Abstract
OBJECTIVE
To review the evidence regarding neuroprotective effects of antenatal exposure to magnesium sulfate.
DATA SOURCES
We conducted database searches of MEDLINE, the Cochrane Library and Controlled Trials Register, as well as the ClinicalTrials.gov and International Clinical Trials Register websites. Bibliographies of all relevant articles were reviewed.
METHODS OF STUDY SELECTION
Randomized controlled trials (RCTs) comparing magnesium sulfate with placebo/other treatment in patients at risk for preterm delivery were evaluated for inclusion and methodological quality. The primary outcome was death or cerebral palsy by 18–24 months corrected age. Secondary outcomes were death, cerebral palsy, moderate-severe cerebral palsy, and death or moderate-severe cerebral palsy. Separate analyses were performed according to the gestational age (GA) at randomization (less than 32 to 34 and less than 30 weeks), and also for studies in which magnesium sulfate was used exclusively for fetal neuroprotection.
TABULATION, INTEGRATION AND RESULTS
Five RCTswere included (5,235 fetuses/infants). When analyzed by GA at randomization, in utero exposure to magnesium sulfate at less than 32–34 weeks did not reduce the rate of death or cerebral palsy (relative risk [RR] 0.92, 95% confidence interval [CI] 0.83–1.03). However, cerebral palsy (RR 0.70, 95% CI 0.55–0.89), moderate-severe cerebral palsy (RR 0.60, 95% CI 0.43–0.84), and death or moderate-severe cerebral palsy were significantly reduced, without an evident increase in the risk of death (RR 1.01, 95% CI 0.89–1.14). Similar results were obtained when the GA at randomization was < 30 weeks. When only neuroprotection trials (4 trials, 4324 fetuses/infants) are analyzed, in utero exposure to magnesium sulfate additionally reduced the primary outcome of death or cerebral palsy. The number needed to treat to prevent one case of cerebral palsy among those who survive until age 18–24 months is 46 in infants exposed to magnesium sulfate in utero before 30 weeks, and 56 in infants exposed to magnesium sulfate in utero before 32 to 34 weeks (95% confidence limits 26–187).
CONCLUSION
Fetal exposure to magnesium sulfate in women at risk for preterm delivery significantly reduces the risk of cerebral palsy without increasing the risk of death.
INTRODUCTION
Magnesium sulfate is one of the most commonly used medications in the United States’ labor and delivery units. For more than 60 years, it has been used for seizure prophylaxis in preeclampsia, and more recently as a tocolytic. A link between antenatal exposure to magnesium sulfate and a reduction in the risk of cerebral palsy (CP) was first suggested by a case-control study of extremely low birth weight infants. (1) Since then, several randomized trials have been conducted to determine its utility as a neuroprotectant. Doyle et al have recently completed a meta-analysis regarding this issue and concluded that antenatal fetal exposure to magnesium sulfate reduces the risk of CP development without affecting the rate of pediatric mortality. (2)
The prevalence of CP is estimated to be 3.6 per 1000, or about 1 in 276 children.(3) It is greatly influenced by the increased rates of prematurity.(4,5) Both economically and emotionally, the burden of CP is enormous. The CDC estimates the lifetime costs including direct medical (physician visits, hospital stays, medications, assistive devices, long term care), direct non medical (home and automobile modifications, special education) and indirect (productivity losses) for all people born with CP in 2000 to be $11.5 billion (2003 dollars). (6)
The objective of this meta-analysis is to systematically review the evidence of fetal neuroprotection by magnesium sulfate from the available randomized trials, and specifically explore the findings at different gestational ages. In addition, we will explore the relation of antenatal exposure to magnesium sulfate on perinatal/infant mortality.
METHODS
a- Data Sources
A literature search was conducted in PubMed (U.S. National Library of Medicine, Jan 1966–Apr 2008) to identify randomized controlled trials (RCTs) with published data on the use of magnesium sulfate for fetal neuroprotection in pregnant women at risk for preterm birth. Keywords included: “magnesium”, “antenatal”, “preterm”, “cerebral palsy” and “neuroprotection”. The “AND” operator was used to combine these terms in varying combinations. No date or language restrictions were employed. Bibliographies of all relevant eligible articles were reviewed for further potential references. In addition the Cochrane Library Database of Systematic Reviews was searched and appropriate data were extracted. The ClinicalTrials.gov, Cochrane Controlled Trials Register, and International Clinical Trials Register websites were also searched to identify any additional ongoing or completed trials.
b- Study Selection and Data Extraction
Studies were included if patients at risk for preterm delivery were randomized to receive antenatal magnesium sulfate or placebo/other treatment. We included trials where the primary aim of magnesium sulfate was for fetal neuroprotection and those where it was used for other aims (e.g. tocolysis or seizure prophylaxis) but long term infant outcomes of CP and perinatal/infant mortality were examined and reported. We excluded observational studies and trials in which there was no long term infant follow-up sufficient for diagnosis of CP. Data were extracted by two reviewers independently and discrepancies were resolved by agreement. Using the QUOROM guidelines for systematic reviews of randomized trials, eligible studies were assessed for methodological quality. Evaluated criteria included the method of randomization, allocation concealment, masking conditions, and adequacy of follow-up. Data were analyzed according to the gestational age (GA) at randomization. A priori, we selected two thresholds for analysis: less than 32–34 and less than 30 weeks at randomization. We also evaluated outcomes according to the primary intent of the included studies, and analyzed those whose aim was to study the neuroprotective effects of magnesium sulfate. The unit of analysis was the fetus/infant.
c- Selection of Outcomes
For the purpose of this meta-analysis our primary outcome is a composite outcome of perinatal or infant death or CP (irrespective of severity) among survivors as assessed at 18–24 months of life (corrected for prematurity). This combination is necessary since CP and death are competing outcomes. Our secondary outcomes include death, CP, moderate-severe CP and a combined outcome of death or moderate-severe CP. For this evaluation we utilized the diagnostic criteria for moderate-severe CP provided by each study. Where this delineation was not made, the following criterion was used: inability of the child to walk independently at age 2 years. This corresponds at two years of age to a severity level of ≥2 on the Gross Motor Function Classification System (GMFCS) which was developed by Palisano et al. (7)
d- Statistical Analysis
Statistical analyses were performed using MIX software version 1.7 (Kitasato Clinical Research Center, Sagamihara, Japan).(8,9) Dichotomous data from each study were extracted and two-way contingency tables constructed to calculate the treatment effects expressed as relative risks (RR) with a 95% confidence interval (CI). Empty cells were treated by adding 0.5 to each cell in the table. Separate contingency tables were made for each outcome, if the data were available, at the two GA cutoffs selected and then according to the intent of the study. Treatment effects were first estimated for each trial and then combined using standard meta-analytic techniques. We used the fixed effects Mantel-Haenszel model (10) to pool RRs from individual trials and the results are presented as such. Publication bias was examined using Begg’s test (11) and by visual inspection of funnel plots. These are plots of the trials’effect estimates against sample size. When biases and heterogeneity are absent, the funnel plots will be symmetrical. Two-sided p values <0.05 were considered statistically significant.
RESULTS
Five RCTs (5235 fetuses/infants) were identified as eligible to be included in this review.(12–17) The studies’ details, methodological quality, and long term infant follow up are summarized in tables 1 and 2. The Magnesium and Neurologic Endpoints Trial (MagNET) (12) consisted of 2 tandem trials in which magnesium sulfate was given either for neuroprotection or tocolysis. This trial is thus divided accordingly in some parts of the meta-analysis {MagNET (P) & MagNET (T)}. Magnesium sulfate was used for fetal neuroprotection in 3 trials (Beneficial Effects of Antenatal Magnesium Sulfate (BEAM)(16), the Australasian Collaborative Trial of Magnesium Sulphate (ACTOMgSO4) (13), and PREMAG (15,17)) and in the preventive arm of the MagNET study.(12) It was used for prevention of eclampsia in the Magnesium Sulphate for the Prevention of Eclampsia (Magpie) trial (14)and for tocolysis in MagNET (T).
Table 1.
Randomized controlled trials included in systematic review.
| Study | Inclusion | Exclusion | Patient characteristics (N women/N fetuses) | Follow up (months) |
|---|---|---|---|---|
| BEAM Rouse (16) 1997–2004 USA |
|
|
|
24 |
| ACTOMgSO4 Crowther (13) 1996–2000 Australia-New Zealand |
|
|
|
24 |
| PREMAG Marret (15,17) 1997–2003 France |
|
|
|
24 |
| Magpie (14) 1998–2001 International (19 countries) |
|
|
|
18 |
| MagNET (12) Mittendorf 1995–1997 USA |
|
|
Preventive arm:
|
18 |
Estimate – uncertain from published data
wks=weeks, PPROM=preterm premature rupture of membrane, PTL=preterm labor, IUFD=intrauterine fetal demise, HTN=hypertension, MgSO4=magnesium sulfate, C/I=contraindication, CD=cesarean delivery, CCB=calcium channel blockers, NRFHT=non reassuring fetal heart tracing, h=hour
Table 2.
Methodological review of randomized controlled trials in systematic review.
| Study | Blinding | Randomization | Stratification | Intent to treat analysis | Infant examiner masked to fetal exposure | Miscellaneous |
|---|---|---|---|---|---|---|
| BEAM (16) |
|
|
|
Yes | Yes |
|
| ACTOMgS O4 (13) |
|
|
|
Yes | Yes |
|
| PREMAG (15,17) |
|
|
|
Yes | Yes |
|
| Magpie (14) |
|
|
|
Yes | Yes |
|
| MagNET (12) |
|
|
|
Yes | Yes |
|
GA=gestational age, USG=ultrasound, CP=cerebral palsy, T arm=tocolytic arm of MagNET trial.
Infant follow-up in the Magpie trial was limited to those patients randomized before delivery and data concerning the outcomes of infants whose mothers received magnesium sulfate antenatally at < 37, 34, and 30 weeks of gestation were obtained from the Cochrane review.(2) The authors of that review reported obtaining the data directly from the Magpie investigators. Although the outcome of severe CP was collected during the Magpie trial, data from the subsets by GA (<34 wks, <30 wks) were not reported. For the BEAM study, we obtained data regarding the outcomes at different GA at randomization directly from the BEAM trial investigators.
The individual studies’ outcomes and their definitions are summarized in table 3. Only 2 of 5 studies detailed the diagnostic criteria for CP (13,16). The BEAM trial used pre-specified criteria of gross motor delay, tone, movement and reflex abnormalities. CP severity was assessed using GMFCS criteria with the diagnosis of moderate-severe CP defined by a GMFCS score ≥2. In the ACTOMgSO4 study the criteria for CP included abnormalities of tone and loss of motor function, and the authors reported that its diagnosis was reflective of “usual clinical practice”. Substantial gross motor dysfunction was defined as “not walking independently” at 2 years, corresponding to a GMFCS score of 2–5. This is consistent with our definition of moderate-severe CP. The primary focus of the PREMAG study was on short term neonatal outcomes; however, long term neurologic morbidities and mortality were reported in the 2-year follow-up study of these infants. The authors did not report the severity of CP; however the data for substantial gross motor dysfunction are included in the recent Cochrane review,(2) and the definition fits our criteria of moderate-severe CP. The MagNET trial did not report the diagnostic criteria for CP or its severity. In the Magpie follow up study, severe CP was defined as “not walking or unlikely to walk unaided by 24 months”, which corresponds to a GMFCS score of 2–5 and is consistent with our definition of moderate-severe CP.
Table 3.
Primary and secondary neonatal outcomes of individual trials and definitions of selected outcomes.
| Primary and secondary neonatal outcome | Definitions of selected outcomes | |
|---|---|---|
| BEAM |
Primary: combined outcome of stillbirth or infant death by 1 year of age or moderate or severe CP at 2 yrs Secondary: neonatal complications, mild, moderate, and severe CP, stillbirth and infant death, Bayley Scales of Infant Development-II |
CP: presence of at least two out of the following three criteria: minimum 30% delay in gross motor developmental milestones; abnormalities in muscle tone, movement, or deep tendon reflexes; or persistence of primitive, or absence of protective reflexes |
| ACTOMgSO4 |
Primary: total pediatric mortality up to age 2, CP, and a combined outcome of death or CP at 2 yrs Secondary: IVH (grade III or IV), cystic PVL, neurosensory disability |
CP: tone abnormalities and loss of motor function |
| PREMAG |
Primary: fetal or neonatal mortality before hospital discharge, severe WMI, combined outcome of death or severe WMI Secondary: severe or moderate WMI, non-parenchymal hemorrhage, periventricular cavitary lesions and their extensions, neonatal complications, motor and cognitive development at 2 yrs |
Severe WMI: cystic PVL, periventricular parenchymal hemorrhage, or large single unilateral porencephalic cyst (caused by ischemic–hemorrhagic infarction) |
| Magpie |
Primary: combined outcome of death or neurosensory disability (blind, deaf, severe CP, or developmental quotient < -2 standard deviations) at 18 months Secondary: death, neurosensory disability, delayed speech, other disability |
Severe CP: not walking or unlikely to walk unaided at 24 months |
| MagNET | Primary: composite of “adverse pediatric health outcomes” (neonatal IVH, PVL, CP or death) by 18 months of age |
CP=cerebral palsy, IVH=intraventricular hemorrhage, PVL=periventricular leukomalacia, WMI=white matter injury
The magnesium regimen used and the actual dosage received varied between studies and between patients within individual studies (Table 4). The statistical analyses are summarized in table 5. There was no significant heterogeneity between the five trials. Begg’s test conducted for each of the outcomes did not reveal any publication bias (p values were between 0.19 and 0.90), and funnel plots appeared symmetrical.
Table 4.
Magnesium sulfate regimen variations between individual trials.
| Study | Magnesium Regimen | Actual amount received (in the magnesium group) |
|---|---|---|
| BEAM |
|
|
| ACTOMgSO4 |
|
|
| PREMAG |
|
|
| Magpie |
|
|
| MagNET | Preventive arm (P): 4 g bolus Tocolytic arm (T): 4 g bolus then 2–3 g/h |
Did not report |
Estimate – uncertain from published data, g=gram, h=hour, IV=intravenous, IM=intramuscular
Table 5.
Summary of Meta-Analysis Results of Individual Outcomes According To Gestational Age At Randomization And Indication For Treatment.
| GA (wks) | Outcome | No. studies | No. infants | No. infants exposed to Magnesium n (%) | No. infants exposed to placebo n (%) | RR | 95% CI |
|---|---|---|---|---|---|---|---|
| < 32–34 | Combined death or CP | 5 | 5225 | 2589 (49.6) | 2636 (50.4) | 0.92 | 0.83–1.03 |
| CP | 5 | 5225 | 2589 (49.6) | 2636 (50.4) | 0.70 | 0.55–0.89 | |
| Death | 5 | 5235 | 2594 (49.6) | 2641 (50.4) | 1.01 | 0.89–1.14 | |
| Moderate-severe CP | 3 | 4250 | 2096 (49.3) | 2154 (50.7) | 0.60 | 0.43–0.84 | |
| Death or moderate-severe CP | 3 | 4250 | 2096 (49.3) | 2154 (50.7) | 0.85 | 0.73–0.99 | |
| < 30 | Combined death or CP | 3 | 3107 | 1522 (49.0) | 1585 (51.0) | 0.91 | 0.81–1.03 |
| CP | 3 | 3107 | 1522 (49.0) | 1585 (51.0) | 0.69 | 0.52–0.92 | |
| Death | 3 | 3107 | 1522 (49.0) | 1585 (51.0) | 1.00 | 0.87–1.15 | |
| Moderate-severe CP | 2 | 2820 | 1378 (48.9) | 1442 (51.1) | 0.54 | 0.36–0.80 | |
| Death or moderate-severe CP | 2 | 2820 | 1378 (48.9) | 1442 (51.1) | 0.84 | 0.71–0.99 | |
| “Neuroprotection” trials only | |||||||
| Combined death or CP | 4 | 4314 | 2130 (49.4) | 2184 (50.6) | 0.86 | 0.75–0.99 | |
| CP | 4 | 4314 | 2130 (49.4) | 2184 (50.6) | 0.71 | 0.55–0.91 | |
| Death | 4 | 4324 | 2135 (49.4) | 2189 (50.6) | 0.95 | 0.80–1.13 | |
| Moderate-severe CP | 3 | 4250 | 2096 (49.3) | 2154 (50.7) | 0.60 | 0.43–0.84 | |
| Death or moderate-severe CP | 3 | 4250 | 2096 (49.3) | 2154 (50.7) | 0.85 | 0.73–0.99 | |
1- Randomization before 32–34 weeks
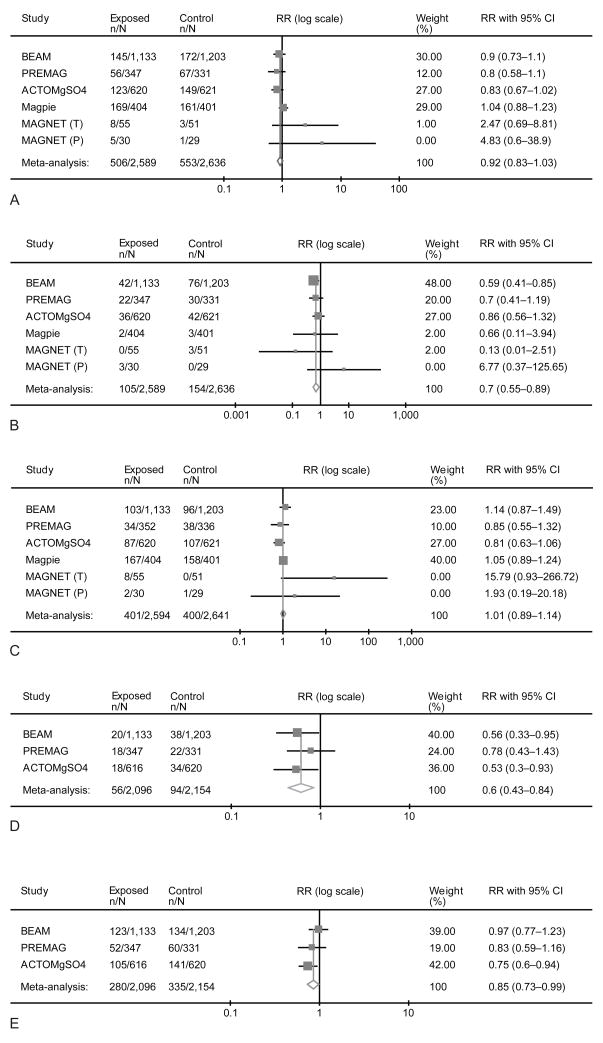
A total of 5235 fetuses/infants were included in the analysis of those whose mothers were randomized at less than 32–34 weeks of gestation (less than 34 weeks in the Magpie trial). We did not find a significant reduction in the primary outcome of death or CP (RR 0.92, 95%CI 0.83–1.03). However, there was no significant effect on death (RR 1.01, 95%CI 0.89–1.14) (Figure 1). Prenatal exposure to magnesium sulfate was associated with significant reductions in the combined outcome of death or moderate-severe CP (RR 0.85, 95%CI 0.73–0.99), CP of any severity (RR 0.70, 95%CI 0.55–0.89), and of moderate-severe CP alone (RR 0.60, 95%CI 0.43–0.84). In this subgroup, the number needed to treat (NNT) to prevent one case of CP among those infants who survive until age 18–24 months is 56 (95%CI 34–164).
Figure 1.
Summation of studies reporting the effect of antenatal exposure of magnesium sulfate at less than 32 to 34 weeks of gestation on death or cerebral palsy (A), cerebral palsy (B), death (C), moderate to severe cerebral palsy (D), and death or moderate-severe cerebral palsy (E). This is a Forest plot with the meta-analysis (fixed effects Mantel-Haenszel model) of the treatment/exposure effect expressed as pooled relative risk (RR) with its 95% confidence interval (CI).
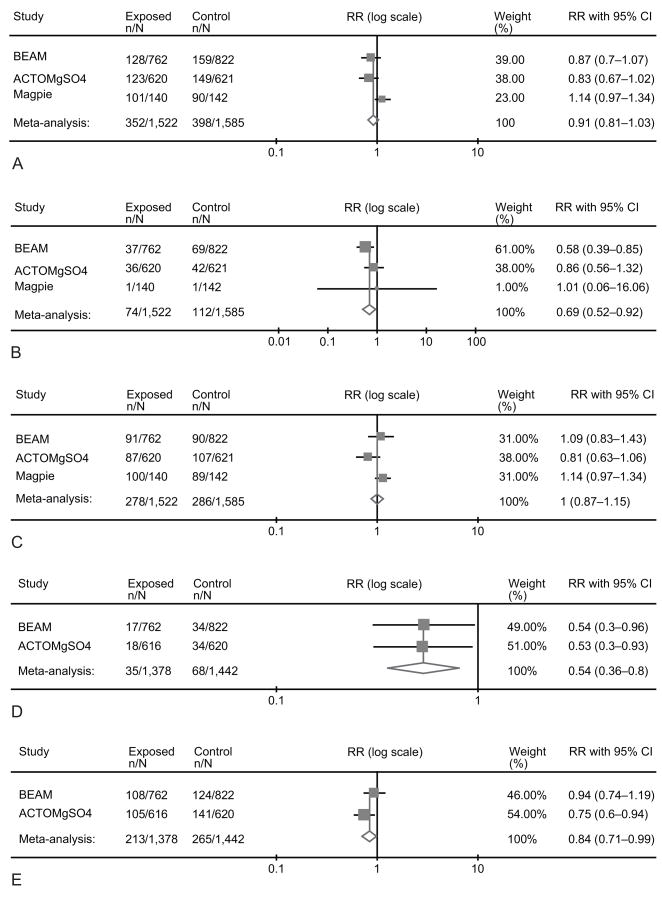
2- Randomization before 30 weeks
Data included a total of 3107 fetuses/infants from 3 trials. (13,14,16) Again we found no significant reduction in the primary outcome of death or CP (RR 0.91, 95%CI 0.81–1.03) or difference in the risk of death (RR 1.00, 95%CI 0.87–1.15) with magnesium exposure. (Figure 2) However, the combined outcome of death or moderate-severe CP was significantly reduced in the magnesium-allocated group (RR 0.84, 95%CI 0.71–0.99). Treatment in utero also significantly decreased the risks of CP of any severity (RR 0.69, 95%CI 0.52–0.92) and moderate-severe CP alone (RR 0.54, 95%CI 0.36–0.80). In this subgroup, the NNT to prevent one case of CP among those infants who survive until age 18–24 months is 46 (95%CI 26–187).
Figure 2.
Summation of randomized controlled trials reporting the effect of antenatal exposure of magnesium sulfate at less than 30 weeks on death or cerebral palsy (A), cerebral palsy (B), death (C), moderate-severe cerebral palsy (D), and death or moderate-severe cerebral palsy (E). This is a Forest plot with the meta-analysis (fixed effects Mantel-Haenszel model) of the treatment/exposure effect expressed as pooled relative risk (RR) with its 95% confidence interval (CI).
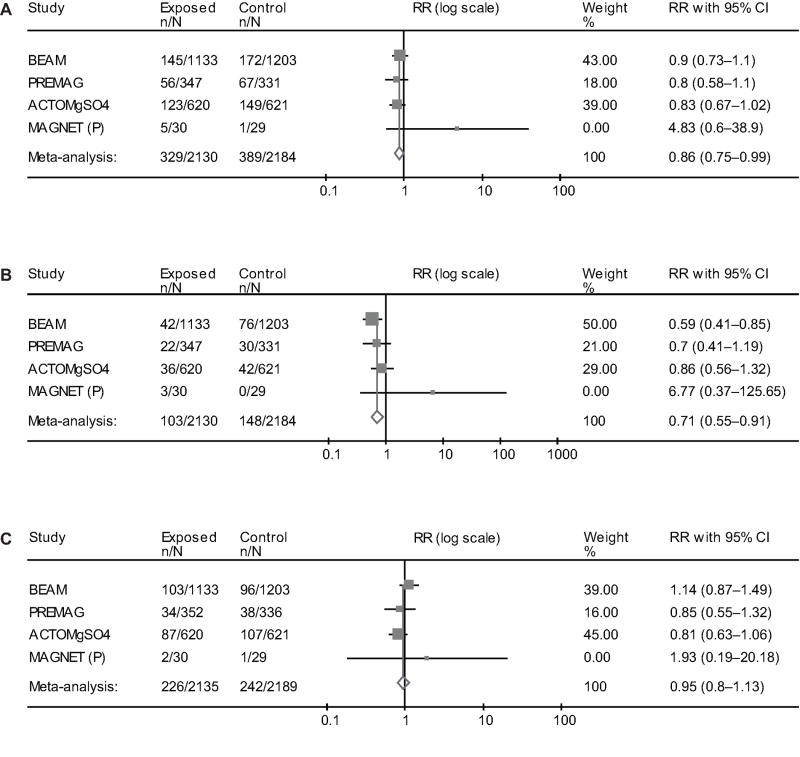
3- Neuroprotection studies only
Magnesium sulfate was used for fetal neuroprotection in 3 trials (13,15,16) in addition to MagNET (P). Its administration was associated with a reduction in the primary outcome of combined death or CP (RR 0.86, 95%CI 0.75–0.99), and also total CP (RR 0.71, 95%CI 0.55–0.91). There was no increase in the risk of death (RR 0.95, 95%CI 0.80–1.13) (Figure 3). The results for moderate-severe CP are identical to those reported in the ‘randomization before 32–34 weeks’ section above. In this subgroup the NNT to prevent one case of CP is 52 (95%CI 30–184). The majority of patients in this group were admitted/enrolled with a diagnosis of preterm labor (PTL) or preterm premature rupture of membranes (PPROM).
Figure 3.
Summation of randomized controlled trials designed for neuroprotection summarizing the effect of antenatal exposure of magnesium sulfate on cerebral palsy or death (A), cerebral palsy (B), and death (C). This is a Forest plot with the meta-analysis (fixed effects Mantel-Haenszel model) of the treatment/exposure effect expressed as pooled relative risk (RR) with its 95% confidence interval (CI).
COMMENT
In utero fetal exposure to magnesium sulfate given to women at risk for preterm delivery significantly reduced the risk of CP of any severity, and handicapping CP (moderate-severe disease) by 30 and 40–45% respectively, without increasing the risk of perinatal or infant death. These findings were consistent and robust throughout the analysis. Moreover; when magnesium sulfate was given solely for fetal neuroprotection, the primary composite outcome of perinatal/infant death or CP was significantly reduced in the magnesium allocated group.
The major strengths of this meta-analysis stem from its inclusion of 4 RCTs specifically designed to study the fetal neuroprotective effect from antenatal exposure to magnesium sulfate. Importantly, this review provides reassurance for obstetricians that antenatal exposure to magnesium sulfate did not increase the risk of perinatal/infant death in over 5235 prospectively evaluated fetuses. Additionally, it demonstrated beneficial effects of antenatal treatment for those recruited before 32–34 weeks as well as before 30 weeks. Thus, it does not appear that treatment should be restricted to the latter. The primary analysis of the Cochrane review (2) included patients randomized up to 37 weeks. However, only Magpie specifically provides outcomes up to this GA. Since the other 4 trials did not randomize anyone beyond 34 weeks, they should not be included in analyses beyond their upper end GA limit for randomization. The benefit of using magnesium sulfate beyond 32–34 weeks for fetal neuroprotection is unproven.
This review does have limitations. First, the magnesium sulfate regimen differed between trials (bolus only, bolus then maintenance for either 12–24 h, repeat treatment or not), and the actual dose received varied between patients within individual studies (Table 4). Moreover, although most of the studies included women with anticipated delivery within 24 hrs, not all patients delivered within that time frame. That raises the issue of the timing of magnesium sulfate infusion for fetal neuroprotection. Magnesium readily crosses the placenta, and can be detected in the fetal serum within 1 hour of maternal infusion, and in the amniotic fluid within 3 hours.(18) Thus the appropriate total dosage, infusion period, need for retreatment and therapeutic window for neuroprotection are not known. Second, there are differences in the patients’ characteristics between the evaluated studies. All patients in the Magpie trial were enrolled with a diagnosis of preeclampsia. However, in the other 4 trials PTL and PPROM were the main indications for admission/delivery. It is plausible that the impact of magnesium sulfate neuroprotection may be different according to the indication for preterm birth. Nevertheless, the studies which were dominated by patients with PPROM and PTL demonstrated beneficial neuroprotective effects of antenatal magnesium sulfate exposure. We were unable to adjust the variance estimates to take into account clustering of multi-fetal gestation, where the outcomes among siblings are not independent. In the two trials where some results were available both with and without adjustment for such clustering, there was little or no measureable difference between the estimated relative risks and variances between the two methods. These two trials, BEAM and ACTOMgSO4, account for at least 50% of the weight of the combined estimates, and therefore it is likely our results are robust.
Magnesium sulfate’s efficacy in prevention of preterm delivery has been questioned.(19) This meta-analysis does not address this issue as most of the patients included in the original studies were not candidates for tocolysis. In addition, neither the original studies, nor this meta-analysis address the combination of magnesium sulfate and other tocolytics. Combination tocolysis may be associated with serious maternal side effects. On the other hand, the number needed to treat (NNT) to prevent one episode of eclampsia is 400 for women with mild preeclampsia and 71 for women with severe disease.(20) Based on the results of this meta-analysis we calculate that 46–56 (95% confidence limits range from 26 to 187) would need to be exposed to magnesium sulfate in utero before 30 or 32–34 weeks of gestation, respectively, to prevent one case of CP. The NNT to prevent one case of CP appears justifiable, particularly given the relative safety of this treatment for the mother, its availability and the lack of evident risk regarding the infant mortality. A concern arises, however, for non-industrialized nations where resources and the availability of magnesium sulfate are frequently limited. In these countries, there is higher concern about the safety of magnesium sulfate use (medication errors, overdose, lack of nursing personnel for adequate supervision. . .etc). Therefore, applying the results from this meta-analysis in developing countries should be balanced with the availability, use of magnesium sulfate as an anticonvulsant in preeclampsia, and the individual safety practices.
CONCLUSION
The data presented here confirm the results from the largest trial (BEAM) that assessed the neuroprotective benefit of magnesium sulfate. Together, this large well designed randomized controlled trial and this meta-analysis provide strong evidence that in utero fetal exposure to magnesium sulfate in mothers at risk for premature delivery reduces the risk of developing cerebral palsy without affecting the rate of perinatal or infant death. Selecting the right patient candidate and identifying the ideal dosing regimen are still unclear and requires further research.
Acknowledgments
Financial Disclosure: The authors did not report any potential conflicts of interest.
The authors thank Dr. George Saade for his contributions to the design, assistance in data review, and manuscript preparation.
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Healthand Human Development (HD53907, HD36801, HD27869, HD34208, HD34116, HD40544, HD27915, HD34136, HD21414, HD27917, HD27860, HD40560, HD40545, HD40485, HD40500, HD27905, HD27861, HD34122, HD40512, HD34210, HD21410, HD19897) and the National Institute of Neurological Disorders and Stroke, Bethesda, MD.
References
- 1.Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birth weight infants? Pediatrics. 1995;95:263–9. [PubMed] [Google Scholar]
- 2.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858/CD004661.pub3. Art. No.: CD004661. [DOI] [PubMed] [Google Scholar]
- 3.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–54. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsson B, Hagberg G, Hagberg B, Ladfors L, Niklasson A, Hagberg H. Cerebral Palsy in preterm infants: a population-based case-control study of antenatal and intrapartal risk factors. Acta Paediatr. 2002;91:946–951. doi: 10.1080/080352502760148685. [DOI] [PubMed] [Google Scholar]
- 5.Clark SM, Ghulmiyyah LM, Hankins GDV. Antenatal antecedents and the impact of obstetric care in the etiology of cerebral palsy. Clin Obstet Gynecol. 2008;51:775–786. doi: 10.1097/GRF.0b013e3181870994. [DOI] [PubMed] [Google Scholar]
- 6.Honeycutt A, Dunlap L, Chen H, al Homsi G, Grosse S, Schendel D. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment. Center for Disease Control and Prevention, United States. MMWR. 2004;53:57–9. [PubMed] [Google Scholar]
- 7.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 8.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. MIX: comprehensive free software for meta-analysis of causal research data. Version 1.7. 2008 doi: 10.1186/1471-2288-6-50. http://mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed]
- 10.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 11.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 12.Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, Tomich PG. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–8. doi: 10.1067/mob.2002.123544. [DOI] [PubMed] [Google Scholar]
- 13.Crowther CA, Hiller JE, Doyle LW, Haslam RR Australasian Collaborative Trial of Magnesium Sulphate (ACTOMgSO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–76. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 14.Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG. 2007;114:289–99. doi: 10.1111/j.1471-0528.2006.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Leveque C, Hellot MF for PREMAG trial group. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial. BJOG. 2007;114:310–8. doi: 10.1111/j.1471-0528.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 16.Rouse DJ, Hirtz DG, Thom E, et al. A randomized trial of magnesium sulfate for the prevention of cerebral palsy. NEJM. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marret S, Marpeau L, Follet-Bouhamed C, Cambonie G, Astruc D, Delaporte B PREMAG trial group. Effect of magnesium sulphate on mortality and neurologic morbidity of the very-preterm newborn with two-year neurologic outcome: Results of the prospective PREMAG trial. Gynecol Obstet Fertil. 2008;36:278–88. doi: 10.1016/j.gyobfe.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Hallak M, Cotton DB. Transfer of maternally administered magnesium sulfate into the fetal compartment of the rat: assessment of amniotic fluid, blood, and brain concentrations. Am J Obstet Gynecol. 1993;169:427–431. doi: 10.1016/0002-9378(93)90101-n. [DOI] [PubMed] [Google Scholar]
- 19.Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. The Cochrane Database of Systematic Reviews. 2002;4:CD001060. doi: 10.1002/14651858.CD001060. [DOI] [PubMed] [Google Scholar]
- 20.Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: evidence from randomized trials. Clin Obstet Gynecol. 2005;48:478–488. doi: 10.1097/01.grf.0000160314.59736.d2. [DOI] [PubMed] [Google Scholar]