SUMMARY
In cultured cells, infection by Group B coxsackieviruses (CVB) is mediated by the coxsackievirus and adenovirus receptor (CAR), but the importance of this molecule in CVB disease has not been determined. We used tissue-specific CAR gene deletion to generate mice that lacked CAR within each of two major CVB target organs, the pancreas and heart. Deletion of CAR from the pancreas resulted in a 1000-fold reduction in virus titers within the pancreas during infection, and a significant reduction in virus-induced tissue damage and inflammation. Similarly, cardiomyocyte-specific CAR deletion resulted in a 100-fold reduction in virus titer within the heart, and a marked reduction in cytokine production and histopathology. Although primary cardiomyocytes from control animals were susceptible to virus infection, CAR-deficient cardiomyocytes resisted infection in vitro. These results demonstrate a critical function for CAR in the pathogenesis of CVB infection in vivo, and in virus tropism for the heart and pancreas.
INTRODUCTION
Receptors have been identified for many of the viruses that cause human disease (Dermody and Tyler, 2005), but our current understanding of their function is based almost entirely on work performed in cultured cells. Further, many viruses are known to interact with multiple receptors in vitro (Helenius, 2007), yet it is unclear how viruses use alternative receptors in the course of infection, and uncertain whether specific identified receptors contribute to in vivo tropism. Several recent observations underscore the importance of extending receptor studies to in vivo models. The marked tropism of adenovirus for the liver has been found to involve none of the identified adenovirus receptors, resulting instead from a complex interaction with coagulation factors and unidentified hepatocyte molecules (Kalyuzhniy et al., 2008; Waddington et al., 2008). Measles virus entry into the body, which had formerly been postulated to involve virus infection of airway epithelial cells, has been shown not to require virus interaction with its epithelial cell receptor (Leonard et al., 2008). And the major reovirus receptor, JAM-A, proves to be dispensible for direct infection of target tissues, but important for hematogenous spread of virus from its primary site of replication in the intestinal epithelium (Antar et al., 2009).
We have now tested the function of the coxsackievirus and adenovirus receptor (CAR) [reviewed in (Bergelson, 2002)] in a well-characterized mouse model of myocarditis and pancreatitis caused by Group B coxsackieviruses (CVB) (Huber and Ramsingh, 2004; Woodruff, 1980). CVB are common human pathogens that cause febrile illnesses as well as diseases of the heart, pancreas, and central nervous system (Modlin, 1995). They are among the major causes of viral myocarditis (Bowles et al., 2003), and they are implicated as a cause of dilated cardiomyopathy (Martino et al., 1994), one of the most common indications for cardiac transplantation. CVB also cause pancreatitis, and, although the association remains controversial, they are thought to contribute to the pathogenesis of childhood-onset diabetes (Drescher and Tracy, 2008).
In cultured cells, CVB have been found to interact with at least three receptors. All tested clinical and laboratory isolates bind to CAR, a 46 kD adhesion molecule, and attachment to CAR on transfected rodent cells is sufficient for infection (Bergelson, 2002). A large subset of CVB isolates also bind to decay-accelerating factor (DAF), a complement regulatory protein, and DAF-induced intracellular signals are required for infection of polarized epithelial cells (Coyne and Bergelson, 2006). In addition, at least one CVB3 isolate has been shown to use a third receptor, heparan sulfate, to infect CAR-deficient cells in vitro (Zautner et al., 2003). Each of these receptors is expressed on a variety of cell types in vivo, but their importance in pathogenesis has not been tested.
To investigate CAR’s role in the pathogenesis of CVB3 pancreatitis and myocarditis, we used tissue-specific gene deletion to ablate CAR expression specifically within the pancreas or the heart. We found that, during infection, virus titers in CAR-deficient tissues were markedly reduced, as were virus-induced tissue destruction and inflammation. The results demonstrate that CAR is the major receptor responsible for CVB3 pathogenesis and tropism in vivo. In addition, they support the idea that tissue damage and inflammation depend on high titer virus replication within target tissues.
RESULTS
Tissue-specific CAR gene deletion
CAR is essential for normal embryonic development, and CAR-null mice do not survive beyond day 11 of embryonic life (Asher et al., 2005; Chen et al., 2006; Dorner et al., 2005). To obtain CAR-deficient mice we used Cre-loxP technology to achieve tissue-specific deletion of the CAR gene in mice that survive into adulthood. We previously reported the generation of mice in which exon 2 of the CAR gene is flanked by recognition sites (loxP) for Cre DNA recombinase (“floxed”) (Chen et al., 2006). Cre-mediated deletion of exon 2 results in a frame shift and premature termination within the CAR leader sequence, creating a null allele. To ablate CAR expression within the pancreas, we bred CAR-floxed mice to transgenic mice expressing Cre under the control of the pancreas-specific pancreas duodenal homeobox gene 1 (pdx) promoter (Gu et al., 2002). To target CAR expression within the heart, we bred CAR-floxed mice to mice expressing Cre under the control of the cardiomyocyte-specific α-myosin heavy chain (MHC) promoter (Gaussin et al., 2002). In all experiments, mice were of mixed genetic background (129/Sv and C57Bl/6), and knockout animals were matched to littermate controls.
As determined by quantitative DNA PCR, CAR gene deletion in the heart (40%, SEM±7% in 8 animals examined) was less extensive than it was in the pancreas (75%, SEM±3% in 28 animals), perhaps because the α-MHC promoter is active only in the cardiomyocyte population within the heart. However, as measured by quantitative RNA PCR, expression of functional CAR mRNA was reduced in the heart by 97% (Figure S1A), suggesting that cardiomyocytes are the predominant CAR-expressing cell type; CAR mRNA in the pancreas was reduced by 94%. Consistent with this, immunoblot analysis and indirect immunofluorescence revealed that CAR protein levels were markedly reduced in the Panc-KO pancreas (Figure S1B, C), and in the Hrt-KO heart (Figure S1D, E). Given the insensitivity of immunoblots, we cannot exclude the persistence of CAR protein at the low, but measurable, levels observed for CAR mRNA. As expected, CAR expression levels in the livers of Panc- and Hrt-KO mice were the same as those in controls.
Pancreatic CAR deletion attenuates pancreatic infection and pancreatitis
To identify the role of CAR in CVB infection of the pancreas, we infected Panc-KO and littermate control mice with CVB3-H3, a strain known to cause both myocarditis (Van Houten et al., 1991) and pancreatitis (Mena et al., 2000). During the acute phase of infection, mice become hypoglycemic, most likely as a result of pancreatitis and digestive dysfunction (Mena et al., 2000). Baseline blood glucose levels were equivalent in uninfected Panc-KO and control mice (Figure 1A). However, by the second day of infection, control mice showed the expected drop in blood glucose levels, whereas Panc-KO animals remained normoglycemic, suggesting that they were protected from pancreatitis.
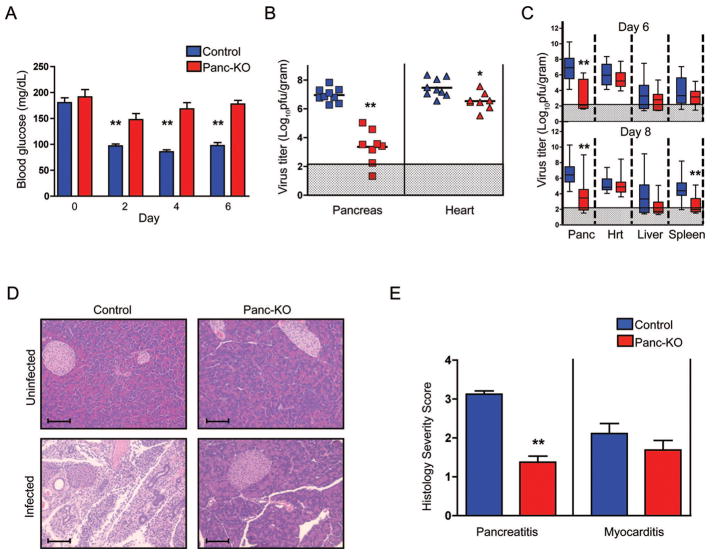
Figure 1.
Reduced virus titers and pancreatitis in infected Panc-KO mice. A) Mice were infected with 1×105 PFU/mouse of CVB3-H3 and blood glucose levels were measured daily. B) After 6 days, virus titers in pancreas and heart were determined by plaque assay. Each symbol represents the virus titer (PFU per gram of tissue) from an individual mouse. Means for each experimental group are indicated by horizontal black lines (n= 8–9 for each genotype). C) Virus titers in mice infected with 1×103 PFU/mouse of CVB3-H3. Titers combined from three separate experiments (n= 12–14 for each experimental group) are shown: the colored box encloses the 25th to 75th percentiles, the mean is depicted as a line, and the range for the entire group is shown as bars. Shaded regions represent the lower limits of viral detection. D) Pancreas sections stained with hematoxylin and eosin. Infected tissues were examined 6 days after infection with 1×105 PFU/mouse. Scale bars, 80 microns. E) Severity scores for pancreatitis and myocarditis (day 6) in control (n=8) and Panc-KO mice (n=9), determined as described in Experimental Procedures. Asterisks indicate a statistically significant difference between the Panc-KO and control groups by Student’s t test (*, p<0.05; ** p<0.005)
Mice were sacrificed six days after infection (the peak of replication, as determined in preliminary experiments), and virus titers in the pancreas and heart were measured by plaque assay. Pancreatic titers in Panc-KO mice were more than 1000-fold lower than those in controls, with titers in some samples below the limits of detection (Figure 1B); titers in the heart were reduced less than 10-fold, although the difference was statistically significant. To confirm these results, we examined a larger number of mice in three independent experiments, examining titers in multiple tissues at both 6 and 8 days after infection (Figure 1C): titers in the pancreas of Panc-KO mice were consistently lower than those in controls, and no differences were seen in titers from the heart and liver. In the spleens, titers were similar on day 6, but for unclear reasons Panc-KO spleens had lower titers than controls on day 8. Together, these results confirm that the Panc-KO mice are protected from viral infection of the pancreas, and suggest that the titer of virus within the pancreas has little effect on the titers found in other organs.
Histologic evaluation revealed that infected control mice had severe pancreatitis, with destruction of the acinar tissue, inflammatory cell infiltration, and ductal proliferation; in contrast, Panc-KO mice exhibited only mild inflammation and little tissue damage (Figures 1D and E). Both Panc-KO and control mice developed chemical pancreatitis after exposure to caerulein (Lampel and Kern, 1977), demonstrating that protection of Panc-KO mice was virus-specific (Figure S2). Taken together, these experiments show that CAR expression is important for virus infection of the pancreas, and for the initiation of viral pancreatitis.
Cardiomyocyte-specific CAR deletion attenuates cardiac infection and myocarditis
We infected Hrt-KO and littermate controls with CVB3-H3 and measured virus titers in the heart and pancreas at day 7 after infection. Heart titers in Hrt-KO mice were more than 100-fold lower than those in controls (Figure 2A); titers in the pancreas were similar in Hrt-KO and control mice. Similar results were obtained when we examined titers in multiple tissues over a time course of infection (Figure 2B). Titers in the hearts of Hrt-KO mice were consistently lower than those in controls, whereas titers in the liver and spleen were similar in Hrt-KO and control animals; a slight reduction of titer in the pancreas of Hrt-KO animals was observed at day 7 in this experiment, but not in an additional experiment involving 5 knockout and 5 control animals (data not shown). Histologic examination of heart sections 7 days post infection revealed that control mice suffered both inflammation and cardiomyocyte necrosis, whereas Hrt-KO mice showed little inflammation and no evidence of necrosis (Figures 2C and D).
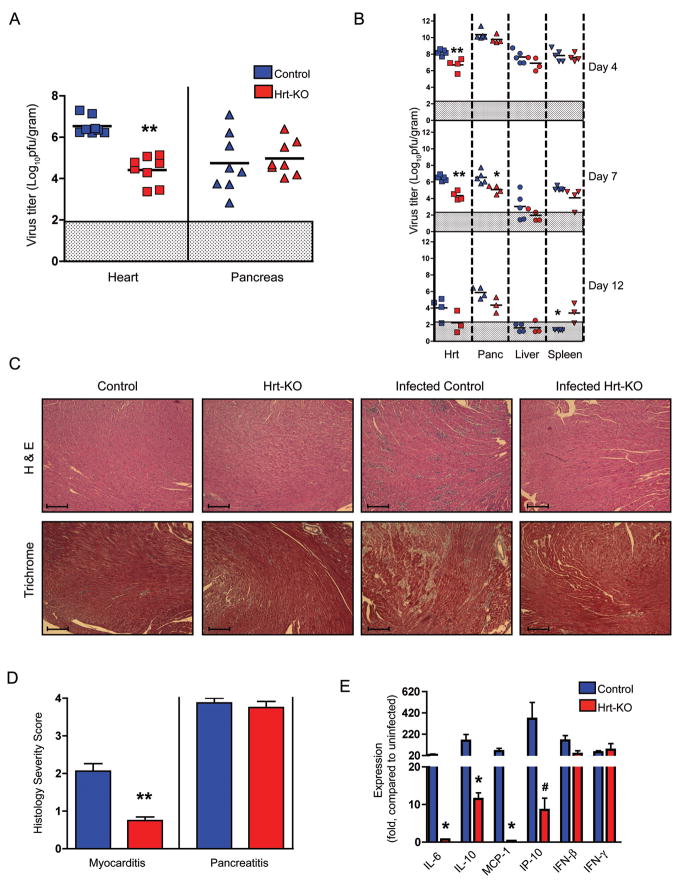
Figure 2.
Reduced virus titers and myocarditis in Hrt-KO mice. A) Mice were infected with 1×105 PFU/mouse of CVB3-H3, and sacrificed 7 days post-infection. Each symbol represents the titer (PFU per gram of tissue) from an individual mouse. Means for each experimental group are indicated by horizontal black lines (n= 8 for each genotype). B) Serial titers in mice infected with 1×105 PFU (n=3–5 mice per experimental group). C) Hematoxylin and Eosin (H & E, top) and Masson’s Trichrome stain (bottom) of heart tissue sections. Uninfected samples are shown in the left-hand panels; sections from infected mice (day 7) are shown on the right. Scale bars, 80 microns. D) Severity scores for myocarditis and pancreatitis in control (n=8) and Hrt-KO mice (n=8), assessed as described in Experimental Procedures. E) Differential induction of cytokine and chemokine expression in heart tissue 7 days post infection (1×105 PFU), measured by quantitative RNA PCR. Expression is measured relative to uninfected control mice of the same genotype. Error bars are SEM, and asterisks indicate a statistical difference between the Hrt-KO and controls (*, p<0.05; ** p<0.005; #, p= 0.058).
We used quantitative RT-PCR to examine expression of cytokines and chemokines that are known to be upregulated in the hearts of mice with CVB3 myocarditis and are believed to be important in regulation of the inflammatory response (Figure 2E). In control mice, as expected, we saw increased expression of IL-6 (Seko et al., 1997), IL-10 (Seko et al., 1997), MCP-1 (Shen et al., 2004), IP-10 (Weinzierl et al., 2008), IFN-β (Schmidtke et al., 2000), and IFN-γ (Seko et al., 1997). In Hrt-KO mice IFN-β and IFN-γ were induced to the levels seen in control mice, but induction of IL-10 and IP-10 was attenuated, and there was no induction of IL-6 or MCP-1. These results suggest that, in many cases, cytokine induction depends on high titer virus replication within cardiomyocytes.
Isolated Hrt-KO cardiomyocytes are protected from infection in vitro
Although virus titers in Hrt-KO hearts were much lower than those in controls, some residual virus was detected. This could result from incomplete CAR deletion in the cardiomyocyte population, infection of non-cardiomyocyte cell types, or infection of cardiomyocytes by a CAR-independent mechanism. To investigate these possibilities we isolated and cultured primary cells from the hearts of newborn knockout and littermate control mice. Cultured cells were stained for sarcomeric myosin, a cardiomyocyte-specific marker, and for CAR (Figure 3A). In control cultures, all cardiomyocytes were seen to express CAR; among the non-cardiomyocyte population, we observed both CAR-positive and CAR-negative cells (top row). In Hrt-KO cultures, cardiomyocytes did not express CAR, and CAR staining was limited to a minority of cells that did not express the cardiomyocyte marker (bottom row).
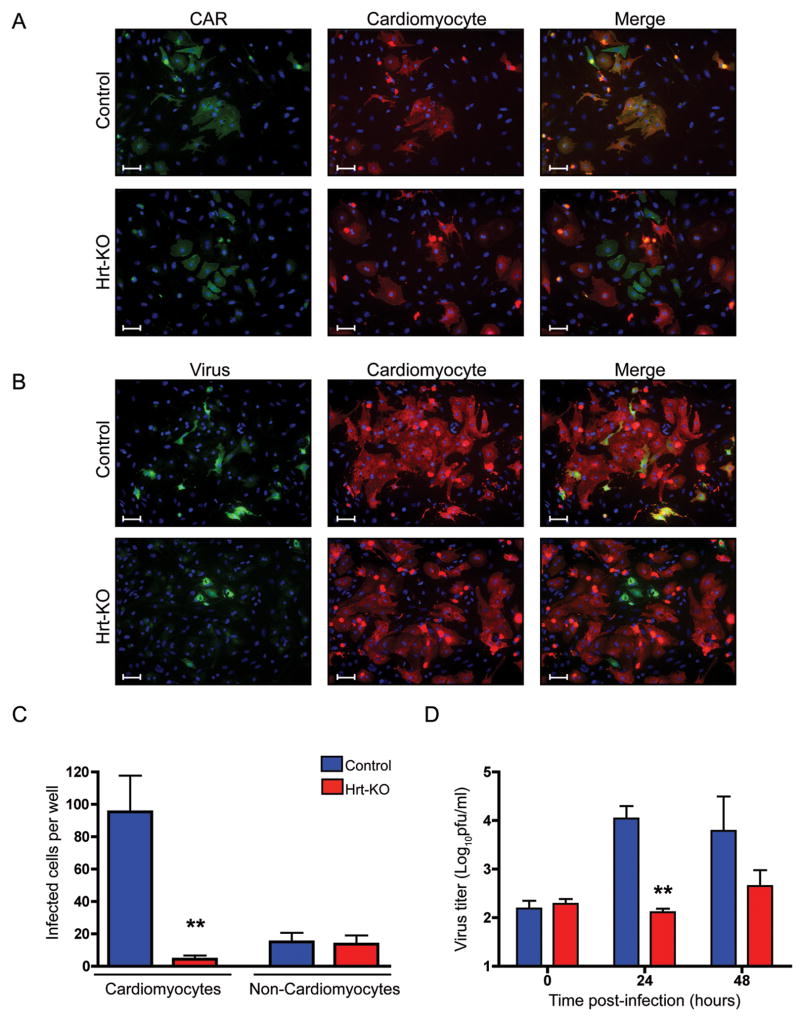
Figure 3.
CAR-deficient cardiomyocytes resist infection in vitro. A) Primary cardiomyocytes were isolated and expression of CAR (green) and the cardiomyocyte-specific marker sarcomeric myosin (red), was detected by indirect immunofluorescence. Cell nuclei were stained with DAPI (blue). Top panels show a representative field of cardiac cells isolated from control hearts; most CAR-positive cells express the cardiomyocyte marker. Bottom panels show an unusual field of Hrt-KO cardiac cells with some CAR-positive cells; note that these CAR-positive cells do not express the cardiomyocyte marker. Scale bars, 20 microns. B) CAR-negative cardiomyocytes are protected from coxsackievirus infection. Primary cardiomyocyte cultures were infected with CVB3-H3 (10 PFU/cell) for 48 hours. Expression of viral protein (green) and the cardiomyocyte-specific marker sarcomeric myosin (red) was detected by indirect immunofluorescence. Top panels show a representative field of cardiomyocytes isolated from control hearts; most of the virus-infected cells are cardiomyocytes. Lower panels show a field of Hrt-KO cardiomyocytes that contains virus-infected cells (most of the fields have no infected cells); note that the virus-infected Hrt-KO cells do not express the cardiomyocyte marker. Scale bars, 20 microns. C) Total numbers of virus-infected cardiomyocytes and non-cardiomyocytes. D) Virus titers produced by infected cardiac cells (Mean ± SEM for triplicate wells; **, p<0.005).
To determine whether cardiomyocytes can be infected in a CAR-independent manner, we exposed primary cultures to CVB3-H3, and identified infected cells by staining for viral protein (Figure 3B). In control cultures many cells became infected; most infected cells were cardiomyocytes, although a few were non-cardiomyocytes (top row). In the Hrt-KO cultures very few cells (and virtually no cardiomyocytes) were infected (bottom row). Quantification of infected cells confirmed that the Hrt-KO cardiomyocytes were unable to support infection, whereas both Hrt-KO and control non-cardiomyocytes were infected to similar levels (Figure 3C); virus titers were lower in cultures from Hrt-KO animals (Figure 3D). These observations show that CAR is essential for CVB3 infection of cardiomyocytes. In addition, they indicate that, within the heart, there is a population of non-cardiomyocytes that express CAR and are potentially susceptible to infection. Taken together with the observation that Hrt-KO mice are protected from myocarditis, the results with primary heart cultures confirm that virus infection of cardiomyocytes is specifically required for the pathogenesis of acute myocarditis.
DISCUSSION
The experiments reported here indicate that CAR is the major receptor mediating CVB infection of the pancreas and heart in vivo, and that CAR is important for the pathogenesis of virus-induced pancreatitis and myocarditis. Specific deletion of CAR from the pancreas resulted in a 1000-fold reduction in virus titers within the pancreas during infection, and a significant reduction in virus-induced tissue damage and inflammation. Similarly, cardiomyocyte-specific CAR deletion resulted in a 100-fold reduction in virus titer within the heart, and a significant reduction in the number and size of histologic lesions.
We did observe some virus replication even in CAR-deficient tissues. Although this may reflect the function of secondary receptor molecules, we believe that it more likely results from a persistence of CAR expression in target tissues. In CAR-deleted tissues, CAR mRNA levels were reduced to approximately 5% of control levels; such low levels of protein expression might not be detected on immunoblots, yet could easily account for the low levels of residual infection. Cre-mediated gene deletion has variable efficiency, and Cre expression is limited to specific cell types within the target tissues. In the heart, the α-MHC promoter drives Cre expression specifically within cardiomyocytes, and but not in endothelial cells, fibroblasts, or other cell types. In primary cultures of cardiac cells, we found that CAR was expressed on a population of non-muscle cells. However, it was clear that CAR expression and susceptibility to infection were virtually ablated in the cardiomyocyte population isolated from Hrt-KO animals.
After intraperitoneal inoculation, CVB3 quickly reaches high titers within the pancreas, and it has been suggested that the pancreas serves as a reservoir from which virus spreads to the heart and other tissues (Horwitz et al., 1998). We found that reduction of virus titer in the Panc-KO pancreas was not accompanied by reduction in virus titer or virus-induced pathology in the heart. In additional experiments (Figure S3) we have studied mice in which CAR is deleted from both the heart and the pancreas. In these dual knockout animals, virus titers in CAR-deleted tissues were the same as those observed in single knockouts. We did observe reduced titers in the spleens of Panc-KO animals at days 7 and 8 (but not day 6) and in dual knockouts at day 7 (Figures 1 and S3). It is thus possible that, late in the course of infection, high titers in the pancreas contribute to viral persistence in the spleen.
The reduction in virus titer in CAR-deficient animals was accompanied by a marked decrease in inflammatory infiltrates and cytokine production in the heart, and reduced tissue damage and inflammation in the pancreas. These results support the idea that, in acute myocarditis and pancreatitis, virus replication in the heart and pancreas is essential for the pathogenesis of necrosis and inflammation. While this paper was under review, other investigators similarly reported an essential role for CAR in the pathogenesis of acute myocarditis (Shi et al., 2009). In genetically susceptible mice, the resolution of acute coxsackievirus myocarditis is followed by the onset of chronic inflammation, which has been attributed both to viral persistence and to autoimmunity (Esfandiarei and McManus, 2008). Chronic pancreatitis is also observed in some experimental models (Ramsingh, 2008). It is not known whether the chronic inflammatory response is initiated within the target organs, or whether virus replication in other tissues is sufficient to induce chronic disease. It will be interesting to determine whether, in susceptible mice, CAR deletion protects against chronic as well as acute pathology.
Receptors have been identified for many of the viruses that cause human disease, but in virtually all cases, the evidence for receptor function is based on in vitro data, and it remains uncertain whether interaction with these receptors explains the tropism of virus for particular tissues. Expression of specific receptor molecules in transgenic animals provides evidence that these molecules can function in infection in vivo (Ren et al., 1990). Targeted gene deletion can determine whether a specific receptor is required for infection and pathogenesis (Antar et al., 2009; Taylor et al., 2007). We have now used tissue-specific gene deletion to show that CAR is important for the pathogenesis of CVB-induced pancreatitis and myocarditis. The Cre-loxP technology we employed makes it possible to examine the importance of receptor expression in specific tissues, and potentially to examine the role of particular tissue compartments during virus spread.
EXPERIMENTAL PROCEDURES
PCR primers
Mouse Lines
For pancreas-specific CAR deletion, heterozygous CAR-floxed mice (Chen et al., 2006) that also carried a transgene permitting Cre expression under control of the pdx-1 promoter (Gu et al., 2002) (CARF/+, pdx-Cre) were bred to mice homozygous for the floxed CAR allele (CARF/F). Offspring homozygous for floxed CAR, and which inherited the pdx-Cre allele (CARF/F -pdx-Cre) were identified by PCR-based genotyping (See Table S1 for primers) and were expected to undergo pancreas-specific CAR deletion. CARF/F littermates without the pdx-Cre transgene were used as controls for all experiments.
We had previously generated mice with heart-specific CAR deletion (CARF/F, MHC-Cre) (Chen et al., 2006). To obtain Hrt-KO mice for these experiments, these were bred to CARF/F. Quantitative analysis of gene deletion and mRNA expression were performed as described in Supplemental Methods.
Animal care and use were in accordance with institutional and NIH guidelines, and approved by the IACUC of the Children’s Hospital of Philadelphia.
Virus Propagation and Mouse Infection
CVB3-H3 (Van Houten et al., 1991) was obtained from Dr. Sally Huber (University of Vermont). Virus titers were determined by triplicate plaque assay on HeLa cells. Mice 6–8 weeks of age were infected by intraperitoneal injection with virus in 100 μl PBS. For glucose measurements, tail-vein blood was analyzed with a One Touch Ultra glucometer (Lifesystems). After euthanasia, portions of each organ were fixed in 10% formaldehyde for histology, or frozen for determination of viral titer. Organs were weighed, then homogenized in 0.5 ml media; tissue debris was spun down and the supernatant was titered, and titers normalized to tissue weight (in grams). For statistical analysis, samples with titers below the limit of detection were assigned a value of 3 PFU/ml (instead of 0), before normalization.
Histology
For histology, deparaffinized sections were stained with Hematoxylin and Eosin or Masson’s Trichrome. The severity of pancreatitis was scored independently by two pathologists (M.G. and M.D.S., who were blinded to the identity of samples), according to the following scale: 0- No inflammation; 1- Mild to moderate interstitial inflammation; 2- Moderate inflammation with and/or acinar loss; 3-Massive inflammation and/or necrosis; 4-Massive inflammation and multifocal ductal proliferation. The severity of myocarditis was scored as follows: 0- No lesions; 1- One or few small lesions; 2-Multiple small or few large lesions; 3-Multiple small and large lesions; and 4-Massive lesions. Lesions were defined as areas of inflammation and/or cardiomyocyte necrosis and loss.
Cardiomyocyte Culture and Infection
Cardiomyocytes were isolated using a neonatal cardiomyocyte isolation kit from Worthington. In brief, pups 1–2 days old were sacrificed and individual hearts were minced and trypsinized overnight. Samples were pooled according to genotype and isolation was carried out according to the manufacturer’s instructions. Cardiomyocytes were enriched by pre-plating two times (75 min) on uncoated tissue culture plates to remove adherent cells. Cardiomyocytes were plated at a density of 5×104 cells/cm2 on collagen coated chamber slides (BD) for immunofluorescence, or in collagen-coated 96-well culture plates for determination of virus titer, in DMEM-F12 supplemented with nonessential amino acids, penicillin-streptomycin, selenium insulin transferrin mix, and 10% fetal bovine serum. 24 hours later, cardiomyocytes were exposed to virus (10 PFU/cell) for 1 hr at room temperature, then washed, given fresh medium, and incubated at 37°C for 48 hrs.
Cells were fixed with cold 50% methanol-acetone for 15 min, then washed twice with PBS before staining. Sarcomeric myosin was detected with monoclonal antibody MF20 (Developmental Studies Hybridoma Bank, dilution 1:2000) and goat anti mouse IgG2b Alexa Fluor 594 (Invitrogen, 1:500). Virus was detected with monoclonal anti-enterovirus VP1 (NCL-ENTERO, Leica, dilution 1:50) and goat anti-mouse IgG2a Alexa Fluor 488 (Invitrogen, 1:500); CAR was detected with polyclonal rabbit anti-CAR (H-300, Santa Cruz Biotechnology; 1:50) and goat anti-rabbit Alexa Fluor 488 (Invitrogen, 1:500)
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R01AI52281 and T32AI07324), the American Heart Association, and the ACVP/STP Coalition for Veterinary Pathology Fellows. We thank Michael Schneider for α-MHC-Cre mice, and Douglas Melton and Guoqiang Gu for pdx-Cre mice. We are grateful for the services of the Pathology and Laboratory Animal core facilities of the Joseph Stokes, Jr. Research Institute at the Children’s Hospital of Philadelphia, and for the advice and assistance of John Le Lay and other members of the Kaestner laboratory. Kunal Patel, Michael Sebert, and Paul Offit provided helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antar AA, Konopka JL, Campbell JA, Henry RA, Perdigoto AL, Carter BD, Pozzi A, Abel TW, Dermody TS. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe. 2009;5:59–71. doi: 10.1016/j.chom.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher DR, Cerny AM, Weiler SR, Horner JW, Keeler ML, Neptune MA, Jones SN, Bronson RT, Depinho RA, Finberg RW. Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development. Genesis. 2005;42:77–85. doi: 10.1002/gene.20127. [DOI] [PubMed] [Google Scholar]
- Bergelson JM. Receptors for coxsackieviruses and echoviruses. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. Washington, DC: ASM Press; 2002. pp. 107–113. [Google Scholar]
- Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- Chen JW, Zhou B, Yu QC, Shin SJ, Jiao K, Schneider MD, Baldwin HS, Bergelson JM. Cardiomyocyte-specific deletion of the coxsackievirus and adenovirus receptor results in hyperplasia of the embryonic left ventricle and abnormalities of sinuatrial valves. Circ Res. 2006;98:923–930. doi: 10.1161/01.RES.0000218041.41932.e3. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Dermody TS, Tyler KL. Introduction to viruses and viral diseases. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingston; 2005. [Google Scholar]
- Dorner AA, Wegmann F, Butz S, Wolburg-Buchholz K, Wolburg H, Mack A, Nasdala I, August B, Westermann J, Rathjen FG, Vestweber D. Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development. J Cell Sci. 2005;118:3509–3521. doi: 10.1242/jcs.02476. [DOI] [PubMed] [Google Scholar]
- Drescher KM, Tracy SM. The CVB and etiology of type 1 diabetes. Curr Top Microbiol Immunol. 2008;323:259–274. doi: 10.1007/978-3-540-75546-3_12. [DOI] [PubMed] [Google Scholar]
- Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Helenius A. Virus entry and uncoating. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 99–118. [Google Scholar]
- Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nature Medicine. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- Huber S, Ramsingh AI. Coxsackievirus-induced pancreatitis. Viral Immunol. 2004;17:358–369. doi: 10.1089/vim.2004.17.358. [DOI] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Leonard VH, Sinn PL, Hodge G, Miest T, Devaux P, Oezguen N, Braun W, McCray PB, Jr, McChesney MB, Cattaneo R. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J Clin Invest. 2008;118:2448–2458. doi: 10.1172/JCI35454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino TA, Liu P, Sole MJ. Viral infection and the pathogenesis of dilated cardiomyopathy. Circulation Research. 1994;74:182–188. doi: 10.1161/01.res.74.2.182. [DOI] [PubMed] [Google Scholar]
- Mena I, Fischer C, Gebhard JR, Perry CM, Harkins S, Whitton JL. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology. 2000;271:276–288. doi: 10.1006/viro.2000.0332. [DOI] [PubMed] [Google Scholar]
- Modlin JF. Picornaviridae. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 1995. [Google Scholar]
- Ramsingh AI. CVB-induced pancreatitis and alterations in gene expression. Curr Top Microbiol Immunol. 2008;323:241–258. doi: 10.1007/978-3-540-75546-3_11. [DOI] [PubMed] [Google Scholar]
- Ren R, Constantini F, Gorgacz E, Lee JJ, Racaniello VR. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- Schmidtke M, Gluck B, Merkle I, Hofmann P, Stelzner A, Gemsa D. Cytokine profiles in heart, spleen, and thymus during the acute stage of experimental coxsackievirus B3-induced chronic myocarditis. J Med Virol. 2000;61:518–526. [PubMed] [Google Scholar]
- Seko Y, Takahashi N, Yagita H, Okumura K, Yazaki Y. Expression of cytokine mRNAs in murine hearts with acute myocarditis caused by coxsackievirus b3. J Pathol. 1997;183:105–108. doi: 10.1002/(SICI)1096-9896(199709)183:1<105::AID-PATH1094>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu W, Chu YW, Wang Y, Liu QS, Xiong SD. Coxsackievirus group B type 3 infection upregulates expression of monocyte chemoattractant protein 1 in cardiac myocytes, which leads to enhanced migration of mononuclear cells in viral myocarditis. J Virol. 2004;78:12548–12556. doi: 10.1128/JVI.78.22.12548-12556.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen C, Lisewski U, Wrackmeyer U, Radke M, Westermann D, Sauter M, Tschö pe C, Poller W, Klingel K, Gotthardt M. Cardiac deletion of the Coxsackievirus-adenovirus receptor abolishes Coxsackievirus B3 infection and prevents myocarditis in vivo. J Am Coll Cardiol. 2009;53:1219–1226. doi: 10.1016/j.jacc.2008.10.064. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten N, Bouchard P, Moraska A, Huber S. Selection of an attenuated coxsackievirius B3 variant, using a monoclonal antibody reactive to a myocyte antigen. J Virology. 1991;65:1286–1290. doi: 10.1128/jvi.65.3.1286-1290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Weinzierl AO, Szalay G, Wolburg H, Sauter M, Rammensee HG, Kandolf R, Stevanovic S, Klingel K. Effective chemokine secretion by dendritic cells and expansion of cross-presenting CD4−/CD8+ dendritic cells define a protective phenotype in the mouse model of coxsackievirus myocarditis. J Virol. 2008;82:8149–8160. doi: 10.1128/JVI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. Viral myocarditis. Am J Pathol. 1980;101:427–479. [PMC free article] [PubMed] [Google Scholar]
- Zautner AE, Korner U, Henke A, Badorff C, Schmidtke M. Heparan sulfates and coxsackievirus-adenovirus receptor: each one mediates coxsackievirus B3 PD infection. J Virol. 2003;77:10071–10077. doi: 10.1128/JVI.77.18.10071-10077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.