Sakkinen P, Abbott RD, Curb JD, Rodriguez BL, Yano K, Tracy RP. C-reactive protein and myocardial infarction. J Clin Epidemiol. 2002;55:445–451. doi: 10.1016/s0895-4356(01)00502-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why zebras don't get ulcers: An updated guide to stress, stress-related diseases, and coping. W.F. Freeman; New York: 1998. [Google Scholar]
- Scheper-Hughes N. Death without weeping: The violence of everyday life in Brazil. University of California Press; Berkeley: 1992. [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. PNAS. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B, Ryff CD, Seeman T. Operationalizing allostatic load. In: Schulkin J, editor. Allostasis, homeostasis, and the costs of physiological adaptation. Cambridge University Press; New York, NY: 2004. pp. 113–149. [Google Scholar]
- Stein AD, Wang M, DiGirolamo A, Grajeda R, Ramakrishnan U, Ramirez-Zea M, Yount K, Martorell R. Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: A prospective study in guatemala. Arch Pediatr Adolesc Med. 2008;162:612–618. doi: 10.1001/archpedi.162.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Super CM, Harkness S. The environment as culture in developmental research. In: Friedman SL, Wachs TD, editors. Measuring environment across the life span: Emerging methods and concepts. American Psychological Association; Washington, DC: 1999. pp. 279–323. [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Ulijaszek S, Johnston FE, Preece MA, editors. The Cambridge encyclopedia of human growth and development. Cambridge University Press; Cambridge, U.K.: 1998. [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- Weaver ICG. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: Let's call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Walley RJ. Statistical evaluation of biomarkers as surrogate endpoints: A literature review. Stat Med. 2006;25:183–203. doi: 10.1002/sim.2319. [DOI] [PubMed] [Google Scholar]
- WHO . Physical status: The use and interpretation of anthropometry. WHO; Geneva: 1995. [Google Scholar]
- Wilkinson R. The impact of inequality: How to make sick societies healthier. New Press: 2005. [Google Scholar]
- Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- Worthman CM. Emotions: You can feel the difference. In: Hinton AL, editor. Biocultural approaches to the emotions publications of the society for psychological anthropology. Cambridge University Press; New York, NY, US: 1999a. pp. 41–74. [Google Scholar]
- Worthman CM. The epidemiology of human development. In: Panter-Brick C, Worthman CM, editors. Hormones, health, and behavior: A socio-ecological and lifespan perspective. Cambridge University Press; Cambridge: 1999b. pp. 47–104. [Google Scholar]
- Worthman CM, Kohrt B. Receding horizons of health: Biocultural approaches to public health paradoxes. Soc Sci Med. 2005;30:698–714. doi: 10.1016/j.socscimed.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Kuzara JL. Life history and the early origins of health differentials. American Journal of Human Biology. 2005;17:95–12. doi: 10.1002/ajhb.20096. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Panter-Brick C. Homeless street children in Nepal: Use of allostatic load to assess the burden of childhood adversity. Dev Psychopathol. 2008;20:233–255. doi: 10.1017/S0954579408000114. [DOI] [PubMed] [Google Scholar]
- Zerhouni E. Medicine. The NIH roadmap. Science. 2003;302:63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]