Abstract
Background
Treatment strategies targeting angiogenesis have revealed promising results in pre-clinical studies and early clinical trials in patients with glioblastomas.
Objective
This review evaluates the preclinical and clinical data for cediranib (AZD2171), a potent oral inhibitor of the vascular endothelial growth factor (VEGF) receptor tyrosine kinase.
Methods
We summarize both pre-clinical and clinical data for cediranib with a focus on the treatment of glioblastomas.
Results/conclusion
Cediranib is an effective drug in patients with glioblastoma acting through inhibition of angiogenesis and normalization of tumor vasculature. Reduction of vasogenic brain edema is a key component of its treatment effect in this patient population. The primary side effects of cediranib include fatigue, diarrhea and hypertension.
Keywords: AZD2171, Cediranib, malignant glioma, glioblastoma, adverse effects, angiogenesis, stem cells, invasion, brain tumors, cerebral edema
1 Introduction
Glioblastomas are among the most challenging solid tumors to treat. Despite advances in diagnostic imaging, surgical techniques, radiation therapy, and the development of new cytotoxic drugs, the survival rate for patients with malignant gliomas has improved only modestly in recent decades. Median overall survival and median progression free survival from time of diagnosis in glioblastoma patients treated with current standard therapy remain between 12–15 months and 6–7 months, respectively [1]. However, an improved understanding of tumor biology has identified a number of promising therapeutic targets. Tumor growth beyond ~1 mm is critically dependent on the formation of new blood vessels. This complex process, also known as angiogenesis, involves activation of endothelial and perivascular cells, tissue remodeling, and dynamic interaction of pro-angiogenic and anti-angiogenic factors. While angiogenesis plays a critical role during development, in adults this process occurs mainly at times of wound healing and during cyclical changes in the female reproductive tract. Given the pivotal role of angiogenesis in cancer biology [2], novel therapeutic strategies have increasingly aimed at targeting this process in cancer patients as a complement to standard chemotherapy and radiation [3]. Vascular endothelial growth factor (VEGF) is one of the most potent pro-angiogenic factors and inhibition of VEGF signaling either through neutralizing antibodies against VEGF, or tyrosine kinase inhibitors has shown promising results in the treatment of cancer patients. Among a number of novel targeted therapeutics evaluated in preclinical studies and recent clinical trials, the oral pan-VEGF receptor tyrosine kinase inhibitor cediranib has emerged as a promising agent with potent anti-angiogenic properties. This review will summarize available pre-clinical and clinical data regarding cediranib in cancer therapy with a special emphasis of its potential in patients with glioblastomas.
2 Angiogenesis, VEGF and gliomas
Among a wide range of factors contributing to angiogenesis, VEGF (vascular endothelial growth factor, formerly known as vascular permeability factor) and its associated signaling cascade are considered of fundamental importance for the biology of solid tumors, including glioblastomas [4,5]. VEGF signaling has been shown to influence numerous processes related to angiogenesis, including activation of endothelial cell precursors, endothelial cell migration and proliferation, modification of protease and integrin expression, capillary tube formation and vascular permeability. VEGF is a secreted dimeric glycoprotein with unique specificity for the vascular endothelium. The production of VEGF is under the influence of changes in oxygen and glucose status, and its expression may be significantly enhanced in solid tumors. VEGF production is driven by hypoxia via activation of hypoxia-inducible factor 1 (HIF-1) [6], a major regulator of tumor cell adaption, hypoxic stress, angiogenesis and tumor cell invasion [7–9].
Several VEGF family members and biologically active splice variants have been characterized, including VEGF-A, placental growth factor, VEGF-B, VEGF-C, VEGF-D, and VEGF-E. Among the splice variants, VEGF-A appears to be the most critical in the process of angiogenesis. VEGF and its family members act through receptor tyrosine kinases (RTK’s) located on endothelial cells. Ligand binding results in receptor dimerization, activation, and auto-phosphorylation of the tyrosine kinase domain. At least three high-affinity receptor subtypes have been identified: VEGFR-1/Flt-1, VEGFR-2/Flk-1/KDR, and VEGFR-3/Flt-4. Among these, activation of VEGFR-2/Flk-1/KDR has been shown to be the most significant stimulator of angiogenesis and vascular permeability [10,11].
Both endothelial and glioma cells may express or up-regulate VEGF and its receptors. Receptor up-regulation may result in both paracrine and autocrine activation, which drives endothelial cell proliferation, tumor cell invasion, migration and vascular permeability [4,12]. Considerable interest has emerged in targeting angiogenic signaling in gliomas in order to disrupt this vital component of tumor survival and progression. This effort has been further stimulated by studies demonstrating that angiogenic factors may stimulate cancer “stem cells”, a subpopulation of cancer cells thought to be critically important in cancer initiation, progression, and resistance to treatment (for review, e.g., [13]). Hence, anti-VEGF therapy has also been shown to target this critical tumor cell population [14].
Due to its dominant role in tumor angiogenesis, targeting VEGF signaling has evolved into a promising therapeutic strategy over the past few years. Bevacizumab, a humanized monoclonal antibody against VEGF-A, was among the first anti-angiogenic drugs to be approved and to become available in the treatment of cancer [15]. As reviewed elsewhere, subsequent studies in various solid tumors have shown that bevacizumab was associated with benefits in response rate and progression free survival when combined with other cytotoxic agents [16].
In patients with recurrent malignant gliomas, treatment with bevacizumab in combination with irinotecan (CPT-11), a cytotoxic topoisomerase I inhibitor, resulted in encouraging results and objective radiographic responses [17–19]. Subsequently, bevacizumab received FDA approval for monotherapy of patients with recurrent glioblastoma. The decision was based on the results of two phase-II clinical trials (AVF3708g and NCI 06-C-0064E) reporting favorable response after bevacizumab in this patient population with response rates between 20–25% and median duration of response of approximately 4 months [20].
In contrast to bevacizumab, which acts through VEGF sequestration, several small-molecule compounds have been developed that function as competitive inhibitors of VEGF receptors and receptor tyrosine kinases for other key angiogenic factors, such as the VEGF-related platelet-derived growth factor (PDGF) family (e.g., [3]). As the inhibition of a single growth factor pathway is not likely to result in complete inhibition of angiogenesis, multi-kinase inhibitors targeting several angiogenic growth factor pathways simultaneously, such as VEGF and PDGF, may yield greater clinical efficacy. The oral pan-VEGF receptor inhibitor cediranib is a small molecule receptor TKI with additional activity against c-kit. Cediranib is currently being tested in the glioblastoma patient population in phase-I, II and III clinical trials.
3 Pre-clinical characteristics and pharmacokinetic data of cediranib
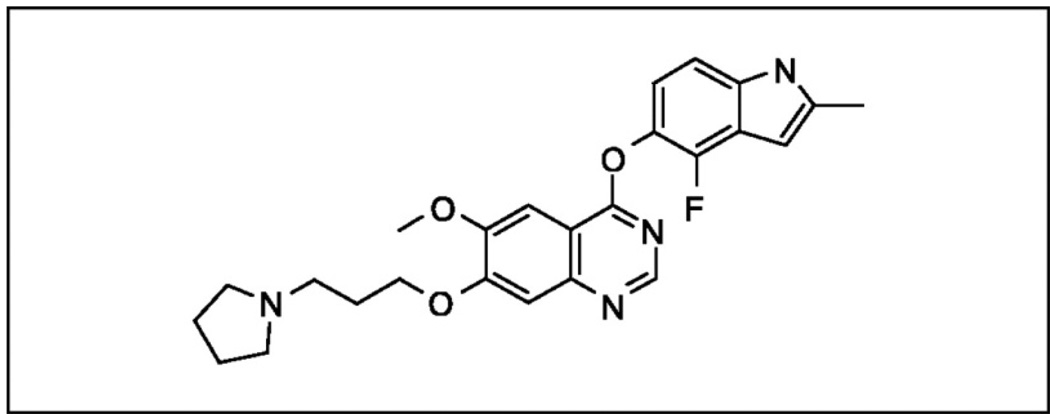
4-[(4-Fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-pyrrolidin-1-yl)propoxy]quinazoline (AZD2171; Cediranib) was developed by AstraZeneca (London, UK), and subsequent studies were performed in conjunction with the National Cancer Institute of the United States and the National Cancer Institute of Canada (Figure 1). It is an indole-ether quinazoline with a molecular weight of 450.51 and potent ATP-competitive inhibition of VEGF signaling by binding to the intracellular domain of all three VEGF receptor tyrosine kinases, but mainly through inhibition of the tyrosine kinase of VEGFR-2/Flk-1/KDR [21]. In addition, cediranib significantly inhibits tyrosine kinase activity for c-Kit, platelet derived growth factor receptor alpha and beta (PDGFR-α, PDGFR-β). IC50 values of recombinant receptor tyrosine kinase inhibition were reported at <0.001µM for VEGFR-2, <0.003µM for VEGFR-3, <0.002µM for c-Kit, <0.005µM for PDGFR-β, <0.036µM for PDGFR-α, and <0.026µM for FGFR-1 based on in vitro assays [21]. Drug concentrations in the sub-nanomolar range were able to effectively inhibit vessel growth and sprouting in co-culture systems of fibroblast and endothelial cells. Dose-dependent inhibition of VEGF-induced angiogenesis and tumor growth with once daily dosing of cediranib was demonstrated in a range of tumor xenograft mouse models, including colon, lung, prostate, breast and ovary [21]. Drug concentrations used in these studies ranged from 0.75mg/kg up to 6mg/kg, and statistical significant tumor growth inhibition was obtained with 1.5mg/kg/day in all tumor models. Significant reduction in tumor vessel density and vascular regression was notable within 52 hours of a once daily administration [21]. Subsequent studies in other human tumor xenografts were consistent with these findings and revealed potent cediranib-associated reduction in tumor microvessel density mediated via VEGFR-2 [22–26]. Further experimental studies in animal models revealed that cediranib may inhibit tumor progression not only through inhibition of VEGFR-2 mediated angiogenesis, but also by concomitantly inhibition of VEGFR-3 mediated lymphangiogenesis [27].
Figure 1.
The biochemical structure of AZD2171
4 Cediranib in clinical trials
Cediranib has shown promising results and encouraging anti-tumor activity in several phase-I clinical trials in patients with various solid tumors. In a phase-I study by Drevs et al., cediranib was administered to 83 patients with a broad range of advanced solid tumors and associated liver metastases [28]. As part of the initial phase of the study, 36 patients were treated with dose escalation ranging from 0.5–60mg. The peak plasma concentration after a single dose was reached after 1 to 8 hours, and the plasma half-life was reported at 22 hours, supporting the use of a once-daily oral dose. The drug was generally well tolerated up to an oral daily dose of 45mg. Similar findings regarding the pharmacokinetic profile of cediranib were reported in patients with hormone refractory prostate cancer, with an effective drug half-life of approximately 27 hours and a peak plasma concentration detected 2–8 hours after oral dosing [29] (Table 1).
Table 1.
Pharmacokinetic and pharmacodynamic parameters of cediranib
| Biological profile and main RTK’s targets | VEGFR-2/VEGFR-1/VEGFR-3>c-Kit>PDGFR [21] |
| Effective oral daily dose in cancer patients as monotherapy | 30mg or 45mg |
| Plasma half-life in patients | 22 hrs [12.5–35.4 hrs] |
| Peak plasma concentration after one single dose in patients | <8hrs |
| Main and typical side effects reported | Fatigue, diarrhea, nausea, dysphonia, hypertension |
In the phase-I study by Drevs et al., a total of 47 patients were subsequently analyzed at doses of either 20mg, 30mg or 45mg. Common side effects included fatigue (57%), diarrhea (47%), nausea (41%), dysphonia (36%) and hypertension (35%). Less frequent side effects included anorexia (29%), headache (22%), peripheral edema (12%) and palmar-plantar erythrodysesthesias (hand-foot-syndrome) (11%). The most common dose-limiting toxicity was hypertension seen at doses of 20mg and higher, observed in 7 patients. Grade-3 hypertension was reported in a total of 13 patients (16%) and Grade-4 hypertensive crisis in three patients (4%). Central nervous system toxicity was seen in one patient with cerebral hemorrhage and two patients with transient ischemic attack, each incident at a daily dose of 45mg. Among the 83 patients, partial responses were seen in one patient with prostate cancer and one patient with renal cancer, and stable disease was reported in 23 patients [28].
In an initial phase-I clinical trial conducted by the National Cancer Institute of Canada Clinical Trials Group, 20 patients with advanced non-small cell lung cancer (NSCLC, clinical stage IIIB/IV) and without prior chemotherapy, including patients with brain metastases, were treated with daily oral cediranib in combination with carboplatin and paclitaxel every three weeks [30]. Cediranib was given on day 2 of cycle 1 at a dose of either 30mg or 45mg. The drug was generally well tolerated at doses of 45mg or less with the main side effects consisting of fatigue (60%), anorexia (35%), hypertension (35%), diarrhea (30%), and mucositis (20%). Dose related changes in blood pressure were observed at doses of 20mg or higher. Of 15 patients evaluated for treatment response, partial responses were observed in 40% and stable disease in 53% of patients.
Based on these promising early results, other phase-II or phase-III clinical trials are currently being conducted in various other malignancies, including colorectal, breast, liver and ovarian cancer, melanoma, and mesothelioma. Taken together, experiences from most recent clinical trials revealed that cediranib has been generally well tolerated at doses of 45mg in monotherapy studies, with 30mg daily being better manageable over long-term, and at a dose of 20mg daily when combined with cytotoxic chemotherapy. The most common adverse events were fatigue, diarrhea, nausea, hoarseness, headache, and hypertension. Dose related hypertension was manageable and usually observed at cediranib doses of 20mg or above.
In patients with recurrent glioblastoma, encouraging results have come from a National Cancer Institute (NCI) sponsored phase-II study. In this study, cediranib was administered as a 45mg single daily dose to 31 patients with recurrent glioblastoma [31]. The regimen was associated with moderate toxicity, requiring temporary drug suspension in 69% of the initial 16 patients. Adverse effects included gastrointestinal toxicity, fatigue and hypertension. Using volumetric analysis of treatment response, decrease in tumor enhancement by more than 50% was notable in 9/16 patients (56%) and by 25–50% in 3/16 patients (19%). The median progression-free survival of the initial cohort was 111 days, comparing favorably to a historic database of a similar patient population treated with standard therapies [32]. Importantly, cediranib resulted in a significant reduction of tumor-associated vasogenic edema as measured by MRI techniques. This effect was paralleled by a potent steroid-sparing effect in most patients.
Advanced MRI studies using gradient echo, spin echo and contrast enhancement (T1+gadolinium) showed that decreased contrast enhancement was associated with reduction in blood vessel size, permeability, blood flow and blood volume, supporting the concept of “vascular normalization” of abnormal tumor blood vessels.
Relative tumor vessel size was significantly decreased as early as 1 day after initiation of cediranib, was more pronounced in larger microvessels (>10µM), and was maintained for at least 28 days. However, this effect was transient, and blood vessel size reversed towards abnormal values in most patients within 2 months of treatment, and after cessation of drug administration. Tumor vessels, however, were shown to re-normalize after resumption of cediranib in those patients on temporary “drug holidays”. Unlike the transient changes in vessel size, vascular permeability remained decreased for up to 112 days, suggesting that this feature of vascular normalization was more prolonged.
One of the most critical challenges in patients treated with anti-angiogenic therapies has been the identification and validation of molecular or biological markers of treatment response. In this regard, the phase-II study in recurrent glioblastoma cited above has provided novel insights in the use of surrogate markers of treatment response. Temporal changes in imaging findings were correlated with blood molecular and cellular biomarkers. Disease progression during ongoing treatment with cediranib correlated with increases in bFGF, SDF1-alpha and viable circulating endothelial cells (CEC). Tumor progression after drug cessation, in contrast, correlated with increase in the number of circulating progenitor cells (CPC), suggesting an independent role of CEC and CPC as biomarkers of treatment response in patients treated with cediranib.
The phenomenon of vascular normalization appears to be one of the critical features of anti-angiogenic therapies. Through selective pruning and maturation of unstable tumor blood vessels, anti-angiogenic agents have been previously shown to promote the formation of more stable and pericyte-coated smaller blood vessels, which may diminish tumor hypoxia and, consequently, improve the efficacy of concurrently administered radiation therapy and cytotoxic chemotherapy [33–36].
The observation that vascular normalization is a transient phenomenon, suggests that a specific treatment window might exist during which chemotherapy and radiation may be most effective. The mechanism responsible for the re-establishment of pathological vascularization is poorly understood, but may offer an explanation for treatment failure due to up-regulation of alternate pro-angiogenic factors, such as bFGF and SDF1-alpha [37].
These encouraging results of cediranib in patients with recurrent glioblastoma have initiated further investigations to validate these initial trial results, and to explore whether cediranib may have a role in first-line chemotherapy in patients with newly diagnosed glioblastoma (Table 2). Preliminary results from a phase-Ib trial using cediranib in combination with standard temozolomide and radiation therapy in patients with newly diagnosed glioblastoma demonstrated that doses of 20mg/day and 30mg/day were well tolerated in this combination and were largely consistent with prior safety and side effect profiles of cediranib [38]. A Phase III, randomized, multi-center clinical trial is currently ongoing comparing the efficacy of cediranib monotherapy versus a combination of cediranib and lomustine (CCNU) and versus CCNU monotherapy in patients with recurrent glioblastoma (REGAL study) (Table 2).
Table 2.
Ongoing clinical trials of cediranib in brain tumors
| Agents | Phase | Diagnosis | Sponsor | Primary Endpoint |
|---|---|---|---|---|
| Cediranib + CCNU | I/III | Recurrent GBM | AZ | MTD (phase I) and PFS (phase III) |
| Cediranib + TMZ + RT | Ib/II | New GBM | NCI | MTD (phase Ib) and PFS (phase II) |
| Cediranib + Cilengitide | Ib/II | Recurrent GBM | NCI | PFS |
| Cediranib | Ib/II | Brain Metastases from NSCLC | NCI | PFS |
CCNU: lomustine; GBM: glioblastoma; AZ: AstraZeneca; PFS: Progression free survival; TMZ: temozolomide; NCI: National Cancer Institute; MTD: maximum tolerated dose; NSCLC: non-small cell lung cancer;
5 Toxicity profiles and potential pitfalls of AZD2171
Cediranib and other anti-angiogenic agents are generally well tolerated in patients with glioblastomas and other solid tumors. Available toxicity data from clinical trials suggest unique patterns of adverse effects associated with this class of anti-cancer agents. The side effect profile includes hypertension, consistent with the physiological role of VEGF in regulating vasomotor tone and blood pressure [39,40]. Patients on anti-VEGF therapies need to be carefully monitored for hypertension, but can be successfully treated with conventional anti-hypertensive agents. Due to the physiological role of VEGF in wound healing and new blood vessel formation, most anti-VEGF/VEGFR agents are associated with a mildly increased risk of bleeding and wound dehiscence. Because of concerns regarding bleeding, prior intratumoral hemorrhage has been considered to be a relative contraindication to the use of cediranib or other anti-VEGF therapy. Other common systemic side effects of this class of drugs include fatigue, nausea, diarrhea, and weight loss.
Another concern has emerged from recent studies on the cell-biological basis of cancer therapy-associated neurotoxicity. Conventional cytotoxic agents target neural progenitor cells that are critically important in the maintenance of normal brain function and white matter integrity [41]. The physiological function of normal neural stem cells and progenitor cells is dependent on a number of factors, such as VEGF, FGF, EGF and PDGF, also employed by cancer stem cells. As much as there is the hope that anti-angiogenic therapies may also be beneficial in targeting cancer stem cells, disruption of those signaling pathways may possibly result in considerable adverse effects, such as cognitive dysfunction, in long-term survivors [42].
6 Assessment of treatment response and biomarkers
Current treatment response criteria in patients with malignant gliomas are based on magnetic resonance imaging (MRI) and are dependent on the degree of contrast enhancement [43]. In patients treated with anti-VEGF therapies, this parameter may be misleading, as the reduction in enhancement may simply be related to the decrease in vascular permeability rather than to an anti-tumor cell effect, complicating the interpretation of radiographic responses and progression with this class of agents. This phenomenon likely is the explanation why compelling radiographic responses in patients treated with anti-VEGF therapies have so far not been translated into prolongation of overall survival. Future trials will incorporate advanced imaging modalities, such as dynamic contrast-enhanced MRI, perfusion imaging, diffusion-weighted imaging, magnetic resonance spectroscopy, or positron emission tomography studies (PET), with novel biomarkers to appropriately evaluate treatment effect and anti-tumor response.
Importantly, there have been increasing concerns about the promotion of tumor infiltration, tumor cell migration and metastasis with anti-angiogenic therapies [44–48]. For example, Rubinstein et al. were able to demonstrate in an orthotopic rodent glioma model that anti-VEGF treatment not only delayed glioblastoma growth, but also resulted in increased tumor cell infiltration and cooption of the host vasculature [45].
Enhanced tumor cell invasion into surrounding brain parenchyma is poorly detected by conventional contrast-enhanced MRI and T2/FLAIR hyperintensities as the latter may reflect not only tumor infiltration but also vasogenic cerebral edema and peritumoral gliosis [49–52]. Other MRI-based techniques including diffusion-weighted imaging may prove more useful for the detection of diffuse tumor infiltration. Based on these observations, anti-angiogenic therapies may ultimately be less effective than initially thought and may need to be combined with other cytotoxic agents, in order to impact overall survival (Table 3).
Table 3.
Benefits and potential risks of cediranib in glioblastoma patients
| Benefits | Risks | ||
|---|---|---|---|
| • | Improved radiographic response rate and progression-free survival, when compared with current standard therapies | • | Cautious interpretation of treatment responses solely based on standard MRI |
| • | Normalization of tumor vasculature, thereby improvement of perfusion and drug delivery of conventional chemotherapeutic agents | • | Risk of increasing tumor cell invasion and migration |
| • | Sensitizing tumor endothelial cells to cytotoxic agents and radiation | • | Toxicity concerns – risk for intracranial hemorrhages, hypertension and thrombembolic complications |
| • | Potential of targeting cancer stem cells | • | Potential risk to develop complications secondary to neural progenitor cell toxicity |
| • | Anti-edema effects | • | Potential rebound edema when cediranib is discontinued |
7 Potent anti-edematous effect of Cediranib
Tumor-associated vasogenic cerebral edema is a direct consequence of the vascular abnormalities seen in patients with malignant gliomas and constitutes a significant cause of morbidity and mortality in this patient population [53].
The degree of vasogenic edema has been considered to be an important factor in treatment failure due to associated hypoxia and due to inadequate tumor penetration of chemotherapy agents secondary to increased interstitial tumor pressure. Conversely, sufficient control of vasogenic edema usually improves quality of life and neurological function in patients with malignant glioma. Corticosteroids are the most widely used agents to treat vasogenic edema, but their use is associated with serious short-term and long-term complications [54]. The mechanisms leading to increased vascular hyperpermeability and fluid leakage from the intravascular space into the brain parenchyma are, at least in part, dependent on the up-regulation and activation of the VEGF signaling pathway in glioblastoma [53]. Consequently, anti-angiogenic agents acting through VEGF blockade have been demonstrated to reduce vasogenic edema through vascular normalization in both preclinical and clinical studies [31,55].
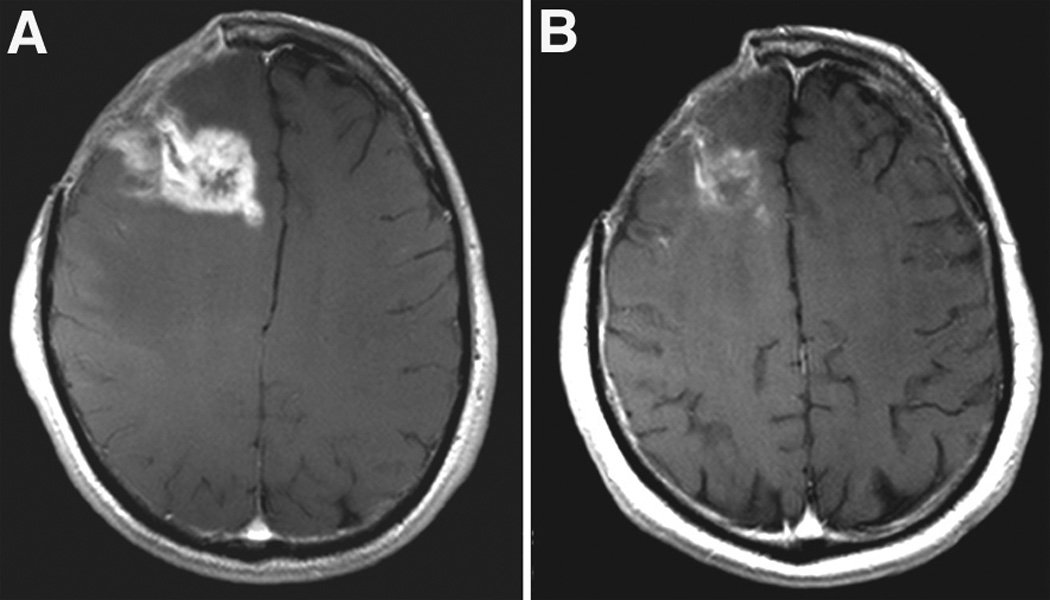
Cediranib has been shown to be associated with significant reduction of vasogenic edema and a steroid-sparing effect in the vast majority of glioblastoma patients [31]. In experimental studies, reduction of vasogenic edema by cediranib prolonged survival in a rodent glioblastoma model, without tumor growth delay [56]. Using intravital microscopy and MRI techniques in orthotopic models of glioblastoma, cediranib significantly decreased vascular permeability with associated reduction in vasogenic edema. Cediranib was able to increase survival despite persistent tumor growth. This study further suggests that anti-VEGF agents may be able to exert beneficial effects on progression-free and overall survival through edema control alone and even in the absence of significant tumor growth inhibition.
8 Expert Opinion and Conclusion
Cediranib has shown promising results in patients with recurrent glioblastoma. The drug is generally well tolerated with manageable and dose-dependent toxicity profile. Potent anti-edema and steroid-sparing effects have been observed in patients treated with cediranib. A temporary normalization effect on the tumor vasculature following therapy with cediranib suggests a unique opportunity for combination therapy with cytotoxic agents. Phase-II clinical trials are ongoing to determine whether cediranib may have a role in combination with radiation and temozolomide in patients with newly diagnosed glioblastoma. Development and validation of novel biomarkers is needed to monitor treatment and toxicity effects of anti-VEGF therapies. Ongoing and future studies of cediranib and other VEGF-targeting agents will determine whether there is a survival benefit and will further define the long-term toxicity profile in cancer patients.
Figure 2.
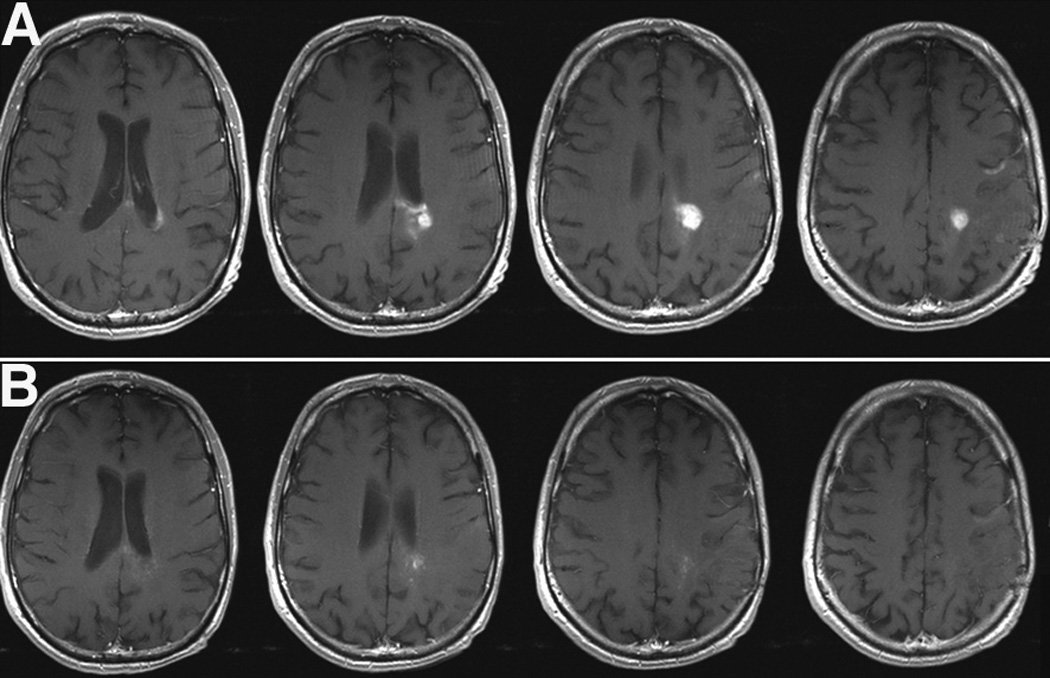
Figure 3.
Acknowledgement
This work has been supported by the NIH (R21CA117079, R01CA129371, KCA125440A to T.T.B), the Richard and Nancy Simches Endowment for Brain Tumor Research and the Montesi Family Fund.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;325(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich J, Norden AD, Wen PY. Emerging antiangiogenic treatments for gliomas - efficacy and safety issues. Curr Opin Neurol. 2008;21(6):736–744. doi: 10.1097/WCO.0b013e3283131370. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 6.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88(11):2606–2618. [PubMed] [Google Scholar]
- 8.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 Suppl 5:10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 9.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer M, Clauss M, Lepple-Wienhues A, et al. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18(2):363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276(5):3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 12.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367(6463):576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich J, Imitola J, Kesari S. Mechanisms of Disease: the role of stem cells in the biology and treatment of gliomas. Nat Clin Pract Oncol. 2008;5(7):393–404. doi: 10.1038/ncponc1132. [DOI] [PubMed] [Google Scholar]
- 14.Bao S, Wu Q, Sathornsumetee S, et al. Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66(16):7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 17.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 18.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 19.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 20.Cloughesy T, et al. American Society of Clinical Oncology (ASCO) 2008. Non-comparative Clinical Trial of Bevacizumab Alone or in Combination with CPT-11 Prolongs 6-Month Progression-free Survival in Recurrent, Treatment-Refractory Glioblastoma. 2010b (abstract) [Google Scholar]
- 21.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65(10):4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 22.Smith NR, James NH, Oakley I, et al. Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther. 2007;6(8):2198–2208. doi: 10.1158/1535-7163.MCT-07-0142. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Rivera F, Santillan-Gomez AA, Younes MN, et al. The tyrosine kinase inhibitor, AZD2171, inhibits vascular endothelial growth factor receptor signaling and growth of anaplastic thyroid cancer in an orthotopic nude mouse model. Clin Cancer Res. 2007;13(15 Pt 1):4519–4527. doi: 10.1158/1078-0432.CCR-06-2636. [DOI] [PubMed] [Google Scholar]
- 24.Takeda M, Arao T, Yokote H, et al. AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res. 2007;13(10):3051–3057. doi: 10.1158/1078-0432.CCR-06-2743. [DOI] [PubMed] [Google Scholar]
- 25.Goodlad RA, Ryan AJ, Wedge SR, et al. Inhibiting vascular endothelial growth factor receptor-2 signaling reduces tumor burden in the ApcMin/+ mouse model of early intestinal cancer. Carcinogenesis. 2006;27(10):2133–2139. doi: 10.1093/carcin/bgl113. [DOI] [PubMed] [Google Scholar]
- 26.Miller KD, Miller M, Mehrotra S, et al. A physiologic imaging pilot study of breast cancer treated with AZD2171. Clin Cancer Res. 2006;12(1):281–288. doi: 10.1158/1078-0432.CCR-05-0219. [DOI] [PubMed] [Google Scholar]
- 27.Heckman CA, Holopainen T, Wirzenius M, et al. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008;68(12):4754–4762. doi: 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- 28.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25(21):3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 29.Ryan CJ, Stadler WM, Roth B, et al. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25(5):445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 30.Laurie SA, Gauthier I, Arnold A, et al. Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced nonsmall-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2008;26(11):1871–1878. doi: 10.1200/JCO.2007.14.4741. [DOI] [PubMed] [Google Scholar]
- 31.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 33.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 35.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671–680. [PubMed] [Google Scholar]
- 36.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 37.Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57(18):3924–3928. [PubMed] [Google Scholar]
- 38.Chi AS, et al. A phase Ib trial of cediranib in addition to standard temozolomide and radiation therapy in patients with newly diagnosed glioblastoma; The 3rd Quadrennial Meeting of the World Federation of Neuro-Oncology and the 6th Meeting of the Asian Society for Neuro-Oncology; Japan: Yokohama; 2009. 10366 (abstract) [Google Scholar]
- 39.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42(18):3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 40.van Heeckeren WJ, Ortiz J, Cooney MM, Remick SC. Hypertension, proteinuria, and antagonism of vascular endothelial growth factor signaling: clinical toxicity, therapeutic target, or novel biomarker? J Clin Oncol. 2007;25(21):2993–2995. doi: 10.1200/JCO.2007.11.5113. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13(12):1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 44.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 45.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15(3):167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lassman AB, Iwamoto FM, Gutin PH, Abrey LE. Patterns of relapse and prognosis after bevacizumab (BEV) failure in recurrent glioblastoma (GBM) J Clin Oncol. 2008;26 doi: 10.1212/WNL.0b013e3181bc0184. 2028 (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuniga RM, Torcuator R, Doyle T, et al. Retrospective analyssis of patterns of recurrence seen on MRI in patients with recurrent glioblastoma multiforme treated with bevacizumab plus irinotecan. J Clin Oncol. 2008;26 13013 (abstract) [Google Scholar]
- 51.Narayana A, Raza S, Golfinos JG, et al. Bevacizumab therapy in recurrent high grade glioma: Impact on local control and survival. J Clin Oncol. 2008;26 doi: 10.3171/2008.4.17492. 13000 (abstract) [DOI] [PubMed] [Google Scholar]
- 52.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;71(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 53.Gerstner ER, Duda DG, di Tomaso E, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol. 2009;6(4):229–236. doi: 10.1038/nrclinonc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutin PH. Corticosteroid therapy in patients with cerebral tumors: benefits, mechanisms, problems, practicalities. Semin Oncol. 1975;2(1):49–56. [PubMed] [Google Scholar]
- 55.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 56.Kamoun WS, Ley CD, Farrar CT, et al. Edema Control by Cediranib, a Vascular Endothelial Growth Factor Receptor-Targeted Kinase Inhibitor, Prolongs Survival Despite Persistent Brain Tumor Growth in Mice. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]