Abstract
The proportion of cardiovascular deaths attributable to sudden cardiac death (SCD) is on the rise. Herein lies the rationale for developing risk stratification strategies to predict who will benefit from prophylactic ICD implantation. Current guidelines recommend prophylactic ICD therapy in patients with reduced left ventricular ejection fraction (LVEF). However, there are clear limitations in using LVEF alone to decide who should receive an ICD. There is mounting evidence that microvolt-level T wave alternans (TWA) is an important marker of arrhythmic risk. TWA is appealing because it non-invasively probes underlying electrophysiological substrate and has been linked to cellular mechanisms for arrhythmias. This review considers the clinical role of TWA for risk stratification of SCD.
Keywords: Repolarization, Electrophysiology, Tachycardia
In recent years cardiovascular death rates have fallen, yet the proportion of cardiovascular death that is attributable to sudden cardiac death (SCD) is on the rise. The most common etiology of SCD is the development of fatal ventricular arrhythmias resulting from complex structural and electrical remodeling that follow myocardial injury, most commonly secondary to coronary artery disease. Though extensively studied, factors responsible for initiating ventricular arrhythmias remain poorly understood. Moreover, ventricular tachyarrhythmia events (VTE) usually occur suddenly, without provocation and almost invariably result in death. Therefore, efforts aimed at predicting which patients are at highest risk for VTE, and therefore would benefit from primary prevention placement of an implantable cardioverter defibrillator (ICD), has emerged as the primary paradigm for addressing this major unresolved public health crisis.
Risk Prediction for Sudden Cardiac Death and Primary Prevention ICD Therapy
Current primary prevention guidelines recommend prophylactic ICD implantation in patients with reduced left ventricular ejection fraction (LVEF) <0.35 due to prior myocardial infarction or non-ischemic cardiomyopathy who are on optimal medical management. These recommendations are based on the fundamental relationship that exists between reduced LVEF and cardiovascular mortality and the findings of the II Multicenter Automated Defibrillator Implantation Trial (MADIT II) and SCD Heart Failure Trial (SCD-HeFT).2; 24 Both MADIT II and SCD-HeFT clearly showed prophylactic ICD therapy saves lives in patients with both ischemic or non-ischemic cardiomyopathy and reduced LVEF (i.e. <0.35). Yet, there are clear limitations to LVEF as the ideal risk-stratification test for deciding whether to implant an ICD for primary prevention of SCD.
Left ventricular ejection fraction is a direct measure of contractile dysfunction yet, only indirectly probes the underlying electrophysiological substrate and hence, inherently non-specific for predicting patients at risk for SCD. For LVEF to be the ideal risk stratification test guiding prophylactic ICD therapy, it should have a very high sensitivity and specificity and a predictive accuracy that remains stable over time.5 Interestingly, both the Maastricht prospective registry and European Autonomic Tone and Reflexes after Myocardial Infarction (ATRAMI) studies suggest that LVEF lacks adequate sensitivity for SCD prediction as both of these studies found that >50% of all SCD in these trials occurred in patients with a LVEF>0.35.17; 21
Determining the exact specificity of LVEF as a predictor of SCD risk is challenging because assigning a mechanism to a cause of death in clinical trials has limitations (i.e. are all sudden deaths arrhythmic?). However, the investigators of the Multicenter Unsustained Tachycardia Trial (MUSTT) provided evidence that LVEF has limited specificity when they showed no difference in the percentage of SCD in patients with LVEF <0.30 compared to 0.30 - 0.40.6 In the ATRAMI study, patients with LVEF <0.35 had no greater risk for SCD than patients with a LVEF >0.35. 21 Interestingly, when the ATRAMI investigators combined LVEF <0.35 with the presence of additional risk markers (i.e. hx. of non-sustained ventricular tachycardia or abnormal baroreflex function) patients were found to be at increased risk of SCD.
Given the low sensitivity and specificity of LVEF for predicting SCD risk, ICD therapy based solely on LVEF is inefficient (i.e. ∼15 ICDs need to be implanted to save 1 life). Also, as LVEF is a marker of contractile impairment and only indirectly assesses underlying electrophysiological substrate, it is not surprising that patients with markedly reduced LVEF may not benefit from prophylactic ICD therapy. The addition of invasive electrophysiological testing to directly investigate electrophysiological substrate can enhance the predictive accuracy of which patients with reduced LVEF will benefit from prophylactic ICD therapy. However, electrophysiological testing is invasive, expensive and not practical for broad application as a screening tool. Therefore, a clear need exists for the identification of non-invasive SCD risk markers that directly probe arrhythmic substrates to improve the accuracy of SCD prediction and enhance the efficiency of prophylactic ICD therapy.
Microvolt-level T wave alternans (TWA) has emerged as a promising non-invasive marker of risk for SCD.25; 29 TWA is appealing because it non-invasively probes underlying electrophysiological substrate and has been linked to cellular mechanisms for arrhythmias.26 In this review, we discuss the electrophysiological basis for TWA and how it can potentially provide prognostically useful information beyond LVEF.
T-wave Alternans Probes Electrophysiological Substrates of Arrhythmogenesis
Beat to beat variation of the amplitude or morphology of a component of the electrocardiogram (ECG) was first described as a marker of electrical instability in the heart more than 100 years ago. 18; 23 Nearly 80 years later, microvolt-level beat to beat variation of the amplitude of the T-wave was shown to be an independent marker of risk for SCD. Given the relationship between TWA and SCD, it is important to understand the cellular mechanisms responsible for TWA. Specifically, what cellular electrophysiological substrates does TWA assess? It is generally accepted that TWA arises from alternans of membrane repolarization (i.e. action potential duration) at the level of the single cardiac myocyte. This was demonstrated by Pastore et al.26 using high-resolution optical mapping in the guinea pig heart to show that with increasing pacing rate, TWA develops on the surface ECG in parallel with the development of action potential alternans at the level of the cardiomyocyte. Interestingly, the magnitude of cellular repolarization alternans is much larger than the corresponding TWA on the surface ECG, explaining why microvolt-level TWA can be physiologically and clinically meaningful. Therefore, at the cellular level, TWA directly probes dysregulation of repolarization.
It is important to understand that repolarization alternans is a normal rate-dependent property of cardiomyocytes in the healthy heart, developing at fast heart rates. However, in diseased hearts (i.e. heart failure), repolarization alternans develops in cardiomyocytes markedly slower heart rates. There are 2 major hypotheses proposed to explain repolarization alternans in the cardiac myocyte: (1) the action potential duration (APD) restitution hypothesis, (2) the calcium cycling hypothesis. Both of these hypotheses support rate dependence for the development of repolarization alternans. Action potential restitution is a normal phenomenon of APD and describes the relationship between the APD of one beat and the diastolic interval (DI) of the preceding beat. Restitution can explain the development of repolarization alternans because a short DI is necessarily followed by a short APD that will increase DI of the next beat leading to a long APD. This cycle will continue indefinitely if the slope of the dynamic APD restitution curve (APD versus DI) is greater than one. Though theoretical models support APD restitution as a primary mechanism underlying repolarization alternans, experimental studies and clinical observations have not confirmed this hypothesis. For example, repolarization alternans has been demonstrated in multiple clinical settings in which the APD restitution curve is flat (i.e. myocardial ischemia).
There is considerable evidence supporting a primary role of alternans of intracellular calcium cycling (i.e. calcium transient alternans) in the development of repolarization alternans. For example, blockade of the ryanodine receptor (RyR) or depletion of sarcoplasmic reticulum (SR) calcium with caffeine suppresses repolarization alternans.19; 30 Alternatively, slowing of SR calcium uptake increases the magnitude of both Ca2+ alternans and repolarization alternans.22; 33 Importantly, Chudin et al. clearly demonstrated that Ca2+ alternans can occur in the absence of repolarization alternans, suggesting that repolarization alternans arises from disturbances of SR Ca2+ cycling.9
What are the subcellular mechanisms underlying alternans of SR Ca2+ cycling and therefore, repolarization alternans? It is postulated that Ca2+ alternans develops anytime heart rate exceeds the capacity of the cardiac myocyte to cycle calcium on a beat to beat basis. Thus, it is predicted that any sustained perturbation to SR Ca2+ cycling will be sufficient to produce Ca2+ alternans. This is supported by the observation that the molecular profile of Ca2+ cycling proteins is markedly different in cardiac myocytes that are prone to alternans when compared to myocytes that are resistant to alternans.33 In particular, both the primary SR Ca2+ reuptake protein, sarcoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA2a) and the primary release protein, RyR are decreased in myocytes that are prone to the development of cardiac alternans. For example, in myocytes with decreased SERCA2a (i.e. decreased SR Ca2+ reuptake capacity), as seen in heart failure, the capacity for SR Ca2+ reuptake is overwhelmed at a lower heart rate than under normal SR Ca2+ reuptake conditions. This hypothesis was recently confirmed when it was shown that SERCA2a overexpression can suppress both Ca2+ and repolarization alternans.14; 34 In addition to altered content of SR Ca2+ handling proteins, abnormal function of these proteins has been proposed to be an important mechanism in the development of Ca2+ alternans. For instance, with increasing heart rate, instability of SR Ca2+ release dynamics can develop without beat to beat variation in SR Ca2+ content, producing Ca2+ alternans.27 It is postulated that the mechanism underlying this phenomenon is secondary to variations in the ability of the RyR to recover from inactivation on a beat by beat basis. Finally, TWA can occur at slow HRs under conditions such as long-QT syndrome. Though the mechanisms underlying the cardiac alternans at slow HRs are unknown, they are likely related to primary alterations of sarcolemmal ionic currents. In summary, TWA provides insight into cellular dysregulation of repolarization, calcium handling and abnormalities of sarcolemmal ionic currents.
Cardiac Alternans a Common Pathway to Arrhythmogenesis
A clear benefit of TWA testing is its ability to non-invasively probe underlying electrophysiological substrate (i.e. repolarization, myocardial calcium cycling and sarcolemmal ionic currents) yet, is the electrophysiological substrate that TWA probes arrhythmogenic? Recently, repolarization alternans was shown to be a mechanism underlying the genesis of ventricular arrhythmias.26 In particular, in an experimental model of TWA, ventricular fibrillation (VF) always precedes the development of discordant repolarization alternans (i.e. alternans occurring with opposite phase between neighboring myocytes). Normally, repolarization alternans develops in a spatially concordant fashion such that all myocytes alternate in the same phase (i.e. long-short-long). Concordant alternans is a marker of changes in the electrophysiological substrate of the heart, yet, it is not particularly arrhythmogenic. However, above a critical heart rate or following an ectopic beat, the phase of repolarization alternans can shift in some cells, producing spatially discordant alternans (i.e. long-short vs. short-long). The onset of discordant alternans markedly amplifies preexisting heterogeneities of repolarization, producing a substrate that is prone to conduction block and in the structurally normal hearts the development of ventricular fibrillation. In contrast, in the structurally abnormal heart (i.e. scar) or under conditions of prolonged QT interval, discordant alternans produces monomorphic ventricular tachycardia or torsades de pointe, respectively.13
Spatially discordant repolarization alternans clearly produces a substrate that is prone to conduction block and therefore creates a common pathway for development of variety of ventricular arrhythmias. However, how discordant repolarization alternans is reflected on the surface ECG remains unclear. It is likely that identifying how discordant repolarization alternans is reflected on the surface ECG could improve the ability of TWA testing to identify patients has risk for SCD.
Methods for Measuring T-wave Alternans in Patients
The most widely applied method for non-invasive measurement of TWA uses the spectral method during controlled heart rate elevation (100 - 110 bpm). This method was developed during the 1980’s as a way to detect and quantify microscopic alternation in the T-wave that is not visible on the surface ECG (i.e., microvolt-level alternans), making it possible to identify and isolate beat-to-beat T-wave fluctuations that repeat every other beat (i.e., alternans) from much larger non-alternating T-wave fluctuations. 29 In particular, measurement of TWA with the spectral method is most commonly performed during graded exercise using specialized noise reducing ECG electrodes. The clinical utility of TWA was first established as a marker of arrhythmic risk using the spectral method, when it was shown that TWA develops at a slower HR in patients at greatest risk for SCD when compared to patients at low risk of SCD.29 In particular, the development of TWA at HR <110 bpm (i.e. positive TWA) as measured by the spectral method is a specific measure of risk for SCD whereas the absence of TWA at HR <110 bpm (i.e. negative TWA) seems to indicate a resistance to SCD in a relevant primary prevention population.4
Recently, a time-domain measurement technique referred to as the modified moving average (MMA) method has been proposed as an alternative to the spectral method for measuring TWA. 12; 32 The scientific and clinical rationale for MMA have not been established, but it is available on commercially available Holter and stress test systems. This method essentially averages the T-wave amplitude and morphology of odd and even beats over a 15-second period and superimposes the odd and even averages to calculate differences in amplitude. This process repeats itself every 15 seconds, creating a modified moving average of TWA.32 Early observation studies have shown that TWA measured using the MMA method may be predictive of cardiovascular mortality yet, it is less clear if it predicts a SCD phenotype.12; 31 Moreover, there has not been rigorous standardization of the definition of an abnormal test, making it difficult to know exactly how to apply TWA measurements made using the MMA method. For example, currents trial using the MMA method for measuring TWA have used a wide range (5 μV - 65 μV) of cut-points to identify a positive TWA test.1 Future investigation will need to prospectively evaluate a standardized definition of an abnormal TWA using the MMA method.
T-wave Alternans as a Clinical Marker of Arrhythmic Risk in Humans
Initially, most studies linking TWA to SCD were performed in high risk patients with a history of myocardial infarction and reduced LVEF. However, several recent observational studies have established TWA as a marker of SCD risk and shown that TWA can improve the efficiency of prophylactic ICD therapy in relevant primary prevention populations3; 8; 11; 15, while others have not.7 Bloomfield et al. demonstrated that using TWA to further risk stratify a MADIT II like population decreased the number needed to treat from eighteen to seven.4 Additionally, in the recently published Alternans Before Cardioverter Defibrillator (ABCD) Trial showed that a TWA directed risk stratification strategy improved the efficiency of prophylactic ICD therapy such that only eleven ICDs needed to be implanted to save 1 life.11
In contrast, the Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients (MASTER) trial failed to demonstrate an increase in ICD-detected VTEs in patients with a non-negative TWA test when compared to patients with a negative test.7 Interestingly, a non-negative TWA test did predict an increase in total mortality. It is not clear why a non-negative TWA test in the MASTER trial was predictive of total mortality but not ICD-detected VTE but may highlight the limitation of ICD-detected VTEs as a surrogate end-point for SCD. For example, Hohnloser et al. recently demonstrated that ICD-detected VTE is an unreliable surrogate end-point for SCD (Figure 1).20 They identified prospective clinical trials evaluating TWA measured using the spectral analytic method in primary prevention populations and analyzed studies in which (1) few patients had implanted ICDs and as a result none or a small fraction (≤ 15%) of the reported endpoint VTEs were ICD-detected VTEs (Low ICD Group) or (2) many of the patients had implanted ICDs and the majority of the reported endpoint VTEs were ICD-detected VTEs (High ICD Group). Interestingly, in the Low ICD Group (n=3,682), the hazard ratio for SCD associated with a non-negative vs negative TWA test was 13.6 compared to 1.6 in the High ICD Group (n=2,234).
Figure 1.
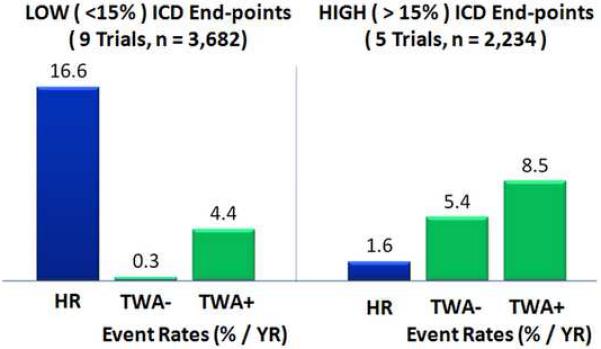
Prediction of events depends on definition of “events”. Comparison of prospective clinical trials evaluating T-wave alternans (TWA) measured using the spectral analytic method in primary prevention populations in which (1) few patients had implantable cardioverter defibrillators (ICD) and as a result none or a small fraction (≤ 15%) of the reported endpoint ventricular tachyarrhythmic events (VTEs) were ICD-detected (Low ICD Group) or (2) many of the patients had implanted ICDs and the majority of the reported endpoint VTEs were ICD-detected (High ICD Group). In the Low ICD Group comprising 3,682 patients, the hazard ratio associated with a TWA + vs TWA - was 13.6 (8.5 - 30.4) and the annual event rate (AER) among the TWA - patients was 0.3% (0.1% - 0.5%). In contrast, in the High ICD Group comprising 2,234 patients, the hazard ratio was only 1.6 (1.2 - 2.1) and the AER among the TWA - patients was elevated to 5.4% (4.1% - 6.7%). Adapted from Hohnloser et al.20
Recently, the TWA substudy of SCD-HeFT, which included 19% of the original study population (mean follow-up was 30 months), suggested that TWA was not predictive of VTEs or mortality.16 However, this study is unique in that it allowed for a comparison of the onset of the “TWA signal” with the onset of the “SCD-phenotype” in the same primary prevention population. In particular, as demonstrated in figure 2, mortality rates between TWA non-negative and TWA negative patients only begin to differ after 14 months.28 Interestingly, this is precisely the same time point that the mortality benefit of ICDs occurred, suggesting the onset of the “SCD-phenotype” in the total SCD-HeFT population. This finding provides clinical evidence to support the hypothesis that TWA probes underlying cellular and molecular electrophysiological substrates that are important in producing a “SCD-phenotype”.
Figure 2.
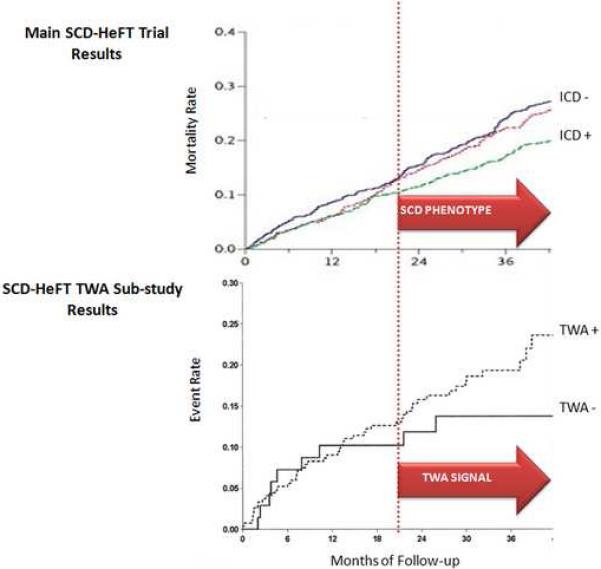
T-wave alternans (TWA) is a marker of the sudden cardiac death phenotype. Upper Panel: Mortality rates from the main SCD Heart Failure Trial (SCD-HeFT). This plot shows that the beneficial effect of ICD therapy in SCD-HeFT begins to emerge between 18 and 24 months (dashed line). Lower Panel: Event rates from the TWA substudy of SCD-HeFT. The time base is scaled to match the survival curves from the main SCD-HeFT trial (upper), permitting comparison of the time course of an apparent prognostic TWA signal (i.e. separation of TWA + and TWA - curves) and the emergence of a detectable SCD phenotype in the same population. These data show that the TWA begins to develop at the same time as the SCD phenotype becomes detectable.28
Role of T Wave Alternans in Risk Stratification for Sudden Cardiac Death
Clearly, the substrates underlying SCD are complex and dynamic. Therefore, it is unlikely that a single test will be adequate to identify patients at highest risk for SCD and who would therefore benefit from prophylactic ICD therapy. Therefore, the optimal risk stratification model for identifying patients at highest risk for SCD will incorporate multiple risk markers. The benefit of risk stratification strategy that combines multiple risk markers is emphasized in figure 3. This figure demonstrates that based on recent primary and secondary prevention trials, patients achieve the greatest benefit from ICD therapy for primary prevention guided by a combined risk stratification strategy (i.e. LVEF + invasive EP testing), even better than secondary prevention. In other words, you are more likely to benefit from an ICD if you have never had an arrhythmia but have positive risk markers, than if you have had a cardiac arrest. Moreover, there appears to be a potential “sweet spot” for identifying patients who are most likely to benefit from prophylactic ICD therapy. Does TWA have a role in identifying this “sweet spot”?
Figure 3.
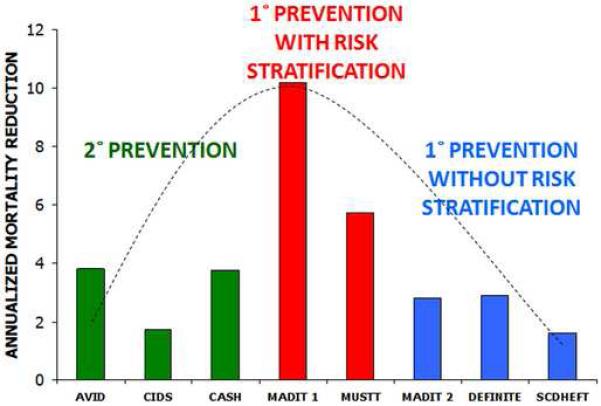
Implantable cardioverter defibrillators (ICD) do not benefit all patients with low left ventricular ejection fraction: Hitting the “sweet spot”. Based on recent primary and secondary prevention trials, patients achieve the greatest benefit from ICD therapy for primary prevention guided by a combined risk stratification strategy (i.e. LVEF + invasive EP testing), even better than secondary prevention. In other words, you are more likely to benefit from an ICD if you have never had an arrhythmia but have a positive risk marker, than if you have had a cardiac arrest.
Since the ABCD trial systematically risk stratified all patients by both EPS and TWA testing, it provides us with a unique opportunity to evaluate different risk stratification strategies (i.e. LVEF, TWA and EPS) for the primary prevention.10 Figure 4 demonstrates analysis of data from the ABCD trial testing various risk stratification scenarios asking how a patient would do with prophylactic ICD therapy in terms of the tradeoff between therapeutic efficiency (i.e. ICD treated patients without an event) and therapeutic risk (i.e. patients who did not receive an ICD, yet had an event). This analysis addresses the question of how risk stratification using combinations of LVEF, TWA, and EP testing can potentially affect the efficiency of primary prevention with ICDs. In particular, if a reduced LVEF alone is the only marker used to guide prophylactic ICD implantation 93% of patients receiving an ICD will never use their device. In contrast, the addition of other risk markers markedly improves therapeutic efficiency (i.e. over-treatment decreases) with a much smaller increase in the risk of under-treatment. Specifically, the addition of TWA reduced the number of ICD treated patients without an event to 65% with only a 1.8% risk that a patient with a VTE is not treated. Moreover, addition of EPS in all patients, a strategy which has been largely abandoned by clinicians, decreases the number of ICD recipients without events to 35%, but increases the risk of having a VTE and not being protected to 2.7%. Taken together, risk stratification strategies using multiple risk markers improve therapeutic efficiency 25 times more than it increases risk of under-treatment, as evidenced by the steep slot of the plot shown in figure 4. These data support a role for TWA testing as a component of a risk stratification strategy aimed at identifying the aforementioned “sweet spot” in primary prevention patients. It is important to emphasize that the aforementioned analysis only accounts for 1 year event rates. Also, the apparent “risk” of not treating patients with ICDs was probably over-estimated because ICD-related events grossly over-estimate clinical events as “appropriate” ICD shocks are not tantamount to a life saved.
Figure 4.

Delivering implantable cardioverter defibrillator (ICD) therapy to those who need it. This figure demonstrates analysis of data from the ABCD trial testing various risk stratification scenarios asking how a patient would do with prophylactic ICD therapy in terms of the tradeoff between therapeutic efficiency (i.e. ICD treated patients without an event) and therapeutic risk (i.e. patients who did not receive an ICD, yet had an event). If a reduced LVEF alone is the only marker used to guide prophylactic ICD implantation 93% of patients receiving an ICD will never use their device. In contrast, the addition of TWA reduced the number of ICD treated patients without an event to 65% with only a 1.8% risk that a patient with a VTE is not treated. Addition of EPS in all patients, a strategy which has been largely abandoned by clinicians, decreases the number of ICD recipients without events to 35%, but increases the risk of having a VTE and not being protected to 2.7%. Taken together, risk stratification strategies using multiple risk markers improve therapeutic efficiency 25 times more than it increases risk of under treatment, as evidenced by the steep slot of the plot.
A major unresolved question is what the acceptable level of therapeutic efficiency is, and what is the acceptable level of under-treatment? The former is a societal question, while the latter is a medical question. To that point, to address the appropriate level of under-treatment, one must also consider competitive risk of morbidity and mortality from nonarrhythmic causes (Figure 4, blue arrow). For example, though an individual patient may meet criteria for prophylactic ICD therapy their risk of mortality from progressive heart failure or non-cardiovascular causes may offset any mortality benefit obtained from implanting an ICD. Therefore, the final decision of whether to implant an ICD for primary prevention must include an individualized assessment of competitive risk.
In conclusion, there are clear advantages to TWA as a risk stratification tool for identifying patients for prophylactic ICD therapy. (1) TWA measured on the surface ECG is linked to an arrhythmia mechanism arising from abnormal intracellular calcium cycling. (2) Patients with reduced LVEF who have a negative TWA test are at considerably lower risk for SCD. (3) In contrast to LVEF that only indirectly probes electrophysiological substrate, TWA probes underlying electrophysiological substrates and has been linked to cellular mechanisms of arrhythmogenesis. (4) Unlike many other risk markers, TWA appears to track arrhythmia susceptibility independent of heart failure progression and is comparably predictive in patients with both ischemic and nonischemic cardiomyopathy. (5) TWA can guide selection of appropriate patients for ICD therapy, particularly when competitive mortality risks are present or patients are reluctant to receive ICD therapy. Going forward, randomized clinical trials are required that use risk stratification strategies, such as TWA to determine therapies such as ICDs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amit G, Costantini O, Rosenbaum DS. Can we alternate between T-wave alternans testing methods? Heart Rhythm. 2009;6:338–40. doi: 10.1016/j.hrthm.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield DM, Bigger JT, Steinman RC, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–9. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter-defibrillator? Not everyone with an ejection fraction < or = 30% should receive an implantable cardioverter-defibrillator. Circulation. 2005;111:2537–49. doi: 10.1161/01.CIR.0000165057.88551.2C. discussion 2537-49. [DOI] [PubMed] [Google Scholar]
- 6.Buxton AE, Lee KL, Hafley GE, et al. MUSTT Investigators Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease - An analysis of patients enrolled in the Multicenter Unsustained Tachycardia Trial. Circulation. 2002;106:2466–2472. doi: 10.1161/01.cir.0000037224.15873.83. [DOI] [PubMed] [Google Scholar]
- 7.Chow T, Kereiakes DJ, Onufer J, Woelfel A, et al. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52:1607–15. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Chow T, Keriakes D, Bartone C, et al. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:1820–7. doi: 10.1016/j.jacc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 9.Chudin E, Goldhaber JI, Weiss J, Kogan B. Intracellular Ca2 dynamics and the stability of ventricular tachycardia. Biophysical Journal. 77:2930–2941. 99. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53:471–9. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 11.Costantini O, Hohnloser SH, Kirk MM, et al. The Alternans Before Cardioverter Defibrillator (ABCD) Trial: Strategies Using T-Wave Alternans to Improve Efficiency of Sudden Cardiac Death Prevention. Journal of the American College of Cardiology. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 12.Cox V, Patel M, Kim J, Liu T, Sivaraman G, Narayan SM. Predicting arrhythmia-free survival using spectral and modified-moving average analyses of T-wave alternans. Pacing Clin Electrophysiol. 2007;30:352–8. doi: 10.1111/j.1540-8159.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 13.Cutler MJ, Rosenbaum DS. Explaining the clinical manifestations of T wave alternans in patients at risk for sudden cardiac death. Heart Rhythm. 2009;6:S22–8. doi: 10.1016/j.hrthm.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler MJ, Wan X, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene overexpression identifies mechanism and potential therapy for arrhythmogenic cardiac alternans. Circulation. 2007;116:II–217. doi: 10.1161/CIRCEP.109.863118. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007;50:2275–84. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Ip JH, Costantini O, Poole JE, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118:2022–8. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–9. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 18.Herring H. Experimentelle Studien an Saugetieren uber das Electrocardiogramm. Ztchr.fd ges exper Med. 1909;7:363. [Google Scholar]
- 19.Hirata Y, Kodama I, Iwamura N, Shimizu T, Toyama J, Yamada K. Effects of verapamil on canine Purkinje fibers and ventricular muscle fibers with particular reference to the alternation of action potential duration after a sudden increase in driving rate. Cardiovasc.Res. 1979;13:1–8. doi: 10.1093/cvr/13.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T-wave alternans. Heart Rhythm. 2009;6:S36–44. doi: 10.1016/j.hrthm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 21.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ, ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 22.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–75. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 23.Lewis T. Notes upon alternation of the heart. Quart.J.Med. 1910;4:141–144. [Google Scholar]
- 24.Moss AJ, Zareba W, Hall WJ, Klein H, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 25.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–81. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 26.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 27.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–8. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum DS. T-wave alternans in the sudden cardiac death in heart failure trial population: signal or noise? Circulation. 2008;118:2015–8. doi: 10.1161/CIRCULATIONAHA.108.818286. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N.Engl.J.Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh H, Bailey J, Surawicz B. Action potential duration alternans in dog purkinje and ventricular muscle fibers. Circulation. 1989;80:1421–1431. doi: 10.1161/01.cir.80.5.1421. [DOI] [PubMed] [Google Scholar]
- 31.Sakaki K, Ikeda T, Miwa Y, et al. Time-domain T-wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: a prospective study. Heart Rhythm. 2009;6:332–7. doi: 10.1016/j.hrthm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Verrier RL, Nearing BD, La Rovere, et al. ATRAMI Investigators Ambulatory electrocardiogram-based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J.Cardiovasc.Electrophysiol. 2003;14:705–711. doi: 10.1046/j.1540-8167.2003.03118.x. [DOI] [PubMed] [Google Scholar]
- 33.Wan X, Laurita KR, Pruvot E, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Xie LH, Sato D, Garfinkel A, Qu Z, Weiss JN. Intracellular Ca alternans: coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–10. doi: 10.1529/biophysj.108.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]


