Abstract
Background
Recent reports suggest that off-label use of drug-eluting stents is associated with an increased incidence of adverse events. Whether the use of bare-metal stents would yield different results is unknown.
Methods
We analyzed data from 6551 patients in the National Heart, Lung, and Blood Institute Dynamic Registry according to whether they were treated with drug-eluting stents or bare-metal stents and whether use was standard or off-label. Patients were followed for 1 year for the occurrence of cardiovascular events and death. Off-label use was defined as use in restenotic lesions, lesions in a bypass graft, left main coronary artery disease, or ostial, bifurcated, or totally occluded lesions, as well as use in patients with a reference-vessel diameter of less than 2.5 mm or greater than 3.75 mm or a lesion length of more than 30 mm.
Results
Off-label use occurred in 54.7% of all patients with bare-metal stents and 48.7% of patients with drug-eluting stents. As compared with patients with bare-metal stents, patients with drug-eluting stents had a higher prevalence of diabetes, hypertension, renal disease, previous percutaneous coronary intervention and coronary-artery bypass grafting, and multivessel coronary artery disease. One year after intervention, however, there were no significant differences in the adjusted risk of death or myocardial infarction in patients with drug-eluting stents as compared with those with bare-metal stents, whereas the risk of repeat revascularization was significantly lower among patients with drug-eluting stents.
Conclusions
Among patients with off-label indications, the use of drug-eluting stents was not associated with an increased risk of death or myocardial infarction but was associated with a lower rate of repeat revascularization at 1 year, as compared with bare-metal stents. These findings support the use of drug-eluting stents for off-label indications.
In 2003, the food and drug administration (FDA) approved drug-eluting stents for the treatment of coronary artery disease. This decision was based on the results of clinical trials that compared a bare-metal stent with a drug-eluting stent in highly selected patients.1-10 Because of the magnitude of the treatment effect of drug-eluting stents in suppressing the recurrence of lesions, consistent positive reports from subsequent trials of small subgroups, and firsthand experiences, physicians extended the use of drug-eluting stents to patients with clinical and anatomical features beyond those of patients in the FDA-approval trials. The use of drug-eluting stents in this context has been termed “off-label.”
Recently, selected reports have questioned the safety of drug-eluting stents.11-13 Consequently, the FDA convened an advisory panel to address this concern. After extended deliberation, the panel concluded that “as compared with on-label use, off-label use is associated with increased risks of stent thrombosis and death or myocardial infarction . . . and that the safety and effectiveness of drug-eluting stents as compared with those of alternative treatments deserve continued study.”14 With these considerations in mind, we evaluated the safety and effectiveness of off-label use of drug-eluting stents and compared our findings with the safety and effectiveness of bare-metal stents used in routine clinical practice. The database of the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry was used for this analysis.
METHODS
The NHLBI Dynamic Registry has been described in detail.15 In brief, the registry involves multi-center recruitment of consecutive consenting patients undergoing percutaneous coronary intervention (PCI) at selected clinical centers in North America during prespecified time intervals or “waves.” Each center received approval from its institutional review board. Five recruitment waves of approximately 2000 patients each have been enrolled and followed over the past 10 years to examine trends in PCI (wave 1: 1997 to 1998, 2524 patients; wave 2: 1999, 2105 patients; wave 3: 2001 to 2002, 2047 patients; wave 4: 2004, 2112 patients; and wave 5: 2006, 2174 patients). Data on baseline demographic, clinical, and angiographic characteristics and procedural characteristics during the index PCI, as well as the occurrence of death, myocardial infarction, and the need for coronary-artery bypass grafting (CABG) during hospitalization, were collected. Follow-up status was ascertained at 1 month, 6 months, and 1 year after intervention. Follow-up rates at 1 year were at least 90% for all waves. With the use of the Social Security Administration's Death Master File (www.ntis.gov/products/ssa-dmf.asp), coordinators periodically evaluated the vital status of patients who were lost to follow-up. If patients underwent subsequent repeat revascularization (either PCI or CABG), vessel-specific and lesion-specific data were collected whenever possible to determine target-vessel revascularization.
Study Population
For this analysis, only patients who received a stent as part of their index PCI were included. The study cohort was divided into two groups: patients receiving only bare-metal stents, and patients receiving only drug-eluting stents. During the recruitment of patients for waves 1, 2, and 3 (1997 to 2002), only bare-metal stents were available; hence, of 6676 patients in these waves, 3858 (57.8%) received at least one bare-metal stent.
During the recruitment of patients for waves 4 and 5 (2004 and 2006), however, both bare-metal and drug-eluting stents were available. Of the 4286 patients in these waves, 2693 (62.8%) received at least one drug-eluting stent, 646 (15.1%) received bare-metal stents only, and 110 (2.6%) received both a drug-eluting stent and a bare-metal stent. Because of the minority of patients in these two waves who received bare-metal stents, and because of the evidence of a large selection bias (i.e., more cardiogenic shock in patients with bare-metal stents), patients in waves 4 and 5 who received bare-metal stents alone or bare-metal and drug-eluting stents were excluded to minimize ambiguity in the results. Thus, the total study population consisted of 6551 patients. Stent use in each patient was classified as standard or off-label.
Definitions
The use of stents was categorized as standard or off-label on the basis of the manufacturers’ instructions for use for the Cypher sirolimus-eluting stent (Cordis) and the Taxus paclitaxel-eluting stent (Boston Scientific), and the classification was applied to both the bare-metal and the drug-eluting stent populations according to lesion criteria. For the Cypher stent, indicated use included treatment of lesions that were 30 mm or less in length in native coronary arteries that had a reference-vessel diameter of 2.5 to 3.5 mm.16 For the Taxus stent, indicated use was for lesions that were 28 mm or less in length in native coronary arteries that had a reference-vessel diameter of 2.5 to 3.75 mm.17 Patients who received stents (either drug-eluting or bare-metal stents or both) and who met these criteria constituted the standard group.
The off-label group included patients who received a stent for reasons that did not meet the standard criteria (e.g., for a restenotic lesion, a lesion in a bypass graft, a lesion length >30 mm, or a reference-vessel diameter <2.5 mm or >3.75 mm), as well as those who had a lesion in the left main coronary artery or ostial, bifurcated, or totally occluded lesions. Acute myocardial infarction as an indication for the procedure was not included in the classification.
End Points
The end points were death from any cause, myocardial infarction, and repeat revascularization (PCI or CABG). Myocardial infarction was diagnosed on the basis of evidence of at least two of the following: typical chest pain lasting more than 20 minutes and not relieved by nitroglycerin; serial electrocardiograms showing changes from baseline or serially in ST and T waves, Q-waves, or both in more than two contiguous leads; a rise in the creatine kinase (CK) level to more than twice the upper limit of the normal range with an increase in CK-MB of more than 5% of the total value; and an elevation in the troponin level to more than twice the upper limit of the normal range.
Repeat revascularization was defined as the combined end point of repeat PCI or CABG during the follow-up period. Repeat PCI was defined as any repeat PCI in the follow-up period, including revascularization of target lesions and target vessels.
Statistical Analysis
For each labeling indication, patient characteristics pertaining to the index PCI, including demographic characteristics, medical history, cardiac presentation, periprocedural medications, procedural characteristics, and in-hospital outcomes were compared between stent types with the use of Student's t-tests or Wilcoxon nonparametric tests for continuous variables and the chi-square test or Fisher's exact test for categorical variables. Similar methods were used for lesion-level analyses. One-year cumulative incidence rates of individual clinical outcomes and composite outcomes were estimated by the Kaplan–Meier method and tested by the log-rank statistic.
Multivariable Cox proportional-hazards regression modeling was used to estimate the independent effect of stent type (drug-eluting stents vs. bare-metal stents) in both the standard group and the off-label group. Sequential models were fit with the initial model including no covariates (unadjusted); the final model included covariates selected by forward stepwise methods and those considered biologically relevant. Proportional-hazards assumptions were evaluated and met for all outcomes with the exception of mortality among patients treated in an off-label fashion. Mortality in the off-label group was reassessed from discharge forward (with in-hospital deaths removed from the analysis) and the proportional-hazards assumption was met; thus, mortality was evaluated both from the time of the PCI (including the in-hospital period) and then again among patients discharged alive. All statistical analyses were performed with the use of SAS software, version 9.1, and a two-sided P value of 0.05 or less was considered to indicate statistical significance.
RESULTS
Baseline Characteristics
According to study definitions, 2110 of 3858 patients who received a bare-metal stent (54.7%) and 1312 of 2693 patients who received a drug-eluting stent (48.7%) were treated in an off-label fashion. Patients receiving stents for off-label indications had more advanced heart disease than did those receiving stents for standard indications, with a greater likelihood of diabetes, hypertension, renal disease, previous PCI or CABG, and myocardial infarction, and a higher prevalence of triple-vessel disease (Table 1).
Table 1.
Baseline Characteristics According to Stent Type and Indications.*
| Variable | Standard Use | Off-Label Use | ||||
|---|---|---|---|---|---|---|
| Bare-Metal Stent (N = 1748) | Drug-Eluting Stent (N = 1381) | P Value | Bare-Metal Stent (N = 2110) | Drug-Eluting Stent (N = 1312) | P Value | |
| Mean age (yr) | 62.6 | 63.7 | 0.02 | 63.4 | 63.4 | 0.80 |
| Female sex (%) | 37.3 | 33.1 | 0.01 | 33.3 | 31.2 | 0.19 |
| Race or ethnic group (%)† | 0.009 | <0.001 | ||||
| White | 79.2 | 74.5 | 82.7 | 76.4 | ||
| Black | 11.1 | 15.2 | 8.9 | 15.4 | ||
| Hispanic | 5.7 | 6.3 | 5.2 | 5.7 | ||
| Asian | 3.8 | 3.4 | 3.1 | 2.3 | ||
| Other | 0.2 | 0.6 | 0.1 | 0.2 | ||
| Smoking history (%) | <0.001 | 0.71 | ||||
| Never | 31.7 | 34.2 | 32.1 | 33.3 | ||
| Current | 29.8 | 23.4 | 25.3 | 25.5 | ||
| Former | 38.6 | 42.4 | 42.6 | 41.2 | ||
| Diabetes (%) | 26.0 | 32.8 | <0.001 | 27.1 | 35.4 | <0.001 |
| Hypertension (%) | 64.4 | 78.1 | <0.001 | 64.6 | 78.1 | <0.001 |
| Renal disease (%) | 4.3 | 8.3 | <0.001 | 5.2 | 9.4 | <0.001 |
| Peripheral vascular disease (%) | 6.0 | 6.9 | 0.31 | 8.5 | 9.7 | 0.24 |
| Cerebrovascular disease (%) | 5.8 | 6.4 | 0.50 | 6.4 | 7.9 | 0.18 |
| Previous PCI (%) | 18.2 | 27.3 | <0.001 | 27.7 | 39.0 | <0.001 |
| Previous CABG (%) | 7.5 | 11.7 | <0.001 | 22.5 | 24.6 | 0.17 |
| Previous myocardial infarction (%) | 28.8 | 20.9 | <0.001 | 33.1 | 27.1 | <0.001 |
| Previous brachytherapy (%)‡ | 0.3 | 0.9 | 0.25 | 0.9 | 1.9 | 0.08 |
| Congestive heart failure (%) | 8.2 | 8.3 | 0.90 | 11.0 | 9.9 | 0.38 |
| Mean left ventricular ejection fraction (%) | 55.0 | 54.1 | 0.12 | 51.1 | 51.1 | 0.80 |
| No. of diseased vessels (%) | <0.001 | <0.001 | ||||
| 0 | 0 | 0.1 | 0.6 | 0.5 | ||
| 1 | 48.0 | 41.3 | 38.1 | 31.9 | ||
| 2 | 33.8 | 32.4 | 31.1 | 30.9 | ||
| 3 | 18.1 | 26.1 | 30.2 | 36.7 | ||
| Primary indication (%) | ||||||
| Acute myocardial infarction | 25.4 | 25.3 | 0.98 | 31.5 | 30.2 | 0.42 |
| Unstable angina | 42.8 | 36.2 | <0.001 | 40.8 | 31.7 | <0.001 |
| Stable angina | 22.1 | 20.1 | 0.20 | 18.5 | 21.3 | 0.04 |
| Ischemia | 5.6 | 12.8 | <0.001 | 5.5 | 12.7 | <0.001 |
| Other | 4.1 | 5.6 | 0.07 | 3.7 | 4.1 | 0.54 |
CABG denotes coronary artery bypass grafting, and PCI percutaneous coronary intervention.
Race and ethnic group were self-reported.
Data were not available for waves 1 and 2.
Characteristics of the Lesions
In the off-label group, differences between patients treated with drug-eluting stents and those treated with bare-metal stents were noted in terms of lesion length and the specific indication for off-label treatment (Table 2). Patients treated with drug-eluting stents had longer lesions (19.6 mm vs. 13.6 mm, P<0.001) and were more likely to have American College of Cardiology/American Heart Association type C lesions (37.1% vs. 22.5%, P<0.001). Patients treated with bare-metal stents more often had very small or very large arteries that were treated but had relatively fewer lesions that were longer than 30 mm or that were located at coronary ostia. There were no significant differences in periprocedural medication use or overall angiographic success between patients treated with drug-eluting stents and those treated with bare-metal stents. One year after treatment, a larger proportion of patients in the drug-elutingstent group than in the bare-metal-stent group remained on dual antiplatelet therapy (71.7% vs. 5.9%, P<0.001).
Table 2.
Lesion and Procedural Characteristics.*
| Variable | Standard Use | Off-Label Use | ||||
|---|---|---|---|---|---|---|
| Bare-Metal Stent (N = 1748) | Drug-Eluting Stent (N = 1381) | P Value | Bare-Metal Stent (N = 2110) | Drug-Eluting Stent (N = 1312) | P Value | |
| Circumstances (%) | 0.62 | 0.13 | ||||
| Elective procedure | 57.6 | 57.8 | 51.4 | 54.9 | ||
| Urgent need | 35.0 | 33.9 | 32.1 | 30.2 | ||
| Emergency | 7.5 | 8.3 | 16.5 | 14.9 | ||
| Cardiogenic shock (%) | 0.9 | 0.4 | 0.14 | 3.0 | 1.1 | <0.001 |
| Glycoprotein IIb/IIIa inhibitors (%) | 35.8 | 31.9 | 0.03 | 42.3 | 41.8 | 0.78 |
| Mean no. of lesions attempted | 1.2 | 1.2 | 0.98 | 1.3 | 1.3 | 0.97 |
| Lesion in left anterior descending artery (%) | 41.5 | 46.2 | 0.009 | 37.3 | 42.1 | 0.005 |
| Mean reference-vessel size (mm) | 3.1 | 3.0 | <0.001 | 3.2 | 3.1 | <0.001 |
| Mean lesion length (mm) | 12.4 | 14.4 | <0.001 | 13.6 | 19.6 | <0.001 |
| ACC/AHA classification (%) | <0.001 | <0.001 | ||||
| A | 18.4 | 19.0 | 10.6 | 5.8 | ||
| B1 | 38.3 | 39.2 | 27.0 | 26.1 | ||
| B2 | 33.7 | 27.4 | 39.9 | 31.0 | ||
| C | 9.6 | 14.5 | 22.5 | 37.1 | ||
| Type of drug-eluting stent (%) | ||||||
| Sirolimus | 0 | 64.5 | 0 | 65.8 | ||
| Paclitaxel | 0 | 36.0 | 0 | 34.9 | ||
| Angiographic success (%) | 99.1 | 99.7 | 0.04 | 98.8 | 99.3 | 0.15 |
| Off-label indications (%) | ||||||
| Restenotic lesion | 11.6 | 16.0 | <0.001 | |||
| Graft lesion | 14.4 | 13.5 | 0.48 | |||
| Reference-vessel diameter | ||||||
| <2.5 mm | 18.1 | 9.2 | <0.001 | |||
| >3.75 mm | 28.2 | 13.7 | <0.001 | |||
| Lesion length >30 mm | 4.3 | 19.0 | <0.001 | |||
| Lesion in left main coronary artery | 2.0 | 4.2 | <0.001 | |||
| Ostial lesion | 13.6 | 19.1 | <0.001 | |||
| Bifurcation | 21.9 | 24.5 | 0.08 | |||
| Total occlusion | 25.6 | 22.4 | 0.03 | |||
Percentages may not total 100 because of rounding. ACC/AHA denotes American College of Cardiology/American Heart Association.
In-hospital Outcomes
Rates of in-hospital death and myocardial infarction were low, and these events occurred more often among patients in the off-label group than among those in the standard group (1.3% vs. 0.4% [P<0.001] and 2.5% vs. 1.3% [P<0.001], respectively). In the off-label group, however, patients treated with drug-eluting stents had a lower mortality rate than did patients treated with bare-metal stents (0.5% vs. 1.9%, P<0.001), whereas there was no significant difference in the rate of myocardial infarction (2.0% vs. 2.8%, P = 0.11) between the two groups. When patients presenting with cardiogenic shock were excluded, the in-hospital rates of death and myocardial infarction were similar between patients treated with drug-eluting stents and those treated with bare-metal stents (data not shown).
End Points
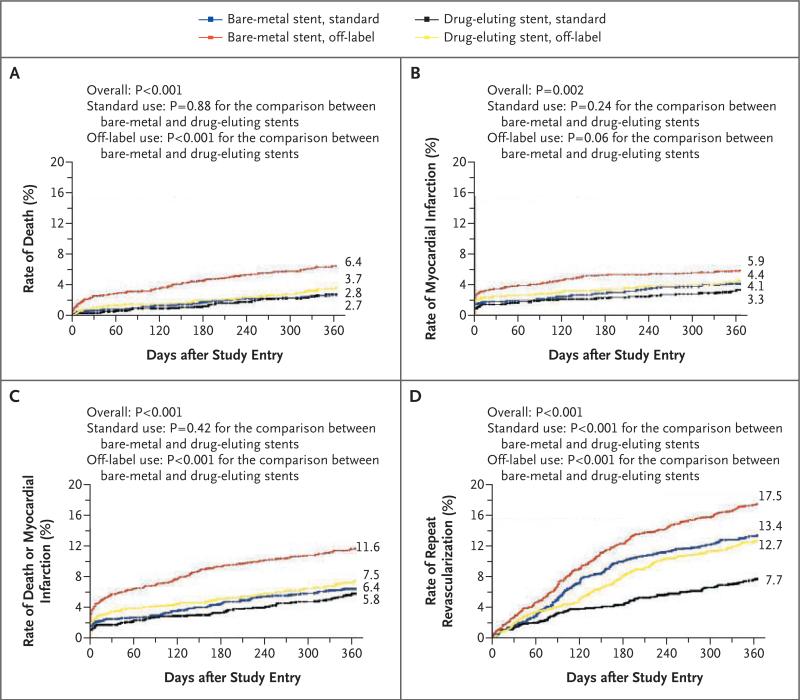
One year after treatment, the following end points were lower in the standard group than in the off-label group: the unadjusted mortality rate (2.7% vs. 5.3%, P<0.001), the rate of myocardial infarction (3.8% vs. 5.3%, P = 0.002), and the rate of death or myocardial infarction (5.2% vs. 10.0%, P<0.001). Within the off-label group, patients with drug-eluting stents, as compared with those with bare-metal stents, had lower rates of death (3.7% vs. 6.4%, P<0.001), death or myocardial infarction (7.5% vs. 11.6%, P<0.001), and myocardial infarction (4.4 vs. 5.9, P = 0.06) (Fig. 1A, 1B, and 1C). As with the in-hospital outcomes, when patients presenting with cardiogenic shock were excluded, the relative unadjusted differences in safety end points according to stent type (drug-eluting stents vs. bare-metal stents) remained similar (data not shown). Furthermore, mortality rates at 1 year across the five recruitment waves were similar (3.1%, 4.0%, 3.1%, 3.4%, and 2.5%, respectively; P = 0.23).
Figure 1. Kaplan–Meier Curves for Outcome According to Stent Type (Drug-Eluting vs. Bare-Metal) and Label Indication (Off-Label vs. Standard Use).
Panel A shows the 1-year incidence of death, Panel B the 1-year incidence of myocardial infarction, Panel C the 1-year incidence of the composite end point of death or myocardial infarction, and Panel D the 1-year incidence of repeat revascularization (percutaneous coronary intervention or coronary-artery bypass grafting).
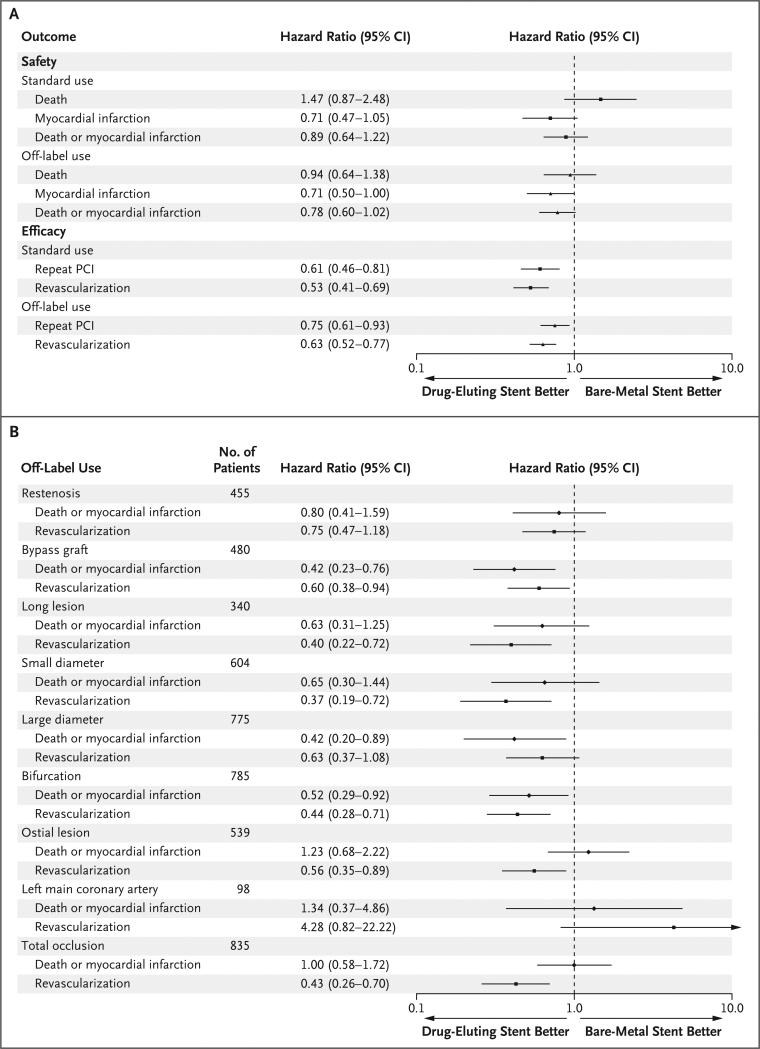
After adjustment for age, sex, circumstances of the procedure (elective, urgent, or emergency), history or no history of PCI, history or no history of CABG, presence or absence of chronic kidney disease, history or no history of congestive heart failure, presence or absence of diabetes mellitus, number of involved vessels, number of lesions for which revascularization was attempted, reason for revascularization, and medical regimen on discharge, off-label use of drug-eluting stents resulted in a lower incidence of myocardial infarction at 1 year than did the use of bare-metal stents (hazard ratio, 0.71; 95% confidence interval [CI], 0.50 to 1.00), but no significant differences were seen with respect to death (hazard ratio, 0.94; 95% CI, 0.64 to 1.38) or the combined end point of death or myocardial infarction (hazard ratio, 0.78; 95% CI, 0.60 to 1.02) (Fig. 2A).
Figure 2. Safety and Efficacy Outcomes at 1 Year of Follow-up.
Shown are the adjusted hazard ratios and 95% confidence intervals (CI) for safety and efficacy outcomes at 1 year for drug-eluting stents and for bare-metal stents (reference category), stratified according to off-label or standard use (Panel A) or stratified according to off-label characteristic (Panel B). Models were adjusted for age, sex, circumstances of the procedure (elective, urgent, or emergency), history or no history of percutaneous coronary intervention (PCI), history or no history of coronary-artery bypass grafting, presence or absence of chronic kidney disease, history or no history of congestive heart failure, presence or absence of diabetes mellitus, number of involved vessels, number of lesions attempted, reason for revascularization, and medical regimen at discharge from the hospital. In both panels, the solid symbols represent the adjusted hazard ratios, and the horizontal lines indicate the 95% CI.
Unadjusted rates of repeat revascularization were lower among patients treated with drug-eluting stents than among those treated with bare-metal stents, regardless of whether the stents were used in standard or off-label fashion (Table 3). No significant difference was noted for the rates of repeat PCI in the off-label group between drug-eluting stents and bare-metal stents (Table 3). After adjustment, however, the risk of repeat PCI (hazard ratio, 0.75; 95% CI, 0.61 to 0.93) and repeat revascularization (hazard ratio, 0.63; 95% CI, 0.52 to 0.77) were significantly lower among patients who received drug-eluting stents than among those who received bare-metal stents (Fig. 2A).
Table 3.
Cumulative 1-Year Event Rates.*
| Variable | Standard | Off-Label | ||||
|---|---|---|---|---|---|---|
| Bare-Metal Stent (N = 1748) | Drug-Eluting Stent (N = 1381) | P Value | Bare-Metal Stent (N = 2110) | Drug-Eluting Stent (N = 1312) | P Value | |
| % | % | |||||
| Safety | ||||||
| Death | 2.7 | 2.8 | 0.88 | 6.4 | 3.7 | <0.001 |
| Myocardial infarction | 4.1 | 3.3 | 0.24 | 5.9 | 4.4 | 0.06 |
| Death or myocardial infarction | 6.4 | 5.8 | 0.42 | 11.6 | 7.5 | <0.001 |
| Efficacy | ||||||
| Repeat percutaneous coronary intervention | 10.5 | 6.5 | <0.001 | 13.6 | 11.4 | 0.07 |
| Coronary-artery bypass grafting | 4.3 | 1.4 | <0.001 | 5.1 | 1.5 | <0.001 |
| Repeat revascularization | 13.4 | 7.7 | <0.001 | 17.5 | 12.7 | <0.001 |
Event rates were calculated with the use of Kaplan–Meier methods.
Subgroup Analyses
In prespecified analyses, adjusted safety and efficacy outcomes were also evaluated for each off-label characteristic. For the outcome of death or myocardial infarction, the use of drug-eluting stents was at least as safe as the use of bare-metal stents in all instances. The risk of repeat revascularization was lower among patients treated with drug-eluting stents than among those treated with bare-metal stents for most subgroups. When the stents were used for restenotic lesions, large-diameter vessels, and lesions in the left main coronary artery, no significant difference was found (Fig. 2B).
DISCUSSION
We compared the safety and efficacy of off-label use of drug-eluting stents with that of bare-metal stents in a large cohort of patients who were undergoing PCI in North America. Our study showed that the use of drug-eluting stents as compared with bare-metal stents was associated with a lower risk of repeat revascularization at 1 year of follow-up. This beneficial effect was achieved without any excess risk of death or myocardial infarction.
Our findings are particularly relevant given recent published reports by Beohar et al.18 and Win et al.19 of worse clinical outcomes with off-label use of drug-eluting stents than with standard use. Both groups reported a very high frequency of off-label use of drug-eluting stents. In the study by Beohar et al., 47% of patients received a drug-eluting stent for either an off-label or an untested indication, and in the study by Win et al., 55% of patients had at least one off-label characteristic. Both studies indicate higher rates of acute and late ischemic complications among patients receiving drug-eluting stents for off-label as compared with standard indications. However, although both studies showed similar findings, the two groups of investigators arrived at different conclusions. Beohar et al., reporting on the D.E.S.cover Registry, concluded that as compared with standard use, off-label use was associated with a higher risk of ischemic complications but that the overall absolute rates were low.18 In contrast, Win et al., reporting on the Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) Registry, noted that ischemic event rates were very high among patients receiving drug-eluting stents for off-label indications and that clinicians should be cautious in extrapolating the results of on-label randomized, controlled trials to higher-risk patients.19 Although all these observations are important and probably valid, the findings of these two groups of investigators need to be taken in the context that neither had a comparison group with an alternative treatment strategy (e.g., bare-metal stents). Such a comparison is vital to evaluate fully the potential adverse effects of drug-eluting stents and to determine specifically whether unfavorable outcomes are related to the clinical status of the patients and the types of lesions treated, rather than to the use of these devices per se.
Our study confirms the findings reported by the investigators of the D.E.S.cover18 and EVENT19 trials — that off-label use of drug-eluting stents is common — since approximately half of the patients in our study had their stent procedure classified as off-label. Furthermore, in keeping with the findings of the two groups of investigators, we found that off-label use in general is associated with worse clinical outcomes than is standard use; specifically, the 1-year event rates of end points such as death and repeat revascularization are similar to those reported by Beohar et al.18 and Win et al.19
Extending beyond those two studies, we observed that off-label use of bare-metal stents also results in increased ischemic complications as compared with standard use. Furthermore, we found that patients treated with drug-eluting stents had significantly lower rates of repeat revascularization than did patients treated with bare-metal stents, without an increased risk of death or myocardial infarction. These findings indicate that the overall poorer outcome with off-label than with standard use is most likely related to characteristics of the patients or lesions, rather than to a specific shortcoming of drug-eluting stents. In fact, if anything, our data indicate that the use of drug-eluting stents for off-label indications is as safe as, and more effective than, the use of bare-metal stents for similar indications.
One potential explanation for our findings may relate to the fact that the bare-metal–stent group in our analysis was treated a few years earlier (1997 to 2002) than was the drug-eluting–stent group (2004 and 2006). Specifically, irrespective of differences in stent type, patients treated more recently could have benefited from changes in the technique of PCI and of adjunctive medical therapy for coronary artery disease. Changes in PCI techniques are unlikely to explain these findings, since the rates of procedural success and in-hospital outcomes in the earlier waves, in which bare-metal stents were used, as compared with the later waves, in which drug-eluting stents were used, were similar. In terms of the effect of medical therapy, although the percentages of patients who underwent PCI and were discharged on statins, angiotensin-converting–enzyme inhibitors, and dual antiplatelet therapy have increased over time and could introduce a temporal bias, these variables were accounted for in the adjusted models and are unlikely to represent a substantial portion of the effect seen with the use of drug-eluting stents.
Our study also shows that the use of drug-eluting stents for off-label indications is more effective than is the use of bare-metal stents in reducing the need for repeat revascularization after the initial stent procedure. The magnitude of this difference, however, is somewhat less than is observed among patients treated for standard indications. This difference is most likely related to the fact that the off-label group represents a patient population with more advanced and less responsive coronary artery disease. Nevertheless, the use of drug-eluting stents for off-label indications still resulted in an estimated 36% relative risk reduction in the need for repeat revascularization as compared with bare-metal stents.
There are some limitations to our investigation. First, our study was based on an observational registry; it was not a randomized trial. Although we adjusted for several variables, it is possible that residual confounding could account for some of the observed differences between bare-metal stents and drug-eluting stents. However, despite our use of a nonrandomized, observational registry, the baseline, angiographic, and procedural characteristics were reasonably well balanced between patients treated with bare-metal stents and those treated with drug-eluting stents in both the standard and the off-label groups. We cannot exclude an unmeasured selection bias toward the use of drug-eluting stents in lower-risk patients in recruitment waves 4 and 5, when both bare-metal stents and drug-eluting stents were available, but the baseline characteristics suggest otherwise. Rather, more adverse characteristics were generally seen in the drug-eluting-stent groups as compared with the bare-metal-stent groups, a finding that without optimal statistical adjustment could artificially attenuate or dilute the benefit seen with drug-eluting stents. Nevertheless, we see the ability to report nonrandomized, real-world registry data from a large cohort of consecutive patients recruited from multiple centers as an advantage, not a weakness, in that these data are complementary to the data reported in randomized, clinical trials.
Another limitation of our study is the relatively short follow-up period. For example, in the Swedish study by Lagerqvist et al.,12 the crossover in events between drug-eluting stents and bare-metal stents did not occur until more than 1 year after treatment. Thus, longer-term data are needed to fully understand the risks and benefits of these treatments for patients with off-label indications.
In summary, our study shows that off-label use of drug-eluting stents as compared with bare-metal stents is not associated with a higher risk of death or myocardial infarction and is associated with a reduced need for repeat revascularization at 1 year. These findings support the use of drug-eluting stents in patients with off-label indications.
Acknowledgments
Supported by grants (HL-33292-14 through HL-33292-22) from the NHLBI.
Dr. Williams reports receiving grant support from Cordis, Boston Scientific, and Abbott Vascular; Dr. Wilensky, grant support from Boston Scientific; Dr. Smith, lecture fees from Cordis; and Dr. Anderson, lecture fees from Boston Scientific. No other potential conflict of interest relevant to this article was reported.
APPENDIX
The following sites and investigators were involved in the NHLBI Dynamic Registry study: Clinical Sites: Boston Medical Center — A. Jacobs, D. Fine, S. Tressel; Emory University Hospital — J. Douglas, P. Block, P. Hyde, E. Block, E. Wright; Lankenau Hospital — P. Coady, T. Shapiro, E. Tarte, H. Criner, A.M. Chikowski; Lenox Hill Heart and Vascular Institute — H. Cohen, C. Brennan, M. Abenoja; Montefiore Medical Center — V.S. Srinivas, M. Galvin; Montreal Heart Institute — M.G. Bourassa, S. Doucet, J.-F. Tanguay, S. Taillefer, L. Robillard, N. St-Jean; New York University Medical Center — J. Slater, E. Weisman, C. Wang; Piedmont Hospital–Fuqua Heart Center of Atlanta — S. King III, J. Mattia, K. Shemwell, J. Creech; Rhode Island Hospital — D. Williams, D. Abbott, J. Muratori, T. Chaffee; Seton Medical Center — R. Shaw, F. Millhouse, M. Murphy, M. Cavanaugh; St. Mary's Hospital–Mayo Clinic — D. R. Holmes, S. Brevig, R. Connelly, P. Sinning; University of Chicago — J. Lopez, E. Holper, P. Bennett, C. Ball; University of Maryland Hospital — J.L. Stafford, B. Reicher, D. Beach; University of New Mexico — W. Laskey, C. Wells; University of Pennsylvania Health System — R. Wilensky, R. Glaser, M. Walsh; University of Pittsburgh Medical Center–UPMC-Presbyterian University Hospital — O. Marroquin, S. Mulukutla, D. Rosenfelder, V. Iouchmanov, T. Vita; Wake Forest University Medical Center — M. Kutcher, T. Young; Coordinating Center: K. Detre (deceased), S. Kelsey, K. Kip, F. Selzer, H. Vlachos, S. Lawlor, E. Passano; NHLBI: G. Sopko, P. Desvigne-Nickens.
REFERENCES
- 1.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–80. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 2.Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–94. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 3.Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 4.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 5.Schofer J, Schlüter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomized controlled trial (E-SIRIUS). Lancet. 2003;362:1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 6.Schampaert E, Cohen EA, Schlüter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol. 2004;43:1110–5. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 8.Dawkins KD, Grube E, Guagliumi G, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005;112:3306–13. doi: 10.1161/CIRCULATIONAHA.105.552190. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–23. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 10.Weisz G, Leon MB, Holmes DR, Jr, et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the Sirolimus-Eluting Stent in de Novo Native Coronary Lesions (SIRIUS) trial. J Am Coll Cardiol. 2006;47:1350–5. doi: 10.1016/j.jacc.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 11.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 12.Lagerqvist B, James SK, Stenestrand S, Lindbäck J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–19. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 13.Mauri L, Hsieh W, Massaro JM, Ho K, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 14.Farb A, Boam AB. Stent thrombosis redux — the FDA perspective. N Engl J Med. 2007;356:984–7. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 15.Williams DO, Holubkov R, Yeh W, et al. Percutaneous coronary intervention in the current era compared with 1985-1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–51. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 16.CYPHER sirolimus-eluting stent: essential prescribing information. Cordis; Miami: (Available at http://www.co.com.) [Google Scholar]
- 17.TAXUS Express stent system: directions for use. Boston Scientific; Natick, MA: (Available at http://www.taxus-stent.com.) [Google Scholar]
- 18.Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA. 2007;297:1992–2000. doi: 10.1001/jama.297.18.1992. [DOI] [PubMed] [Google Scholar]
- 19.Win HK, Caldera AE, Maresh K, et al. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 2007;297:2001–9. doi: 10.1001/jama.297.18.2001. [DOI] [PubMed] [Google Scholar]