Abstract
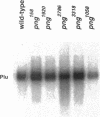
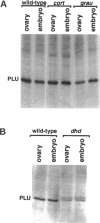
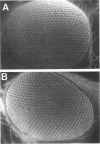
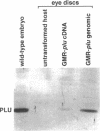
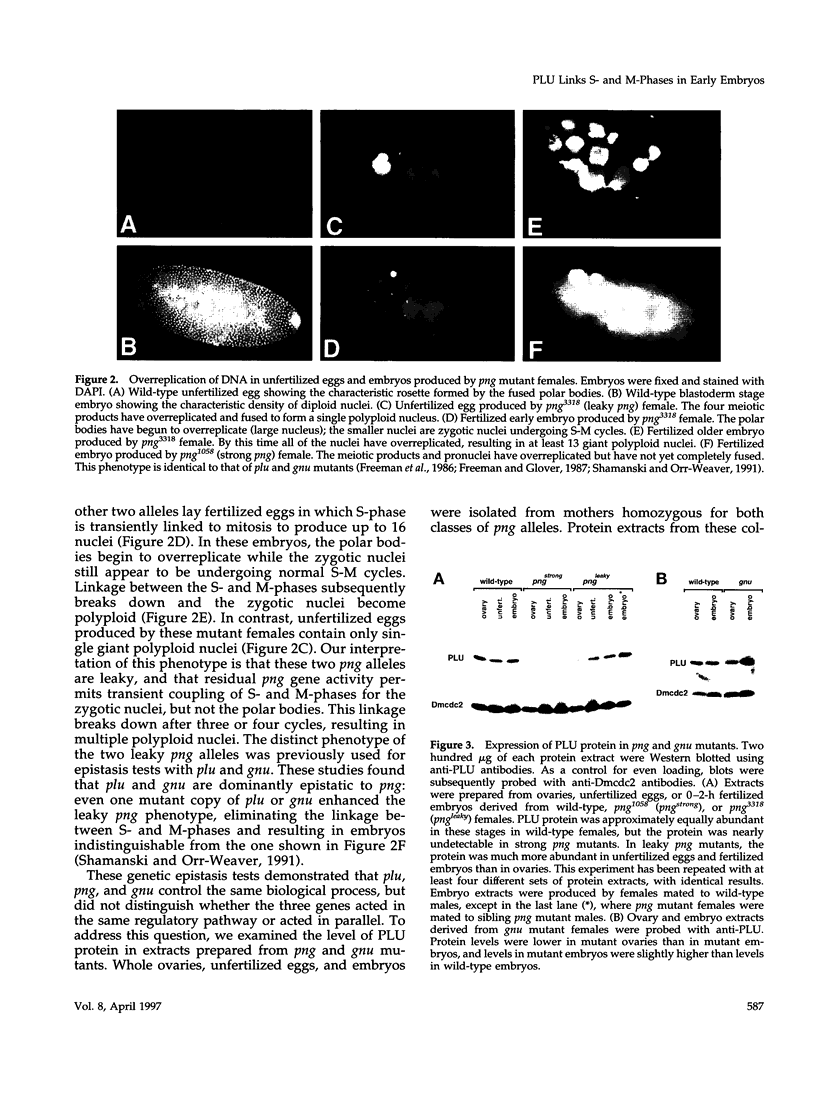
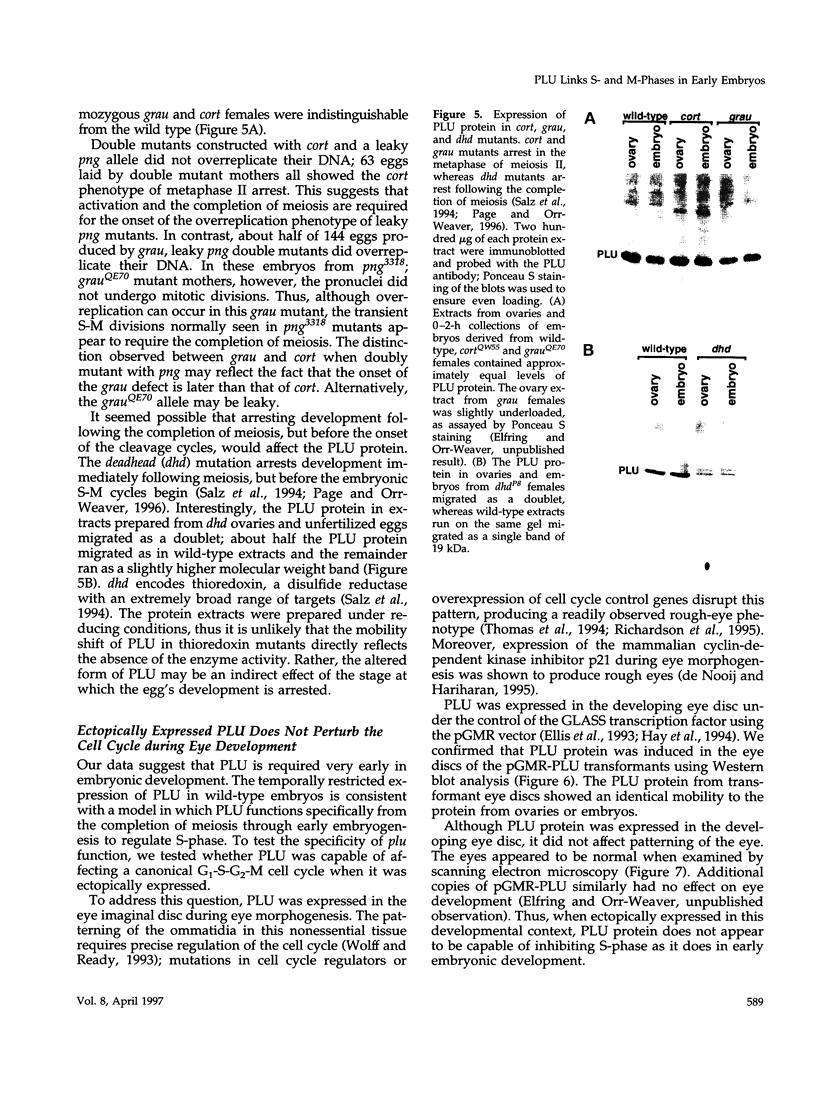
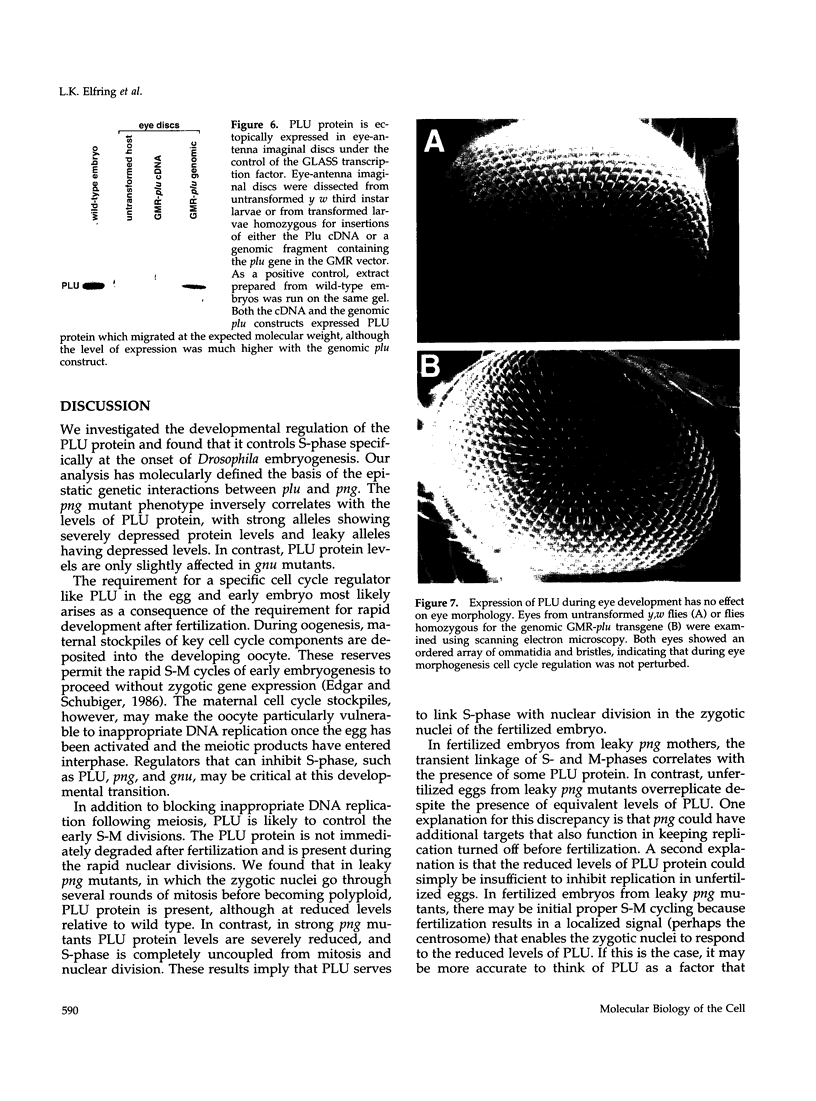
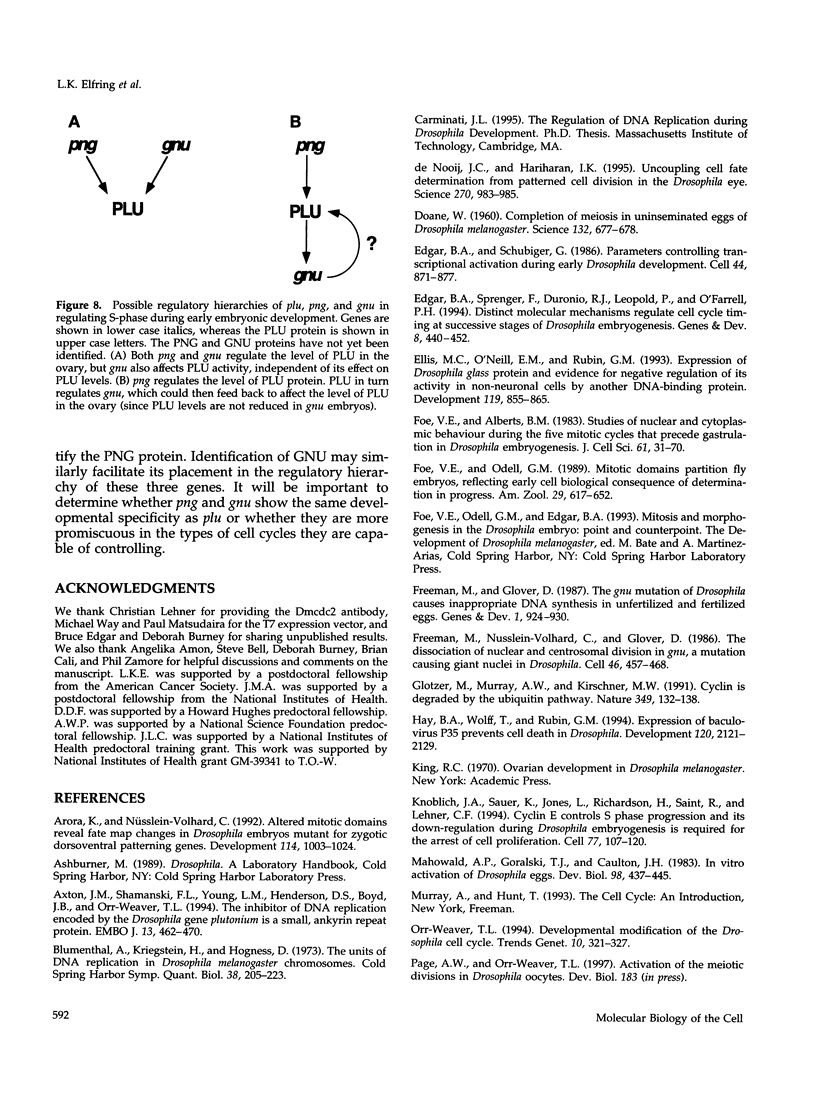
Unfertilized eggs and fertilized embryos from Drosophila mothers mutant for the plutonium (plu) gene contain giant polyploid nuclei resulting from unregulated S-phase. The PLU protein, a 19-kDa ankyrin repeat protein, is present in oocytes and early embryos but is not detectable after the completion of the initial rapid S-M cycles of the embryo. The persistence of the protein during the early embryonic divisions is consistent with a direct role in linking S-phase and M-phase. When ectopically expressed in the eye disc, PLU did not perturb the cell cycle, suggesting that PLU regulates S-phase only in early embryonic development. The pan gu (png) and giant nuclei (gnu) genes also affect the S-phase in the unfertilized egg and early embryo. We show that functional png is needed for the presence of PLU protein. By analyzing png mutations of differing severity, we find that the extent of the png mutant phenotype inversely reflects the level of PLU protein. Our data suggest that PLU protein is required at the time of egg activation and the completion of meiosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora K., Nüsslein-Volhard C. Altered mitotic domains reveal fate map changes in Drosophila embryos mutant for zygotic dorsoventral patterning genes. Development. 1992 Apr;114(4):1003–1024. doi: 10.1242/dev.114.4.1003. [DOI] [PubMed] [Google Scholar]
- Axton J. M., Shamanski F. L., Young L. M., Henderson D. S., Boyd J. B., Orr-Weaver T. L. The inhibitor of DNA replication encoded by the Drosophila gene plutonium is a small, ankyrin repeat protein. EMBO J. 1994 Jan 15;13(2):462–470. doi: 10.1002/j.1460-2075.1994.tb06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- DOANE W. W. Completion of meiosis in uninseminated eggs of Drosophila melanogaster. Science. 1960 Sep 9;132(3428):677–678. doi: 10.1126/science.132.3428.677. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., Schubiger G. Parameters controlling transcriptional activation during early Drosophila development. Cell. 1986 Mar 28;44(6):871–877. doi: 10.1016/0092-8674(86)90009-7. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., Sprenger F., Duronio R. J., Leopold P., O'Farrell P. H. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994 Feb 15;8(4):440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. C., O'Neill E. M., Rubin G. M. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development. 1993 Nov;119(3):855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- Foe V. E., Alberts B. M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983 May;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Freeman M., Glover D. M. The gnu mutation of Drosophila causes inappropriate DNA synthesis in unfertilized and fertilized eggs. Genes Dev. 1987 Nov;1(9):924–930. doi: 10.1101/gad.1.9.924. [DOI] [PubMed] [Google Scholar]
- Freeman M., Nüsslein-Volhard C., Glover D. M. The dissociation of nuclear and centrosomal division in gnu, a mutation causing giant nuclei in Drosophila. Cell. 1986 Aug 1;46(3):457–468. doi: 10.1016/0092-8674(86)90666-5. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wolff T., Rubin G. M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994 Aug;120(8):2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Knoblich J. A., Sauer K., Jones L., Richardson H., Saint R., Lehner C. F. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994 Apr 8;77(1):107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Mahowald A. P., Goralski T. J., Caulton J. H. In vitro activation of Drosophila eggs. Dev Biol. 1983 Aug;98(2):437–445. doi: 10.1016/0012-1606(83)90373-1. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L. Developmental modification of the Drosophila cell cycle. Trends Genet. 1994 Sep;10(9):321–327. doi: 10.1016/0168-9525(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Page A. W., Orr-Weaver T. L. The Drosophila genes grauzone and cortex are necessary for proper female meiosis. J Cell Sci. 1996 Jul;109(Pt 7):1707–1715. doi: 10.1242/jcs.109.7.1707. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992 Feb 7;68(3):573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- Rhyu M. S., Jan L. Y., Jan Y. N. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994 Feb 11;76(3):477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Richardson H., O'Keefe L. V., Marty T., Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995 Oct;121(10):3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Sagata N. Meiotic metaphase arrest in animal oocytes: its mechanisms and biological significance. Trends Cell Biol. 1996 Jan;6(1):22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- Salz H. K., Flickinger T. W., Mittendorf E., Pellicena-Palle A., Petschek J. P., Albrecht E. B. The Drosophila maternal effect locus deadhead encodes a thioredoxin homolog required for female meiosis and early embryonic development. Genetics. 1994 Mar;136(3):1075–1086. doi: 10.1093/genetics/136.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanski F. L., Orr-Weaver T. L. The Drosophila plutonium and pan gu genes regulate entry into S phase at fertilization. Cell. 1991 Sep 20;66(6):1289–1300. doi: 10.1016/0092-8674(91)90050-9. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Gunning D. A., Cho J., Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994 Jul 1;77(7):1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- de Nooij J. C., Hariharan I. K. Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science. 1995 Nov 10;270(5238):983–985. doi: 10.1126/science.270.5238.983. [DOI] [PubMed] [Google Scholar]