Abstract
Hematopoietic stem/progenitor cells (HSPC) transition in location during development1 and circulate in mammals throughout life2, moving into and out of the bloodstream to engage bone marrow (BM) niches in sequential steps of homing, engraftment and retention3–5. We show here that HSPC engraftment of BM in fetal development is dependent upon the guanine nucleotide binding protein stimulatory alpha subunit (Gsα). Adult Gsα−/− HSPCs differentiate and undergo chemotaxis, but also do not home to or engraft in the BM in adult mice and demonstrate marked inability to engage the marrow microvasculature. If deleted after engraftment, Gsα did not lead to lack of retention in the marrow, rather cytokine-induced mobilization into the blood was impaired. Testing whether activation of Gsα affects HSPC, pharmacologic activators enhanced homing and engraftment in vivo. Gsα governs specific aspects of HSPC localization under physiologic conditions in vivo and may be pharmacologically targeted to improve transplantation efficiency.
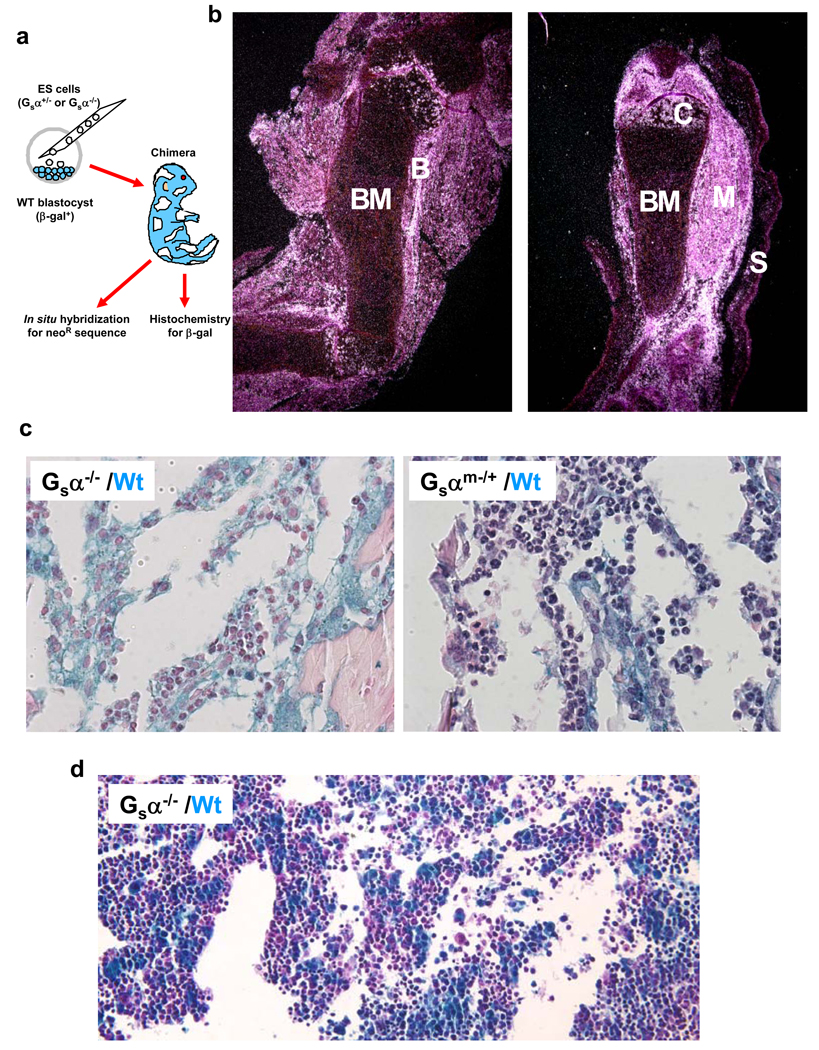
Gsα is essential for normal development with gene deficient embryos exhibiting fatal defects before day E10.56. To overcome this limitation, chimeric mice were generated by blastocyst injection of Gsα−/− embryonic stem cells7 injected into a blastocyst transgenic for β-galactosidase enabling resultant embryos to be assessed for tissue chimerism using in situ hybridization against neo in the targeting vector to score for Gsα−/− (KO) cells and histochemical staining for β-galactosidase to score for Gsα+/+ (WT) cells (Fig.1a). Examining E17.5 embryos by in situ hybridization, chimerism could be readily detected. Limb sections revealed neo expressing Gsα−/− cell involvement of skin, muscle, bone and cartilage, but without evidence of contribution to BM (Fig.1b). In contrast, as a positive control for the in situ hybridization, we examined limb sections from mice chimeric for an unrelated knock-out and could readily detect BM chimerism (Suppl. Fig.1). Independent analysis using β-gal staining for WT cells revealed only β-gal+ cells in the BM of Gsα−/− chimeras, but both β-gal+ and β-gal− cells in the BM of Gsα+/m− chimeras (100% vs. 44% β-gal+, respectively; Fig.1c). Other tissues of Gsα−/− chimeras demonstrated both β-gal+ and β-gal− cells (Suppl. Fig.2). Therefore, the BM appeared distinctive in its lack of contribution from Gsα−/− cells.
Figure 1. Gsα is required for HSPC engraftment of bone marrow in development.
(a) Chimeric mice were created by injection of Gsα−/− or Gsα−/+ embryonic stem cells into wild-type or β-galactosidase transgenic blastocysts. At E17.5, the mice were killed and the organs were probed for Gsα−/− cells (using a neo probe). (b) In situ hybridization of femur and tibia is shown. Bone marrow (BM), bone (B), muscle (M), skin (S) and chondrocytes (C) are indicated. (c) Mice were assessed by histochemistry for β-galactosidase (blue stain) in Gsα−/− (left panel) or Gsαm−/+ (right panel) chimeric animal bone marrow. (d) Histochemical analysis of β-galactosidase expression in fetal liver from E13.5 Gsα−/− chimeric mice.
Evaluation of E13.5 fetal liver demonstrated significant chimerism with Gsα−/− cells by β-gal (48% β-gal+; Fig.1d) and no difference in colony forming unit-cell (CFU-C) content between Gsα+/+ and Gsα−/− chimeric MNCs (Suppl. Fig.3). Therefore, HSPC form and have intact hematopoietic potential in the fetal liver even in the absence of Gsα, but without Gsα, there is a failure to engraft BM by E17.
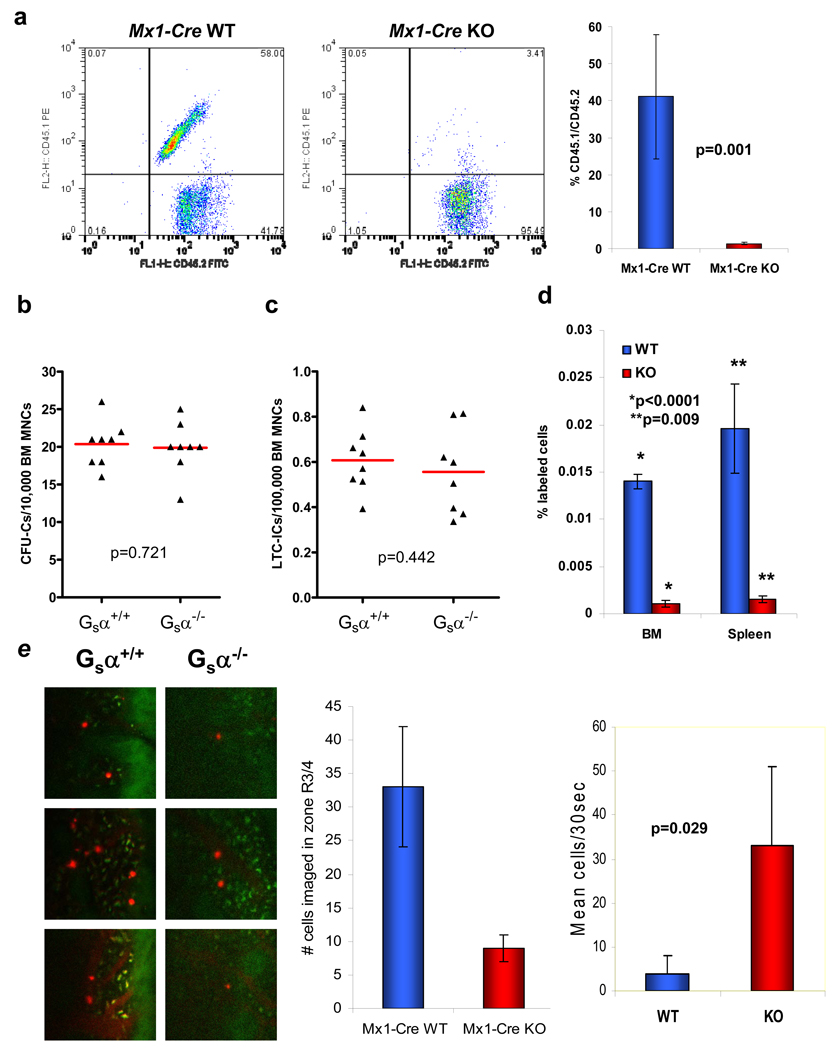
To evaluate whether this developmental requirement was also present in adult HSPC, we crossed Gsαfl/fl mice with Mx1-Cre mice, a strain that enables high efficiency polyI.polyC induced Cre expression in HSPC. Competitive repopulation was examined using BM mononuclear cells (BM MNCs) obtained from 6 week old Gsα+/+Mx1-Cre+ or Gsαfl/flMx-1-Cre+ mice treated with polyI.polyC for 5 days (hereafter referred to as Mx1-Cre WT and Mx1-Cre KO, respectively), and transplanted in competition (1:1) with wild-type cells. Deletion of the Gsα gene in primitive cells was confirmed by PCR (Suppl. Fig.4). Analysis of the number of HSPCs in the BM MNCs, phenotypically defined as lin−c-Kit+Sca-1+Flk-2− was also identical between the Mx1-Cre WT and Mx1-Cre KO animals (WT: 0.031% +/− 0.012; KO: 0.038% +/− 0.002; p=0.545). Cells from the Mx1-Cre WT animals were able to efficiently engraft the BM, while BM MNCs from the Mx1-Cre KO mice were essentially absent from the BM at early (4 weeks) and late (16 weeks) time points following transplantation (Fig.2a). Furthermore, when animals were transplanted with BM cells from Mx1-Cre KO mice in the absence of competing wild type cells, spleen engraftment was significantly reduced as assessed by CFU-S8 number (Suppl. Fig.5), and all animals died within two weeks indicating a failure of extramedullary engraftment. Gsα is therefore required for HSPC engraftment in adult as well as in embryonic settings.
A failure to successfully engraft may reflect multiple abnormalities that we sequentially examined. As others had previously found that pharmacologic modifiers of the adenylyl cyclase pathways influenced myeloid differentiation in vitro8,9, BM MNCs were obtained from Mx1-Cre WT and KO mice and progenitor cell activity was analyzed using the CFU-C assay. No significant differences were observed between the genotypes in colony number (Fig.2b), morphology or size. Similarly, analysis of the more primitive cell fraction using the long-term culture initiating cell (LTC-IC) assay10, demonstrated no significant differences between the WT and KO cells (Fig.2c). Taken together, these data demonstrate that deletion of the Gsα gene does not alter the differentiation potential of primitive BM MNCs in vitro.
Figure 2. Gsα is required for HSPC engraftment of bone marrow in adults.
Gsα was conditionally deleted in hematopoietic cells of 6-week old Mx1-Cre Gsαfl/fl mice and cells were then assessed for engraftment capability. (a) Representative flow cytometric analysis at 16 weeks and collated data of engraftment of Mx1-Cre WT (Gsα+/+) or Mx1-Cre KO (Gsα−/−) cells in competitive repopulation assay (n=8 from 2 independent experiments; error bars=s.e.m.). BM MNCs were assessed for their in vitro growth potential using (b) CFU-C and (c) LTC-IC assays. (d) In vivo homing to WT bone marrow and spleen of Mx1-Cre WT and KO LKS+ cells (n=3; error bars represent s.e.m.). (e) Intravital imaging of calvarium bone marrow of WT recipient mice injected by tail vein with Mx1-Cre WT or KO LKS+ cells stained with DiD. Representative images (red: DiD, green: autofluorescence) and quantitation (graphs) of the number of cells visualized over a 30 second interval at ~35 min. post-infusion to be stably localized or circulating is shown (n=3; error bars=s.d.).
Migratory capacity was next assessed using the primitive lin−c-Kit+Sca-1+ (LKS+) or more mature lin−c-Kit+Sca-1− (LKS−) BM MNCs of Mx1-Cre WT or Mx1-Cre KO mice. In vitro chemotaxis toward SDF-1α, the only known in vitro chemotactic agent for HSPCs11, demonstrated comparable migratory capacity for each genotype of LKS+ cells for all but the lowest concentration of SDF-1α (Suppl. Fig.6a). In addition, there was no significant change in the chemotaxis of the more mature LKS− subset of cells (Suppl. Fig.6b), or lymph node lymphocytes to SDF-1α (data not shown) indicating that there was no generalized impairment of in vitro cell motility related to the Gsα defect.
Primitive cells isolated from BM MNCs of Mx1-Cre WT or KO mice were then evaluated for in vivo capacity to home to the BM environment. Fluorescently-labelled LKS+ cells were injected into the peripheral circulation of mice and the numbers of cells present in the BM and spleen were measured using flow cytometry at 6 hours post-injection. Deletion of Gsα in LKS+ BM MNCs resulted in a marked impairment in their ability to home to both the BM and spleen (Fig.2d). To examine whether this was specific to primitive cell homing to the BM, or a more general defect, we examined the ability of the more mature LKS− and lymph node lymphocytes to home to hematopoietic organs. While LKS− cells demonstrated a similar impairment as LKS+ cells (Suppl. Fig.7a), no defect in Gsα−/− lymphocytes (genotype confirmed by PCR) homing to lymph node, BM or spleen was observed (Suppl. Fig.7b). These data argue against a generalized defect in cell homing associated with a deficiency of Gsα and identify a specific BM homing defect of primitive hematopoietic cells. To assess the specific homing defect of primitive Gsα−/− cells, we visualized HSPC homing in the calvarial BM endothelium using intravital confocal/two-photon videomicroscopy12. We found that Gsα−/− LKS+ cells were defective in their interaction with the BM endothelium with significantly fewer cells observed rolling on the endothelial cells, while the number of cells retained in the circulation was significantly increased (Fig.2e and Suppl. Movies 1 and 2). Therefore, Gsα is critical for the engagement of the BM vasculature by primitive hematopoietic cells thereby enabling subsequent steps in stem cell engraftment.
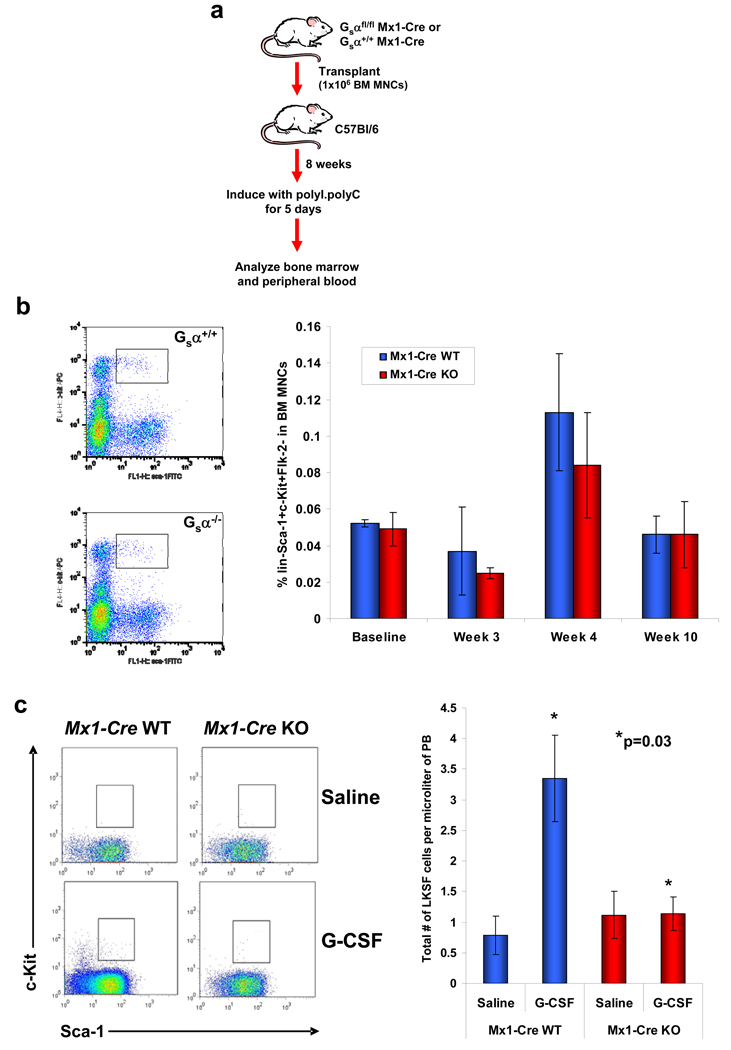
Deletion of the Gsα subunit resulted in significant reductions in the ability of primitive cells to establish hematopoiesis in the BM environment. Therefore, we examined whether the same deletion had any effects once engraftment of the cells in the BM had been achieved. To study this, BM MNCs from Gsα+/+Mx1-Cre+ or Gsαfl/flMx-1-Cre+ mice were transplanted into wild-type mice. Eight weeks following transplantation, the mice were treated with polyI.polyC to induce gene deletion (Fig.3a). Longitudinal analysis demonstrated no significant differences between mice with Mx1-Cre WT or KO cells in terms of BM CBC, CFU-C frequency (Suppl. Fig.8a–c) or HSPC frequency as assessed by immunophenotype (Fig.3b). Specific analysis of HSPC cell cycling status or number of apoptotic cells also indicated that there were no differences between the two different cell genotypes (Supp. Fig.9a,b). Further, there was no evidence of HSPC enriched cell movement from the BM to the peripheral blood by immunophenotypic analysis to indicate a retention defect. Rather, we did observe a striking defect in the ability of the Mx1-Cre KO cells to be mobilized using G-CSF (Fig.3c). These data demonstrate that Gsα signalling is not required for HSPC retention in the marrow, but under conditions of stress, mimicked here by G-CSF stimulation, trafficking out of the marrow space is impaired.
Figure 3. Gsα signalling is not required for retention of the HSPCs in the BM, but does influence mobilization by G-CSF.
(a) Diagrammatic representation of the procedure to assess Gsα signalling in HSPC BM retention. Mx1-Cre Gsα+/+ or Gsαfl/fl cells were injected into wild-type mice; after 8 weeks, deletion of the Gsα gene was induced by polyI.polyC and BM and PB evaluated. (b) Retention of primitive LKS Flk-2− Mx1-Cre WT or KO cells in the bone marrow. Representative flow cytometric analysis at week 10 and mean collated data shown (n=5; error bars=s.e.m.). (c) Representative flow cytometric plots (left) and quantitation (right) of HSPC mobilization into the peripheral circulation following 5 days of G-CSF. Sca-1 and c-Kit expression of gated Lin−Flk-2− cells is shown (n=5; error bars=s.e.m.).
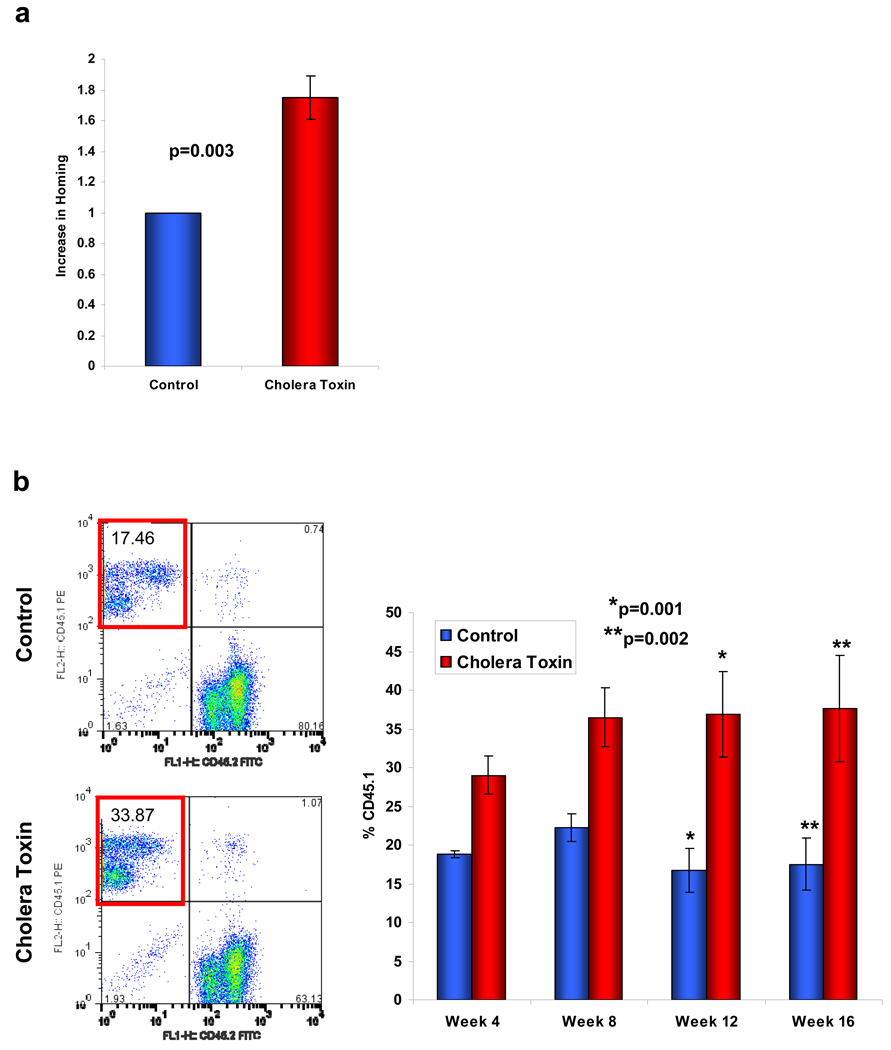
Genetic alteration of Gsα signalling demonstrated a central role in BM homing of HSPCs. However, to determine if these findings might be relevant for medicine, we tested whether pharmacologic modifiers of Gsα signalling could alter stem cell function in vivo. We treated BM MNCs from wild-type C57Bl/6 mice with cholera toxin, a compound known to constitutively activate Gsα by preventing GTP hydrolysis from the ADP-ribose-Gsα-GTP complex. Using a simple ex vivo exposure of cells for 1hour, we observed increased intracellular cAMP concentrations within hematopoietic cells by ELISA (data not shown). This resulted in an enhancement in the ability of treated cells to both home and engraft in the BM with approximately two-fold increased engraftment at 16 weeks (Fig.4a,b). To test whether the enhancement by cholera toxin was due to a non-specific action of the compound, homing of Gsα−/− cells treated with cholera toxin was assessed; however no improvement in the homing defect was observed (Suppl. Fig.10). Since stimulation of Gsα-coupled receptors has also been noted to increase proliferation of HSPCs in developing zebrafish13, we tested whether murine LKS+ cells treated with cholera toxin demonstrated increased cell cycling following transplantation. Cell cycle evaluation of labelled cells two days following injection into lethally irradiated hosts did not detect any difference between untreated and cholera toxin treated cells (Suppl. Fig.11). Therefore, cholera toxin acting at least in part through the Gsα-coupled pathway, augments the homing of the cells and engraftment in the BM. This effect on engraftment was durable and without distortion of mature cell subsets, indicating that the cholera toxin induced improvement in HSPC engagement of the niche did not compromise cellular function.
Figure 4. Pharmacological modulation of Gsα affects homing and engraftment of primitive wild-type BM MNCs.
(a) Primitive lin− BM MNCs pre-treated with cholera toxin or mock treated for 1 hour ex vivo were assessed for their in vivo homing potential (n=3 from 3 individual experiments; error bars represent s.e.m.). (b) Engraftment potential in a competitive transplant model (n=9 from 2 individual experiments; error bars represent s.e.m.).
Elucidating the mechanisms by which HSPCs home to the BM environment has biologic and medical implications that has made it an area of intense investigation. A number of molecules have been implicated, often they were studied because of their effects on other hematopoietic populations such as lymphocytes. Here we show that Gsα plays a key role in the specific homing of HSPCs to the BM, in a manner not shared by lymphocytes.
The inability of stem cells to migrate to the BM in development strongly resembles the phenotype observed with the deletion of CXCR4 or SDF-114,15. This, coupled with an impairment in SDF-1α responsive migration at specific concentrations in vitro, suggests that CXCR4 may be using Gsα as its predominant signalling pathway in that cell type. The data presented here do not rule out this possibility for fetal hematopoiesis. However, two important distinctions between the Gsα and CXCR4 null phenotypes exist for stem cell function in adult animals. First, pharmacological interruption of CXCR4 signalling results in prompt mobilization of stem cells into the circulation16. Genetic disruption of Gsα resulted in no such change in location. Indeed, G-CSF induced mobilization of cells from the BM was impaired in the absence of Gsα suggesting that egress from the marrow may be diminished. Therefore, while CXCR4 is a key component of HSPC retention in the BM17, it is not exerting its effect through Gsα. Second, CXCR4 null cells engraft in the BM, while Gsα null cells do not18. It is therefore unlikely that the role of Gsα is simply downstream of CXCR4 as this molecule is dispensable for HSPC engraftment in the transplant setting.
Our results demonstrate that Gsα is critical at the HSPC homing stage, enabling engagement of the niche, however continued Gsα signalling is not required to retain the cells there. There is then meaningful molecular distinction between the homing, engraftment and retention processes. It could be hypothesized that the deficiency in the ability of the Gsα−/− cells to home to the BM could lead to a depletion of the HSPCs in the marrow space and an accumulation in the blood. However, the decreased egress from the marrow may balance the decreased ingress. Alternatively, there may be compensatory changes in resident cells that mask changes in the population.
Another unknown from these studies is the potential upstream receptor utilizing this pathway. The candidates are numerous and may include adrenergic and prostaglandin receptors. The report of prostaglandin E2 (PGE2) serving as a means of enhancing stem cell transplantation in mice13 indicates that this is likely at least one receptor capable of activating the pathway. Whether PGE2 mediates an effect physiologically in mammals could not be discerned from the data of North and colleagues, however, our data indicate that the downstream pathway is critical for physiologic processes in development.
The localization of stem cells following transplantation is a critical determinant of success of that clinical procedure. Currently, massive numbers of stem cells are required in clinical transplantation in part due to the limited efficiency of homing and engraftment. This is particularly problematic in umbilical cord blood transplantation where the number of stem cells is limited. This problem is being approached by dual unit transplantation, an expensive undertaking that indicates how a modest (2-fold) increase in stem cells can profoundly affect clinical outcomes. Alternatives to the use of multiple unit infusions might be to increase the homing efficiency of the cells, increase the nurturing capacity of the niche or increase the number of cells by ex vivo expansion. Each approach has potential and with new information about the means of affecting each parameter, the possibility of translation to clinical trial. Transient exposure to agents stimulating Gsα is one such candidate approach.
Methods Summary
Gsα−/− chimeric mice
Mice were created as described previously7. Gsα−/− embryonic stem cells were injected into a blastocyst transgenic for β-galactosidase. Resultant embryos were then assessed for tissue chimerism using in situ hybridization against neo to detect Gsα−/− cells or histochemical staining for β-galactosidase to detect Gsα+/+ cells.
Gsα conditional knockout adult mice
Gsαfl/+ mice were crossed with Mx1-Cre+ mice to create Mx1-Cre+Gsαfl/+ mice. These mice were then bred to obtain Mx1-Cre+Gsαfl/fl mice and Mx1-Cre+Gsα+/+ littermates. Deletion of the Gsα gene was achieved following exposure of the mice to polyI.polyC for 5 days. Analysis of the primitive BM MNCs used standard in vitro immunophenotyping, CFU-C, LTC-IC and chemotaxis assays, or in vivo homing and engraftment assays.
Gsα deletion following engraftment
Bone marrow MNCs from Gsα+/+Mx1-Cre+ or Gsαfl/flMx-1-Cre+ mice were transplanted into wild-type mice. Eight weeks following transplantation, the mice were treated with polyI.polyC to induce deletion of the gene. Peripheral blood and BM MNCs were then evealuated at various time points post-deletion.
Cholera toxin treatment
Bone marrow MNCs from wild-type C57Bl/6 mice were treated with cholera toxin (10µg/ml) for 1 hour ex vivo. Treated cells were then used in in vivo homing and engraftment studies.
Methods
Flow cytometric, CFU-C and LTC-IC analyses
As described previously21,22,23.
Induction of deletion
Mx-1 promoter was induced by 3 intra-peritoneal injections of polyI.polyC (Amersham Biosciences, Piscataway, NJ) 250µg over 5 days. Deletion was confirmed by PCR.
Engraftment studies
For the competitive transplantation experiments, 2.5×105 BM MNCs were mixed with 2.5×105 BM MNCs cells from wild-type mice and relative contributions to hematopoiesis assessed as described previously21.
In vitro transmigration
Chemotaxis assays used 5 µm pore transwells (Corning-Costar Corp.). BM cells or lymph node lymphocytes (5×104) were added to the upper well. Chemotaxis towards murine SDF-1α (PeproTech Inc.) in the lower chamber was scored at 3h.
Homing in vivo
Purified BM cells or lymph node lymphocytes were labelled with 5 µM DiI or DiO (Molecular Probes Inc.) in accordance with the manufacturer’s instructions. Cells were injected by tail vein and assessed by flow cytometry of BM and spleen at 6h, of lymph nodes at 24h.
Cholera Toxin treatment
Purified cells were resuspended in fully supplemented medium at 1×106 cells/ml. Cholera toxin (10µg/ml; Sigma) was added, incubated at 37°C for 1h, washed ×3 with PBS and used in the in vivo homing and engraftment studies.
In vivo microscropy
Two-photon microscopy was performed as previously described12. Video imaging was performed 30–40 minutes after intravenous injection of labelled cells for 10 minutes; supplemental movies represent a 10 second window in the middle of the recording period. Still images of cells were obtained 60 minutes after injection and cells were quantitated as stably homed if no movement was evident over a several minute observation interval.
Supplementary Material
Acknowledgements
Financial support for this work was provided by the Burroughs Wellcome Fund, Doris Duke Charitable Trust (D.T.S.), the Harvard Stem Cell Institute (C.P.L.) and the National Institutes of Health (G.B.A., C.P.L., H.M.K., D.T.S.).
References
- 1.Mikkola HKA, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 2.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 3.Mazo IB, et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J. Exp. Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of β4β1 over β2-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 5.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 6.Yu S, et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc. Natl. Acad. Sci. USA. 1998;95:8715–8720. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastepe M, et al. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:14794–14799. doi: 10.1073/pnas.0405091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dexter TM, Whetton AD, Heyworth CM. Inhibitors of cholera toxin-induced adenosine diphosphate ribosylation of membrane-associated proteins block stem cell differentiation. Blood. 1985;65:1544–1548. [PubMed] [Google Scholar]
- 9.Long MW, Heffner CH, Gragowski LL. Cholera toxin and phorbol diesters synergistically modulate murine hematopoietic progenitor cell proliferation. Exp. Hematol. 1988;16:195–200. [PubMed] [Google Scholar]
- 10.Ploemacher RE, van der Sluijs JP, van Beurden CA, Baert MR, Chan PL. Use of limiting-dilution type long-term marrow cultures in frequency analysis of marrow-repopulating and spleen colony-forming hematopoietic stem cells in the mouse. Blood. 1991;78:2527–2533. [PubMed] [Google Scholar]
- 11.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 15.Ma Q, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, et al. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann. N. Y. Acad. Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 17.Foudi A, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107:2243–2251. doi: 10.1182/blood-2005-02-0581. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata K, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc. Natl. Acad. Sci. USA. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods References
- 19.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 20.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 21.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99:2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.