Abstract
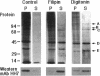
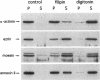
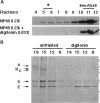
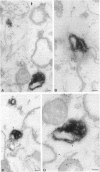
Annexin II is an abundant protein which is present in the cytosol and on the cytoplasmic face of plasma membrane and early endosomes. It is generally believed that this association occurs via Ca(2+)-dependent binding to lipids, a mechanism typical for the annexin protein family. Although previous studies have shown that annexin II is involved in early endosome dynamics and organization, the precise biological role of the protein is unknown. In this study, we found that approximately 50% of the total cellular annexin was associated with membranes in a Ca(2+)-independent manner. This binding was extremely tight, since it resisted high salt and, to some extent, high pH treatments. We found, however, that membrane-associated annexin II could be quantitatively released by low concentrations of the cholesterol-sequestering agents filipin and digitonin. Both treatments released an identical and limited set of proteins but had no effects on other membrane-associated proteins. Among the released proteins, we identified, in addition to annexin II itself, the cortical cytoskeletal proteins alpha-actinin, ezrin and moesin, and membrane-associated actin. Our biochemical and immunological observations indicate that these proteins are part of a complex containing annexin II and that stability of the complex is sensitive to cholesterol sequestering agents. Since annexin II is tightly membrane-associated in a cholesterol-dependent manner, and since it seems to interact physically with elements of the cortical actin cytoskeleton, we propose that the protein serves as interface between membranes containing high amounts of cholesterol and the actin cytoskeleton.
Full text
PDF



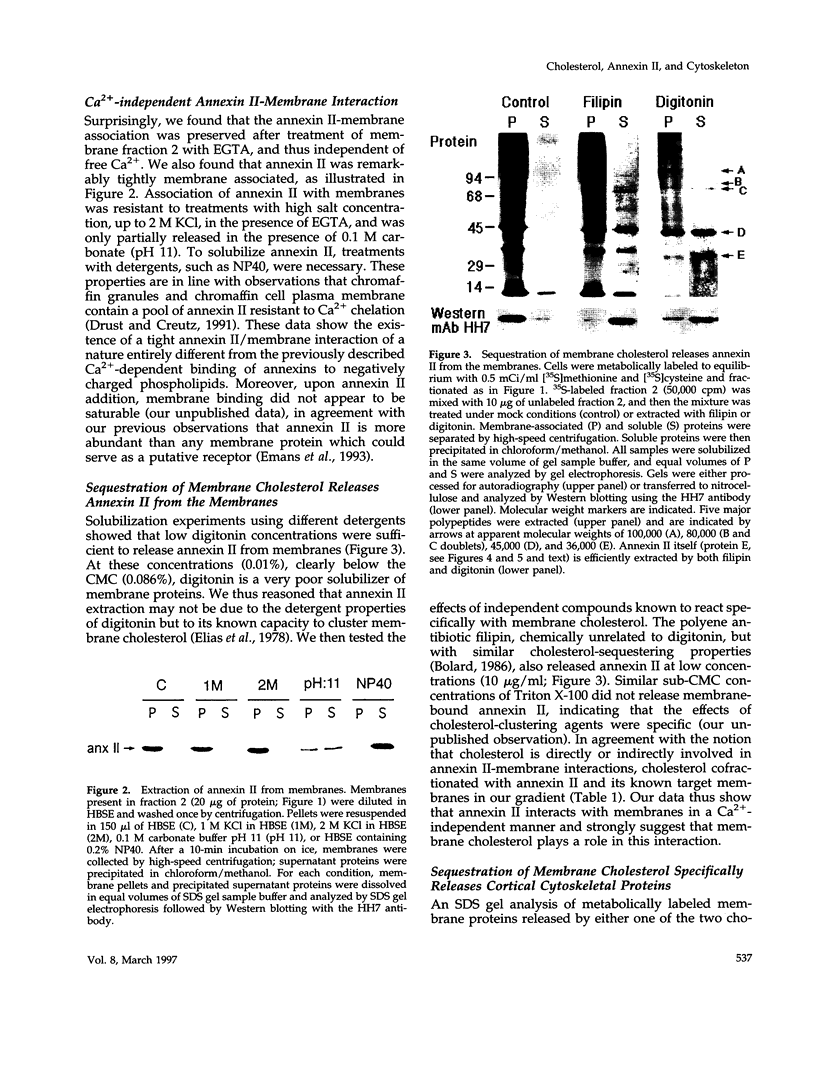
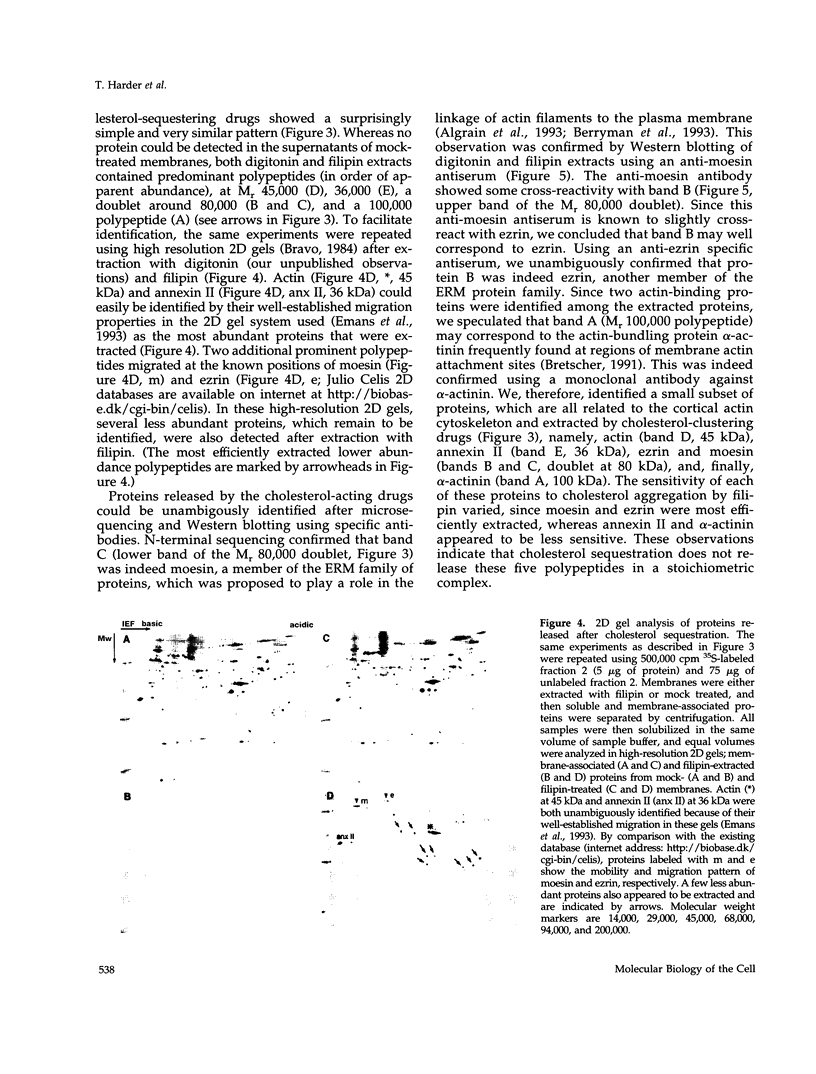
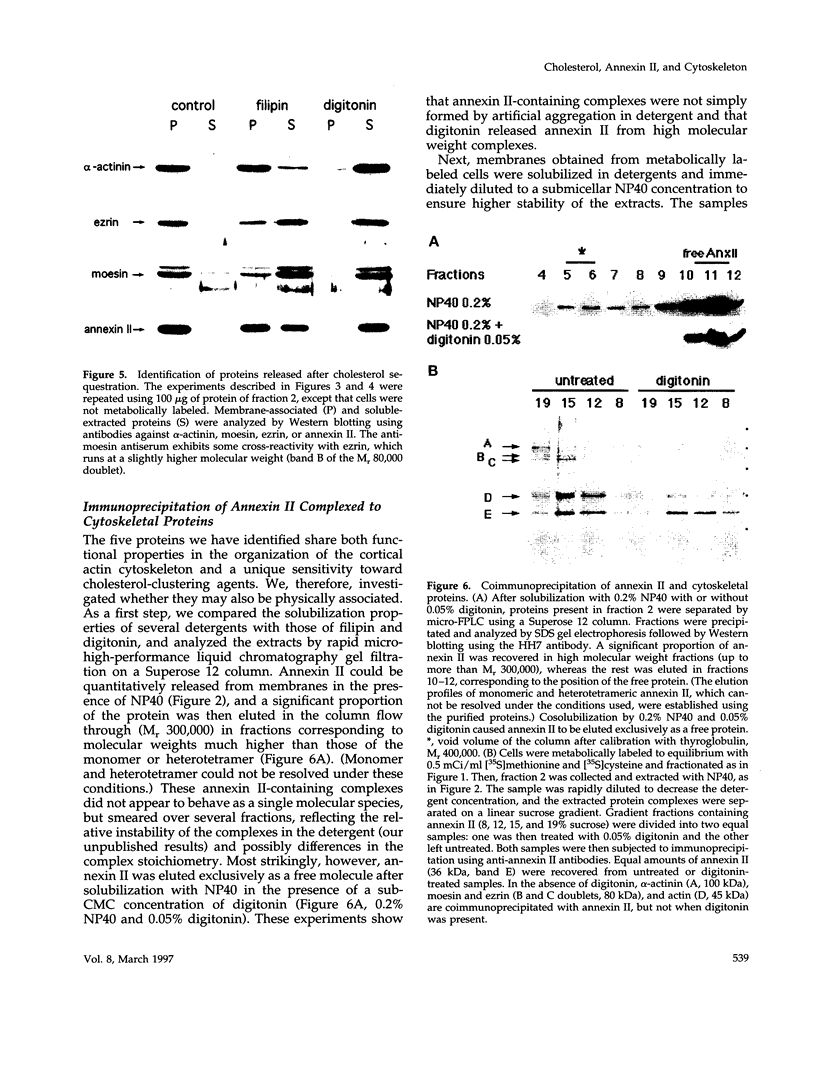

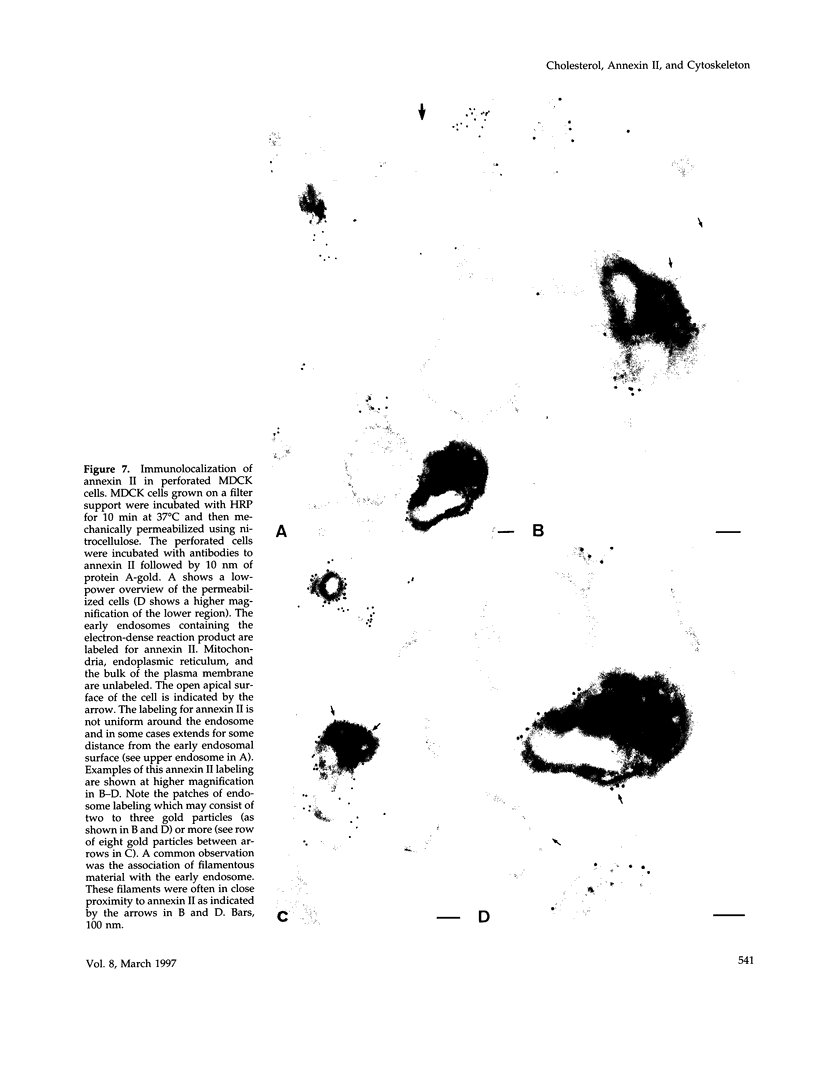




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algrain M., Turunen O., Vaheri A., Louvard D., Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993 Jan;120(1):129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Aniento F., Gu F., Parton R. G., Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996 Apr;133(1):29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman M., Franck Z., Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993 Aug;105(Pt 4):1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Berryman M., Gary R., Bretscher A. Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol. 1995 Dec;131(5):1231–1242. doi: 10.1083/jcb.131.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol. 1991;7:337–374. doi: 10.1146/annurev.cb.07.110191.002005. [DOI] [PubMed] [Google Scholar]
- Chasserot-Golaz S., Vitale N., Sagot I., Delouche B., Dirrig S., Pradel L. A., Henry J. P., Aunis D., Bader M. F. Annexin II in exocytosis: catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J Cell Biol. 1996 Jun;133(6):1217–1236. doi: 10.1083/jcb.133.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E. The annexins and exocytosis. Science. 1992 Nov 6;258(5084):924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Deckert M., Ticchioni M., Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996 May;133(4):791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Differential subcellular distribution of p36 (the heavy chain of calpactin I) and other annexins in the adrenal medulla. J Neurochem. 1991 Feb;56(2):469–478. doi: 10.1111/j.1471-4159.1991.tb08174.x. [DOI] [PubMed] [Google Scholar]
- Durrbach A., Louvard D., Coudrier E. Actin filaments facilitate two steps of endocytosis. J Cell Sci. 1996 Feb;109(Pt 2):457–465. doi: 10.1242/jcs.109.2.457. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Goerke J., Friend D. S., Brown B. E. Freeze-fracture identification of sterol-digitonin complexes in cell and liposome membranes. J Cell Biol. 1978 Aug;78(2):577–596. doi: 10.1083/jcb.78.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N., Gorvel J. P., Walter C., Gerke V., Kellner R., Griffiths G., Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol. 1993 Mar;120(6):1357–1369. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Fiedler K., Lafont F., Parton R. G., Simons K. Annexin XIIIb: a novel epithelial specific annexin is implicated in vesicular traffic to the apical plasma membrane. J Cell Biol. 1995 Mar;128(6):1043–1053. doi: 10.1083/jcb.128.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C. E., Felder S., Schlessinger J., Ullrich A., Hopkins C. R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993 Jan;120(1):77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991 Mar 8;64(5):915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Graeve L., Drickamer K., Rodriguez-Boulan E. Polarized endocytosis by Madin-Darby canine kidney cells transfected with functional chicken liver glycoprotein receptor. J Cell Biol. 1989 Dec;109(6 Pt 1):2809–2816. doi: 10.1083/jcb.109.6.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Emans N. Annexins in membrane traffic. Trends Cell Biol. 1993 Jul;3(7):224–227. doi: 10.1016/0962-8924(93)90116-i. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Harder T., Gerke V. The annexin II2p11(2) complex is the major protein component of the triton X-100-insoluble low-density fraction prepared from MDCK cells in the presence of Ca2+. Biochim Biophys Acta. 1994 Sep 29;1223(3):375–382. doi: 10.1016/0167-4889(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Harder T., Gerke V. The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J Cell Biol. 1993 Dec;123(5):1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwald H., Dedio J., Kellner R., Loos M., Müller-Esterl W. Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. Identity with the gC1q receptor. J Biol Chem. 1996 May 31;271(22):13040–13047. doi: 10.1074/jbc.271.22.13040. [DOI] [PubMed] [Google Scholar]
- Huber R., Schneider M., Mayr I., Römisch J., Paques E. P. The calcium binding sites in human annexin V by crystal structure analysis at 2.0 A resolution. Implications for membrane binding and calcium channel activity. FEBS Lett. 1990 Nov 26;275(1-2):15–21. doi: 10.1016/0014-5793(90)81428-q. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Parton R. G., Lafont F., Simons K. Analysis of the role of p200-containing vesicles in post-Golgi traffic. Mol Biol Cell. 1996 Jun;7(6):961–974. doi: 10.1091/mbc.7.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Hartmann E., Dupree P. Guilty by insolubility--does a protein's detergent insolubility reflect a caveolar location? Trends Cell Biol. 1995 May;5(5):187–189. doi: 10.1016/s0962-8924(00)88990-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liscum L., Underwood K. W. Intracellular cholesterol transport and compartmentation. J Biol Chem. 1995 Jun 30;270(26):15443–15446. doi: 10.1074/jbc.270.26.15443. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Berón W., Sarrouf M. N., Colombo M. I., Creutz C., Stahl P. D. Calcium-dependent fusion among endosomes. J Biol Chem. 1994 Dec 9;269(49):30927–30934. [PubMed] [Google Scholar]
- Morgan R. O., Fernández M. P. Molecular phylogeny of annexins and identification of a primitive homologue in Giardia lamblia. Mol Biol Evol. 1995 Nov;12(6):967–979. doi: 10.1093/oxfordjournals.molbev.a040290. [DOI] [PubMed] [Google Scholar]
- Muallem S., Kwiatkowska K., Xu X., Yin H. L. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995 Feb;128(4):589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Peränen J., Schreiner R., Wieland F., Kurzchalia T. V., Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Johnsson N., Wehland J., Weber K. The submembranous location of p11 and its interaction with the p36 substrate of pp60 src kinase in situ. Exp Cell Res. 1988 Mar;175(1):81–96. doi: 10.1016/0014-4827(88)90257-1. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Joggerst B., Simons K. Regulated internalization of caveolae. J Cell Biol. 1994 Dec;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Simons K. Digging into caveolae. Science. 1995 Sep 8;269(5229):1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Oh P., Pinney E., Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994 Dec;127(5):1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C., Osborn M., Gerke V. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11- and Ca(2+)-binding sites. J Cell Sci. 1992 Nov;103(Pt 3):733–742. doi: 10.1242/jcs.103.3.733. [DOI] [PubMed] [Google Scholar]
- Thiel C., Weber K., Gerke V. Characterization of a Ca(2+)-binding site in human annexin II by site-directed mutagenesis. J Biol Chem. 1991 Aug 5;266(22):14732–14739. [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wice B. M., Gordon J. I. A strategy for isolation of cDNAs encoding proteins affecting human intestinal epithelial cell growth and differentiation: characterization of a novel gut-specific N-myristoylated annexin. J Cell Biol. 1992 Jan;116(2):405–422. doi: 10.1083/jcb.116.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Ca(2+)-dependent annexin self-association on membrane surfaces. Biochemistry. 1991 Oct 8;30(40):9607–9615. doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]