Abstract
Once a virtually unknown nitrogen oxide, nitroxyl (HNO) has emerged as a potential pharmacological agent. Recent advances in the understanding of the chemistry of HNO has led to the an understanding of HNO biochemistry which is vastly different from the known chemistry and biochemistry of nitric oxide (NO), the one electron-oxidation product of HNO. The cardiovascular roles of NO have been extensively studied, as NO is a key modulator of vascular tone and is involved in a number of vascular related pathologies. HNO displays unique cardiovascular properties and has been shown to have positive lusitropic and ionotropic effects in failing hearts without a chronotropic effect. Additionally, HNO causes a release of CGRP and modulates calcium channels such as ryanodine receptors. HNO has shown beneficial effects in ischemia reperfusion injury, as HNO treatment during reperfusion reduces infarct size. In addition to the cardiovascular effects observed, HNO has shown initial promise in the realm of cancer therapy. HNO has been demonstrated to inhibit GAPDH, a key glycolytic enzyme. Due to the Warburg effect, inhibiting glycolysis is an attractive target for inhibiting tumor proliferation. Indeed, HNO has recently been shown to inhibit tumor proliferation in mouse xenografts. Additionally, HNO inhibits tumor angiogenesis and induces cancer cell apoptosis. The effects seen with HNO donors are quite different from NO donors and in some cases are opposite. The chemical nature of HNO explains how HNO and NO, although closely chemically related, act so differently in biochemical systems. This also gives insight into the potential molecular motifs that may be reactive towards HNO and opens up a novel field of pharmacological development.
Keywords: Nitroxyl, nitric oxide, heart failure, ischemia reperfusion injury
Introduction
Nitrogen oxides are important components of many physiological processes and with respect to cardiovascular diseases, have the potential to be useful pharmacological agents. The advent of nitrovasodilators such as nitroglycerin and the discovery that nitric oxide (NO) is endothelial derived relaxation factor (EDRF) has lead to an increase of research in this field. In the cardiovascular system, nitric oxide has been shown to regulate vascular tone, platelet function, leukocyte adhesion and extravasations of leukocytes [1, 2]. Dysfunction in the NO/cGMP pathway leads to a number of cardiovascular disorders [3, 4]. In addition to the critical functions of NO in the circulatory system, nitric oxide plays a role in the control of heart function [5].
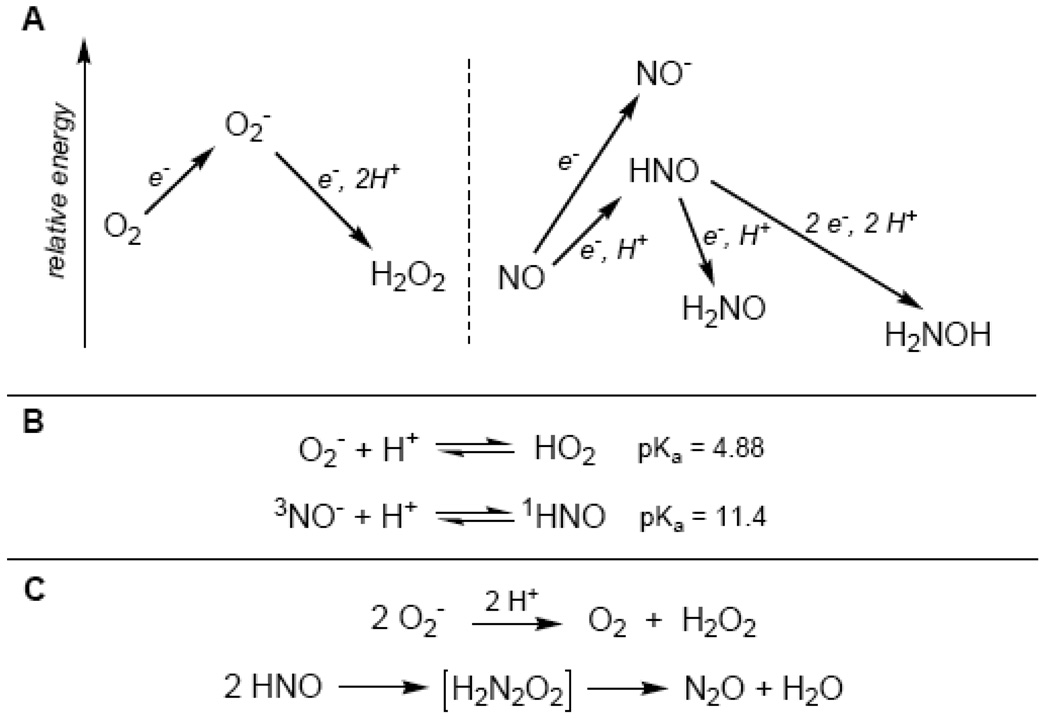
One of the complexities of studying NO is the diverse array of other nitrogen oxides that must be considered since NO metabolism can result in species such as nitrate, nitrite, peroxynitrite, nitrogen dioxide, hydroxylamine and ammonia. One of the most overlooked, yet chemically simple nitrogen oxide is HNO (colloquially termed nitroxyl; nitrosyl hydride in the IUPAC nomenclature), which is the one-electron reduction product of NO. In comparison, HNO is to NO as superoxide (O2−) is to molecular oxygen (O2) (Figure 1). Both NO and O2 are stable paramagnetic gases with neutral charge and one-electron reduction to HNO/NO− or O2−, respectively, results in the formation of an anion. In aqueous conditions, the formed anion has a pKa associated with the equilibrium of protonation of the anion. For superoxide, a pKa of 6.8 has been measured, while the pKa of NO− has been calculated to be around 11.4 (the HNO/NO− acid-base equilibrium is more complicated than common Brönsted-Lowry acid-base conjugates and will be discussed below in more detail). The electrochemical reduction of O2 to O2− is thermodynamically unfavorable (E°= −0.33 V) and similarly, the reduction of NO to NO− is thermodynamically very unfavorable (E°= −0.8 V) while reduction to HNO is still unfavorable (E°= −0.6 V) [6]. Just as superoxide is easily reduced to hydrogen peroxide (E= +0.89 V vs. NHE, pH 7), HNO can also be reduced to hydroxylamine as the two-electron reduction potential has been calculated to be approximately E°= +0.8 V [7]. Additionally, the one-electron reduction of HNO to aminoxyl radical (NH2O) has been calculated and is also thermodynamically favorable (E°= +0.6 V) [7]. Another comparison lies with the self-destruction of both superoxide and HNO; superoxide is known to undergo spontaneous disproportionation (i.e. two molecules of superoxide react to form molecular oxygen and hydrogen peroxide) while two molecules of HNO react to form essentially an HNO dimer (hyponitrous acid), which decomposes to nitrous oxide and water. The disproportionation reaction of superoxide proceeds with a relatively slow rate constant of ≥2 M−1·s−1 (and is presumably why superoxide dismutase enzymes exist) while the dimerization reaction of HNO proceeds with a rate constant of ~ 8×106 M−1s−1 [8]). For this reason, HNO is an inherently unstable molecule with respect to decomposition and must be generated in situ from donor compounds.
Figure 1. Comparison of HNO and Superoxide Chemistry.
(A) Molecular oxygen (O2) reduction to superoxide (O2−) is thermodynamically unfavorable, as is the reduction of NO to HNO/NO−. Further reduction of O2− to hydrogen peroxide is thermodynamically favorable, as is the reduction of HNO. (B) Reduction of O2 or NO results in an anionic species that has an associated pKa in aqueous conditions. Superoxide has a pKa of 4.88 and in neutral conditions the anion is the predominant species. Nitroxyl has a pKa of 11.4 and the dominant species in biology will be HNO. (C) Superoxide and HNO both auto-react resulting in the destruction of these species.
HNO Donor Compounds
Due to the fleeting nature of nitroxyl, vis-à-vis dimerization and formation of N2O, HNO must be generated in situ from donor compounds (Table 1). The most common donor currently used is Angeli’s salt (AS), which releases HNO with a half-life of approximately 2–3 minutes at physiological pH and temperature. HNO release from this compound is observed between pH 4–8. The mechanism of HNO release from Angeli’s salt has received a theoretical computational treatment [10]. The decomposition of Angeli’s salt to yield HNO also produces one equivalent of nitrite. Nitrite is not an innocuous bystander in physiological processes and reports on the biological effects of nitrite are rapidly accumulating [9]. Thus the formation of nitrite from Angeli’s salt can be a confounding component in studying HNO biology and pharmacology. Another drawback to Angeli’s salt use in a physiological context is the rapid kinetics of decomposition and lends itself to almost a “bolus dose” of HNO and nitrite. This can often be a technical problem when studying biochemical and physiological processes that can be several orders of magnitude slower. An often-overlooked chemical feature of Angeli’s salt decomposition is that theoretical calculations predict that a very small amount of NO is produced [10].
Table 1.
Common HNO Donors Used in Biology
| Donor | Chemical Structure |
Notes |
|---|---|---|
| Angeli's salt | 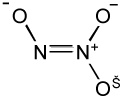 |
- Fast release of HNO |
| - Nitrite is also formed | ||
| Piloty's acid | 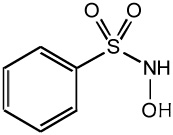 |
- Releases HNO only at high pH |
| Acyloxo nitroso "Blue compounds" | 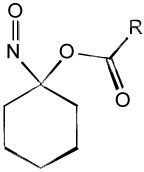 |
- Releases HNO upon cleavage of ester bond |
| - Rate of HNO release is controled by R substituent | ||
| - Reacts with thiols | ||
| IPA/NO | 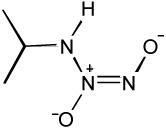 |
- Primary amine "NONOate" |
| - NO donor at lower pH | ||
| hetero-Diels-Alder cycloadduct | 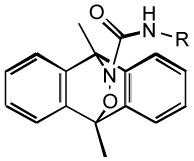 |
- Releases HNO upon photo activation |
| - Requires UV -A radiation (330 –380 nm) |
Another long known HNO donor is Piloty’s acid (or N-hydroxy-benzene sulfonamide). Piloty’s acid releases HNO upon deprotonation and requires rather high pH to yield HNO. This is the major issue with using Piloty’s acid in biological studies as such high pH is rarely, if ever, encountered [11]. However a potential area of research involves the development of structural analogs of Piloty’s acid that alter the HNO release pH profile by changing the electronic constituents on the phenyl ring. It is hypothesized that electron withdrawing groups may allow HNO release at more physiological conditions.
A new class of HNO donors, acyloxy nitroso compounds, has been developed by King et al. that release HNO upon cleavage of the ester bond [12]. Modifying the electronic and/or steric properties of the acetyl group position changes the rate of ester cleavage and thus HNO release. One of the major benefits of these HNO donors, besides controlling the HNO release rate, is that nitrite is not a product as is the case with Angeli’s salt. Another feature of these compounds that lends itself to biochemical studies is the blue color that is a result of the π→n electronic transition of the N-O bond. Thus biochemical reaction kinetics can be easily monitored. As novel and exciting as these compounds are, there are other reactions beyond HNO release. A major route of decomposition in biological systems is the reactivity of the donor compound with thiols. The characteristic blue color of these compounds due to the N-O bond implies that the relatively low energy LUMO is associated with this bond and is susceptible to nucleophilic attack. Thus HNO release from these compounds must compete with thiol reactivity at the electrophilic nitroso functional group. Since the majority of HNO biochemistry and pharmacology is associated with thiol reactions, the reactivity of “blue” HNO donors with thiols may complicate the interpretation of cellular or in vivo experiments. However the elegant chemistry of these compounds make it a very attractive choice for chemical and biochemical HNO donation and future generations of “blue” compounds may limit the thiol decomposition route.
Another potential HNO donor compound is IPA/NO, a primary amine NONOate. NONOates of secondary amines have traditionally been used as NO donors and have become the standard for NO donating compounds in chemistry and biology. However primary amine NONOates have the potential to release HNO under certain conditions [11]. IPA/NO decomposition has received theoretical consideration, and in concert with empirical observations, it is currently agreed that HNO is released above pH 7.8 while IPA/NO is believed to be exclusively a NO donor at pH <7 [13].
Temporal control of HNO release from a donor compound is a feature that is lacking from the types of donors described above. Compounds that release HNO via a retro-Diels-Alder reaction are activated by UV-A light [14]. This gives researchers control over when HNO is released and the only other product to consider from decomposition is a primary amine. Despite the control of these compounds with respect to HNO release, there have been no biological reports using these compounds, perhaps due to the use of UV irradiation.
While there are currently only a few reasonable HNO donors available to researchers, it must be noted that the chemical, biochemical and physiological study of HNO is achievable, if not readily straightforward. The use of multiple HNO donors in a study is strongly encouraged due to the confounding factors of each HNO donor (i.e. nitrite from Angeli’s salt, thiol reactions of “blue” compounds, NO release from IPA/NO). If multiple HNO donors are used and elicit the same biological response, then it is safe to assume that the effect is due to HNO as HNO release is the only common characteristic of these diverse chemical compounds (Table 1). Thus when it comes to HNO donors, fear not but be thorough.
HNO Chemistry and Biochemistry
The orthogonal relationship between NO and HNO was not predicted from the literature prior to 2000. Rampant misconception on the chemical nature of nitroxyl came from the reported pKa of 4.7, which suggests that NO−, not HNO, was the most relevant species at physiological concentration [15]. This also suggested that NO− and NO would readily interconvert with reduction potential of NO to 3NO− of +0.39 V and 1NO− of −0.2 V [16]. If these potentials were accurate, NO should be rapidly be converted to 3NO− which then would be expected to react with O2 with diffusion controlled rate constants to form peroxynitrite. This implies that NO would not be detectable in biological systems and that peroxyntrite would be the primary effector molecule of NOS metabolism. However, NO is directly detected in vivo and from the enzymatic turnover of NOS [17–19]. Additional experiments showed that NO donors in vivo give different effects than HNO donors indicating that these species do not interconvert but rather have orthogonal and distinct processes [20].
These observations taken together required a re-examination of the chemistry of HNO/NO−. Two studies using different methods re-evaluated the pKa finding that it was 11.5 instead of 4.7 [8, 21]. This reverses several previous conceptions about nitroxyl. The first is that HNO, and not NO−, is the predominant species in vivo. The second is that the reduction potential of NO is high < −0.8 V which is higher than reducing system occur in mammalian systems. This explains why NO is not converted in vivo to NO− (and then to peroxynitrite via reaction with O2) and this, in part, explains the orthogonal behavior of NO and HNO donors observed.
Though the revised pKa of 11.5 suggests that HNO and not NO− is the dominant species at physiological pH, this does not discount the possibility of the reactions involving the intermediacy of 3NO−. Normally, reactions that are involved in acid base equilibrium, i.e. protonation/deprotonation reactions, are so rapid that it does not affect the overall kinetics of a reaction. However, it has been shown that the deprotonation of HNO involves intersystem crossing (vibrational relaxation from one energy state to a lower energy state of differing quantum spin states) between 1HNO and 3NO−. This causes the deprotonation reaction (HNO ↔ H+ + NO−) to be very slow compared to a spin allowed process (e.g. 2 H2O ↔ H3O+ + OH−). This slow deprotonation of HNO has effects on its overall reactivity. For example 3NO− can react with O2 with rate constants of >109 M−1 s−1. However at neutral pH, HNO is the predominant species and deprotonation to 3NO− proceeds with a rate constant of ~105 M−1 s−1 and HNO has a half-life of 5000 seconds [22]. It has been argued that the 3NO−/O2 reaction is not kinetically viable at neutral pH, even though the rate constant for this reaction is near diffusion controlled, due to this spin-forbiden deprotonation [20, 22]. This kinetic barrier suggests that HNO cannot be converted to 3NO− that could be converted to peroxynitrite via oxygen reaction. Therefore, NO− and HNO have sufficient kinetic and thermodynamic barriers that they cannot be interconverted. These vastly different chemical entities (HNO and NO−) also display very different chemical reactivities. While NO− predominately undergoes outer sphere electron transfer (much like static electricity where the electron jump from one atom to another) and is therefore a nucleophile, HNO is a good electrophile preferring addition reaction to nucleophiles, especially thiols, as opposed to hydrogen atom donation [20, 23], although HNO can potentially serve as a hydrogen atom donor to high energy radicals such as a lipid radical.
With this new chemical view of HNO, many of the biochemical targets can be illuminated. The kinetics of HNO reactivity with a number of potential biomolecules revealed that HNO reacts with ferric heme proteins, CuZnSOD, MnSOD and thiols with bimolecular rate constant of ~106 M−1 s−1 [20, 23]. HNO reacts with ferrous heme proteins approximately 100 times slower (k = ~104 M−1 s−1) than ferric heme proteins. Interestingly the rate constant for the reaction of HNO with O2 is <103 M−1 s−1, although the precise mechanism or the intermediates of this reaction are not currently known [20, 23]. In contrast, NO reacts preferentially with ferrous heme protein and high-energy radicals (e.g. lipid radicals, O2−) [24]. One feature of HNO is the relatively weak H-N bond strength of ~ 50 kcal/mol, making HNO a decent H atom donor and thus able to quench high-energy radicals. Indeed, HNO has been shown to inhibit lipid peroxidation in a yeast model system, demonstrating that HNO also acts as an anti-oxidant [25]. The differences in reactivity with biochemical targets suggest that the biological orthogonality of NO and HNO can be rationalized based on the chemical reactivity of these related but vastly different chemical species, summarized in Table 2.
Table 2.
Comparison of NO and HNO Reactivity with Biological Reactants
| Biological Reactant |
NO | HNO |
|---|---|---|
| NO | no reaction | Forms N2O2−/HN2O2 unknown chemistry |
| HNO | Forms N2O2−/HN2O2 unknown chemistry | Dimerization and decomposition to N2O |
| O2 | Autoxidation leading to nitrosative species (i e. NO2 and N2O3) | Forms a potent 2 e− oxidant that is not ONOO− |
| RSH /RS− | no reaction | Forms sulfinamide or disulfide + H2NOH |
| Fe2+ heme | Very stable Fe2+-NO | Forms coordination complexes |
| Fe3+ heme | Forms unstable electrophic nitrosyl; first step in reductive nitrosylation | Very stable Fe2+-NO (except sGC) |
| Cu2+ | no reaction | Reduces Cu2+ to Cu+ yielding NO |
| Lipid Radical | Yields Lipid -NO | Yields Lipid -H + NO |
Given the preference of HNO to react with ferric heme proteins and acidic thiols, this should guide investigators when studying the biological effects of HNO. The facile and irreversible reaction between HNO and the active site thiol of GAPDH represents a unique biochemical molecular motif for further investigation [25]. Similarly, HNO inhibits cathepsin B activity, which is a cysteine protease [26, 27]. HNO also has been shown to react with metal bound thiolates, as Angeli’s salt was used to disrupt a yeast Cu-thiolate transcription factor protein [28]. These examples demonstrate the reactive nature of HNO in a biological setting by targeting critical reactive thiolates (RS−). Other enzymes such as caspases, fatty acid acyl transferase and ubiquitin ligases use acidic thiols in their respective catalytic processes and are potentially targets of HNO. Furthermore, calcium channels, which are altered by HNO, have critical thiols that are presumably the chemical motif that is responsible for the observed effects of HNO. Additionally, metalloproteins are subject to reactivity with HNO. These potential biochemical targets have not yet been investigated, but represent a large body of work that needs to be addressed in the development of HNO biochemistry and pharmacology.
HNO in Biological Systems
Similar to nitric oxide, HNO has been shown to induce vasorelaxation. This effect has been known for some time as HNO, from either Angeli’s salt or cyanamide, is a potent vasodilator similar to NO and EDRF [29, 30]. HNO was also a candidate for EDRF, as thiol compounds inhibit the vasorelaxation of EDRF, which is consistent with HNO chemistry [31]. More recent work has shown that HNO is selectively a venodilator in dogs [32]. In contrast, NO donors equally dilate both the arterial and venal side of the circulatory system. Administration of baroreceptor blockers resulted in balanced dilation suggesting that the preference for AS mediated venodilation was through constriction of the arterial side through a sympathetic response [32]. The molecular mechanisms of HNO mediated vasodilation have been reported. Recent studies have shown that HNO mediates relaxation in rodent models through a cGMP mechanism as well as modulating voltage-gated potassium channels [33, 34]. In rat coronary vasculature, Angeli’s salt is a potent vasodilatory agent by sGC dependent CGRP release and by activating KATP channels [35]. Of clinical relevance, HNO from Angeli’s salt does not develop tolerance in isolated rat aorta, which is a concern with traditional therapies for angina pecoralis such as nitroglycerin. [36].
An important property of HNO in the cardiovascular system is that HNO donors cause an increase in contractility in a canine model. HNO has been shown to have positive inotropic (force of muscle contraction) as well as lusitropic (relaxation of cardiac muscle) properties; both properties contribute to increased cardiac output [32]. Furthermore, in failing cainine hearts, HNO also has positive lusitropic and inotropic effects that are independent of beta-adernergic signaling [32]. Additionally, it was found that HNO donors increased circulating CGRP levels, which increases the contractility of the heart (positive inotropy) without an increase in the chronotropic effect (i.e. without an increase in the rate of cardiac contraction) [32]. It was presumed that CGRP was released from NANC neurons. However, under heart failure conditions, CGRP did not play a major role in the HNO induced contractility suggesting another molecular target [37]. Additional mechanisms for HNO induced contractility have been proposed. Sarcoplasmic reticulum calcium cycling has been implicated as HNO donors were shown to modulate the RyD receptors and SERCA that result in increasing the contractility of isolated cardiomyocytes [38]. Mechanistically, HNO has been shown to target a critical cysteine residue of SERCA (cysteine 674) that causes an increase in SERCA activity [39]. Furthermore, SERCA activation is achieved by dephosphorylation by phospholamban (PLN), as HNO causes the formation of a disulfide on PLN, which removes the inhibition of the Ca2+ pump [40]. Additionally, HNO alters myofilament- Ca2+ interaction and results in increased cardiac contraction force [41]. Collectively, these finding indicate that nitroxyl may have a positive effect in heart failure with a number of potential targets and mechanisms. Furthermore this represents opportunity to develop HNO donors for the pharmacological treatment of heart failure.
HNO has been shown to have effects in the realm of ischemia reperfusion (IR) injury. IR injury occurs when tissue is deprived of adequate blood flow for a period of time causing among other effects hypoxia, which is the ischemic event. The reperfusion of oxygenated blood causes the injury and results in necrosis of the tissue. IR injury can be alleviated by preconditioning the tissue, which involves brief occlusions of the vasculature prior to the actual cessation of blood flow. NO donors have been shown to be protective in cardiac ischemia reperfusion injury when given during the reperfusion phase [42]. However, HNO administration during reperfusion was found to dramatically increase the infarct size. These results again indicate that in vivo, NO and HNO have very different effects [43]. However, additional studies have shown that HNO is a powerful preconditioning agent. Pre-treating hearts with Angeli’s salt followed by an ischemic event results in a dramatic decrease in the infarct size [44]. In contrast, NO donors are modest preconditioning agents. These in vivo results clearly suggest that HNO and NO donors have very different pharmacological effects.
In addition to cardiovascular effects, HNO has shown promising anti-cancer effects. Most solid tumors thrive in hypoxic conditions and rely upon glycolysis as a major energy source. One of the critical enzymes in the glycolytic pathway is GAPDH. HNO has been shown to inhibit GAPDH activity in an irreversible manner [45]. Furthermore, HNO inhibits breast and neurobalstoma cancer proliferation in mouse xenografts as well as in vitro cultures [46, 47]. HNO also caused an increase of apoptosis in the breast cancer xenografts and inhibited tumor angiogenesis [46]. In addition to the direct effects of HNO on cancer cells, HNO-donors may be useful adjuvant agents to cancer chemotherapy. Angeli’s salt has been shown to inhibit poly(ADP-Ribose) polymerase (PARP) in a breast cancer cell line [48]. PARP is an important component of the DNA repair machinery and is thus a potentially important molecular target in the treatment of cancer, since a number of chemotherapies and radiation therapy are based on inducing DNA damage of the cancer cell. Inhibition of PARP by HNO donors would then increase the efficacy of these therapies. These observations indicate that HNO donors may be effective agents in cancer chemotherapy.
Due to the unique physiological properties of HNO, the endogenous formation of HNO in vivo is an intriguing possibility and one that has been suggested for several decades. A number of potential mechanisms have been proposed for the endogenous production of HNO (Figure 2); however no mechanism has been clearly established to date, mainly due to a lack of detection (vide infra). Several studies implicated that HNO could be an intermediate in catalytic process of nitric oxide synthase (NOS) [49–51]. Detection of N2O and NH2OH, products of HNO metabolism, was an indication that HNO was part of the enzymatic turnover of NOS [50, 52]. Furthermore, the presence of CuZnSOD caused an increase in NO from NOS [53]. Also, Fe(II)-NO was observed to be formed during enzymatic turnover in NOS which is normally in a ferric resting state. Taken together, these findings imply that nitroxyl could be an intermediate in the metabolism of NOS, however this has not been conclusively established.
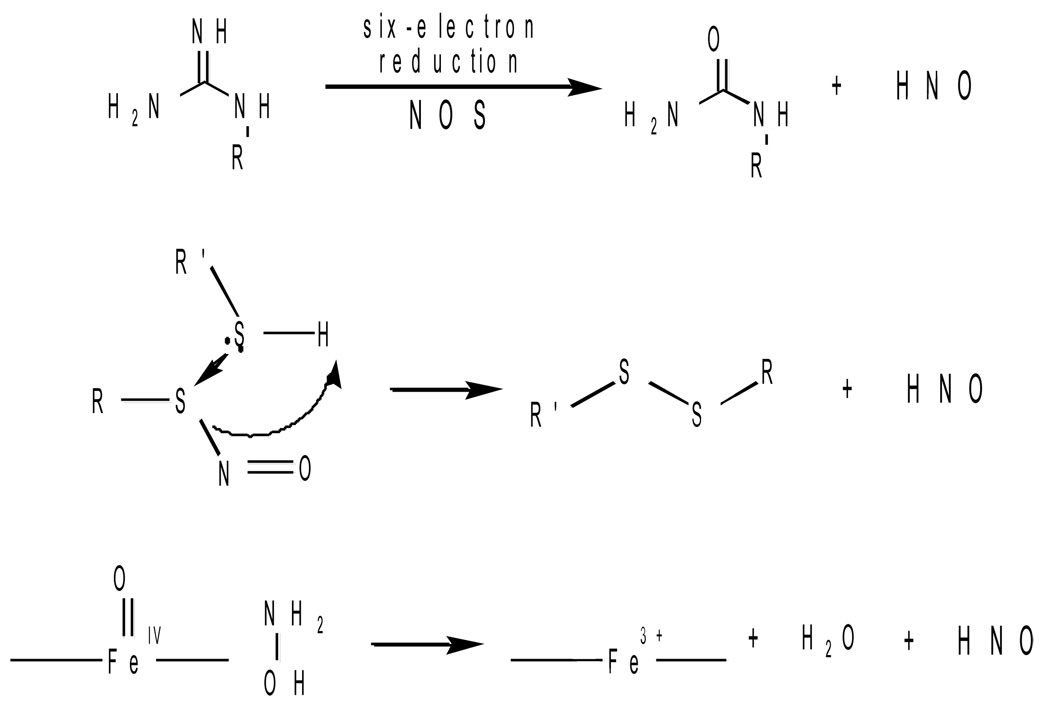
Figure 2. Potential Mechanisms of Endogenous HNO Formation.
HNO has been postulated to be formed from the enzymatic activity of nitric oxide synthase (NOS) in which the substrate arginine is reduced by six electron to yield HNO instead of five electrons to yield NO. Another route is from the decomposition of nitrosothiol by thiols such as glutathione. HNO can also be generated by the oxidation of hydroxylamine from the peroxidase activity of heme proteins.
Another potential mechanism of HNO generation in biological systems is through the decomposition of RSNO. Thioredoxin has been shown to generate HNO and NH2OH though the reduction of these adducts [54]. HNO is also formed from the nitrosation of dithiol compounds (i.e. DTT and lipoic acid) [55]. Thus one of the secondary products of nitrosative stress involves the intermediacy of HNO and eventually NH2OH. These processes have not yet been established in vivo, but the chemistry implies that RSNO is not an evolutionary signaling motif like phosphorylation, as has been suggested, due to the reactivity of excess thiols to form disulfide and HNO [56].
The oxidation of NH2OH is another potential source of HNO. The two-electron oxidation of NH2OH whose oxidation potential is +0.7 V (vs. Ag/AgCl) results in HNO. The generation of high-valent iron-oxo complexes from the reaction of hydrogen peroxide with ferric hemes can oxidize NH2OH to HNO [55]. The formation of glutathione sulfinamide was used as a marker of HNO migration from the heme pocket [55]. Since the resulting ferric heme that is formed after the oxidation of hydroxylamine can react with HNO to form a ferrous nitrosyl complex, there is some question as to whether HNO would be capable of escaping the heme pocket to react with another substrate. A survey of heme proteins showed that Mb and MPO having proximal hisitidine residues to the heme site could generate HNO that is able to migrate from the heme pocket. Interestingly, HNO activity has been primarily shown to be associated with the heart and under conditions of inflammation where hydrogen peroxide generation is known.
One of the current challenges with expanding HNO research is in developing reliable detection methods of HNO generation and/or reactivity in vivo. Currently, the formation of N2O and NH2OH are used to indicate that HNO is involved in a particular process; however this is only indirect evidence of nitroxyl. The formation of sulfinamide with proteins such as GAPDH may provide a unique footprint for the formation and activity of this compound [45, 57]. Recently a novel proteomic approach offers a potentially very useful tool in analyzing the reactive targets of HNO from ex vivo samples [58]. The development of analytical methods is critically needed for this promising pharmacological agent to advance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Ignarro LJ. Physiology and pathophysiology of nitric oxide. Kidney Int. 1996;55 Suppl.:S2–S5. [PubMed] [Google Scholar]
- 3.Bian K, Doursout MF, Murad F. Vascular system: role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. (Greenwich) 2008;10:304–310. doi: 10.1111/j.1751-7176.2008.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoli C, de NF, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15:265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Paolocci N, Lopez BE, Jackson MI, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM. The pharmacology of nitroxyl (HNO) and its therapeutic potential: not just the Janus face of NO. Pharmacol. Ther. 2007;113:442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuto JM, Switzer CH, Miranda KM, Wink DA. Nitroxyl (HNO): chemistry, biochemistry, and pharmacology. Annu. Rev. Pharmacol. Toxicol. 2005;45:335–355. doi: 10.1146/annurev.pharmtox.45.120403.095959. [DOI] [PubMed] [Google Scholar]
- 7.Dutton AS, Fukuto JM, Houk KN. Theoretical reduction potentials for nitrogen oxides from CBS-QB3 energetics and (C)PCM solvation calculations. Inorg. Chem. 2005;44:4024–4028. doi: 10.1021/ic048734q. [DOI] [PubMed] [Google Scholar]
- 8.Shafirovich V, Lymar SV. Nitroxyl and its anion in aqueous solutions: spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc. Natl. Acad. Sci. USA. 2002;99:7340–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 10.Dutton AS, Fukuto JM, Houk KN. Mechanisms of HNO and NO production from Angeli's salt: density functional and CBS-QB3 theory predictions. J. Am. Chem. Soc. 2004;126:3795–3800. doi: 10.1021/ja0391614. [DOI] [PubMed] [Google Scholar]
- 11.Miranda KM, Nagasawa HT, Toscano JP. Donors of HNO. Curr. Top. Med. Chem. 2005;5:649–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 12.Sha X, Isbell TS, Patel RP, Day CS, King SB. Hydrolysis of acyloxy nitroso compounds yields nitroxyl (HNO) J. Am. Chem. Soc. 2006;128:9687–9692. doi: 10.1021/ja062365a. [DOI] [PubMed] [Google Scholar]
- 13.Miranda KM, Katori T, Torres dHCL, Thomas L, Ridnour LA, McLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, Tocchetti CG, Saavedra JE, Keefer LK, Houk KN, Fukuto JM, Kass DA, Paolocci N, Wink DA. Comparison of the NO and HNO donating properties of diazeniumdiolates: primary amine adducts release HNO in Vivo. J. Med. Chem. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y, Nakagawa H, Matsuo K, Suzuki T, Miyata N. Photoactivatable HNO-releasing compounds using the retro-Diels-Alder reaction. Chem. Commun. (Camb) 2008;41:5149–5151. doi: 10.1039/b811985f. [DOI] [PubMed] [Google Scholar]
- 15.Gratzel M, Taniguchi S, Henglein A. Pulsradiolytische Untersunchung kurzlebiger zwischenprodukte der NO-reduktion in wassriger losung. Ber. Bunsen-Ges. Phys. 1970;74:292–297. [Google Scholar]
- 16.Stanbury DM. Reduction potentials involving inorganic free radicals in aqueous solution. Adv. Inorg. Chem. 1989;33:69–136. [Google Scholar]
- 17.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibbs JBJ, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 19.Lewis RS, Tamir S, Tannenbaum SR, Deen WH. Kinetic analysis of the fate of nitric oxide synthesized by macrophages in vitro. J. Biol. Chem. 1995;270:29350–29355. doi: 10.1074/jbc.270.49.29350. [DOI] [PubMed] [Google Scholar]
- 20.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartberger MD, Liu W, Ford E, Miranda KM, Switzer C, Fukuto JM, Farmer PJ, Wink DA, Houk KN. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc Natl. Acad. Sci. USA. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafirovich V, Lymar SV. Spin-forbidden deprotonation of aqueous nitroxyl (HNO) J. Am. Chem. Soc. 2003;125:6547–6552. doi: 10.1021/ja034378j. [DOI] [PubMed] [Google Scholar]
- 23.Liochev SI, Fridovich I. The mode of decomposition of Angeli's salt (Na2N2O3) and the effects thereon of oxygen, nitrite,superoxide dismutase, and glutathione. Free Radic. Biol. Med. 2003;34:1399–1404. doi: 10.1016/s0891-5849(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 24.Wink DA, Miranda KM, Katori T, Mancardi D, Thomas DD, Ridnour L, Espey MG, Feelisch M, Colton CA, Fukuto JM, Pagliaro P, Kass DA, Paolocci N. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: a novel redox paradigm. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2264–H2276. doi: 10.1152/ajpheart.00531.2003. [DOI] [PubMed] [Google Scholar]
- 25.Lopez BE, Shinyashiki M, Han TH, Fukuto JM. Antioxidant actions of nitroxyl (HNO) Free Radic. Biol. Med. 2007;42:482–491. doi: 10.1016/j.freeradbiomed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Vaananen AJ, Salmenpera P, Hukkanen M, Miranda KM, Harjula A, Rauhala P, Kankuri E. Persistent susceptibility of cathepsin B to irreversible inhibition by nitroxyl (HNO) in the presence of endogenous nitric oxide. Free Radic. Biol. Med. 2008;45:749–755. doi: 10.1016/j.freeradbiomed.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Vaananen AJ, Salmenpera P, Hukkanen M, Rauhala P, Kankuri E. Cathepsin B is a differentiation-resistant target for nitroxyl (HNO) in THP-1 monocyte/macrophages. Free Radic. Biol. Med. 2006;41:120–131. doi: 10.1016/j.freeradbiomed.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Cook NM, Shinyashiki M, Jackson MI, Leal FA, Fukuto JM. Nitroxyl-mediated disruption of thiol proteins: inhibition of the yeast transcription factor Ace1. Arch. Biochem. Biophys. 2003;410:89–95. doi: 10.1016/s0003-9861(02)00656-2. [DOI] [PubMed] [Google Scholar]
- 29.Fukuto JM, Hszieh R, Gulati P, Chiang KT, Nagasawa HT. N,O-diacylated-N-hydroxyarylsulfonamides: nitroxyl precursors with potent smooth muscle relaxant properties. Biochem. Biophys. Res. Commun. 1992;187:1367–1373. doi: 10.1016/0006-291x(92)90453-r. [DOI] [PubMed] [Google Scholar]
- 30.Fukuto JM, Nagasawa HT. Involvement of nitroxyl (HNO) in the cyanamide-induced vasorelaxation of rabbit aorta. Biochem. Pharmocol. 1994;47:922–924. doi: 10.1016/0006-2952(94)90495-2. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Furchgott RF. Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J Pharmacol. Exp. Ther. 1993;267:371–378. [PubMed] [Google Scholar]
- 32.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc. Natl. Acad. Sci. USA. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favaloro JL, Kemp-Harper BK. Redox variants of nitric oxide (NO* and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2009 doi: 10.1152/ajpheart.00008.2009. [DOI] [PubMed] [Google Scholar]
- 34.Irvine JC, Favaloro JL, Kemp-Harper BK. NO- activates soluble guanylate cyclase and K+ channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 35.Favaloro JL, Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc. Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 37.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proc. Natl. Acad. Sci. USA. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di BG, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ. Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N, Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 41.Dai T, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. Nitroxyl increases force development in rat cardiac muscle. J. Physiol. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X-L, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after mycardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ. Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 43.Ma XL, Gao F, Liu GL, Lopez BL, Christopher TA, Fukuto JM, Wink DA, Feelisch M. Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proc. Natl. Acad. Sci. USA. 1999;96:14617–14622. doi: 10.1073/pnas.96.25.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolocci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 45.Lopez BE, Wink DA, Fukuto JM. The inhibition of glyceraldehyde-3-phosphate dehydrogenase by nitroxyl (HNO) Arch. Biochem. Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Norris AJ, Sartippour MR, Lu M, Park T, Rao JY, Jackson MI, Fukuto JM, Brooks MN. Nitroxyl inhibits breast tumor growth and angiogenesis. Int. J. Cancer. 2008;122:1905–1910. doi: 10.1002/ijc.23305. [DOI] [PubMed] [Google Scholar]
- 47.Stoyanovsky DA, Schor NF, Nylander KD, Salama G. Effects of pH on the cytotoxicity of sodium trioxodinitrate (Angeli's salt) J. Med. Chem. 2004;47:210–217. doi: 10.1021/jm030192j. [DOI] [PubMed] [Google Scholar]
- 48.Sidorkina O, Espey MG, Miranda KM, Wink DA, Laval J. Inhibition of poly(ADP-RIBOSE) polymerase (PARP) by nitric oxide and reactive nitrogen oxide species. Free Radic. Biol. Med. 2003;35:1431–1438. doi: 10.1016/j.freeradbiomed.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Feelisch M, te Pool M, Zamora R, Deussen A, Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994;368:62–64. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt HH, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. No. NO from NO synthase. Proc. Natl. Acad. Sci. USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adak S, Wang Q, Stuehr DJ. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J. Biol. Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 52.Ishimura Y, Gao YT, Panda SP, Roman LJ, Masters BS, Weintraub ST. Detection of nitrous oxide in the neuronal nitric oxide synthase reaction by gas chromatography-mass spectrometry. Biochem. Biophys. Res. Commun. 2005;338:543–549. doi: 10.1016/j.bbrc.2005.07.202. [DOI] [PubMed] [Google Scholar]
- 53.Hobbs AJ, Fukuto JM, Ignarro LJ. Formation of free nitric oxide from L-arginine by nitric oxide aynthase: direct enhancement of generation by superoxide dismutase. Proc. Natl. Acad. Sci. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoyanovsky DA, Tyurina YY, Tyurin VA, Anand D, Mandavia DN, Gius D, Ivanova J, Pitt B, Billiar TR, Kagan VE. Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J. Am. Chem. Soc. 2005;127:15815–15823. doi: 10.1021/ja0529135. [DOI] [PubMed] [Google Scholar]
- 55.Donzelli S, Espey MG, Thomas DD, Mancardi D, Tocchetti CG, Ridnour LA, Paolocci N, King SB, Miranda KM, Lazzarino G, Fukuto JM, Wink DA. Discriminating formation of HNO from other reactive nitrogen oxide species. Free Radic. Biol. Med. 2006;40:1056–1066. doi: 10.1016/j.freeradbiomed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 56.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 57.Shen B, English AM. Mass spectrometric analysis of nitroxyl-mediated protein modification: comparison of products formed with free and protein-based cysteines. Biochemistry. 2005;44:14030–14044. doi: 10.1021/bi0507478. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman MD, Walsh GM, Rogalski JC, Kast J. Identification of nitroxyl-induced modifications in human platelet proteins using a novel mass spectrometric detection method. Mol. Cell Proteomics. 2008 doi: 10.1074/mcp.M800230-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]