Summary
The vertebrate lens provides an excellent model with which to study the mechanisms required for epithelial invagination. In the mouse, the lens forms from the head surface ectoderm. A domain of ectoderm first thickens to form the lens placode and then invaginates to form the lens pit. The epithelium of the lens placode remains in close apposition to the epithelium of the presumptive retina as these structures undergo a coordinated invagination. Here, we show that F-actin-rich basal filopodia that link adjacent presumptive lens and retinal epithelia function as physical tethers that coordinate invagination. The filopodia, most of which originate in the presumptive lens, form at E9.5 when presumptive lens and retinal epithelia first come into close contact, and have retracted by E11.5 when invagination is complete. At E10.5 - the lens pit stage - there is approximately one filopodium per epithelial cell. Formation of filopodia is dependent on the Rho family GTPase Cdc42 and the Cdc42 effector IRSp53 (Baiap2). Loss of filopodia results in reduced lens pit invagination. Pharmacological manipulation of the actin-myosin contraction pathway showed that the filopodia can respond rapidly in length to change inter-epithelial distance. These data suggest that the lens-retina inter-epithelial filopodia are a fine-tuning mechanism to assist in lens pit invagination by transmitting the forces between presumptive lens and retina. Although invagination of the archenteron in sea urchins and dorsal closure in Drosophila are known to be partly dependent on filopodia, this mechanism of morphogenesis has not previously been identified in vertebrates.
Keywords: Cdc42, IRSp53 (Baiap2), Eye development, Filopodia, Lens development, Morphogenesis, Mouse
INTRODUCTION
A fundamental process in determining the shape of organs is cellular sheet folding and invagination (Lecuit and Lenne, 2007) as exemplified by the developing neural tube, eye, ear and heart. Invagination of cellular sheets begins when a few cells become conical or wedge-shaped as a result of apical constriction. Models suggest that this creates a local mechanical stress that promotes invagination (Odell et al., 1981; Hardin and Keller, 1988; Kimberly and Hardin, 1998). Tissue invagination through the formation of apically constricted cells is a highly conserved feature of tissue morphogenesis and is observed in the developing fly embryo during mesoderm invagination (Leptin and Grunewald, 1990), in the sea urchin in early gastrulation (Davidson et al., 1995) and in vertebrate neural tube closure (Schroeder, 1970). Dynamic actin rearrangements have been implicated in these processes.
The Rho family of small GTPases are essential regulators of actin dynamics (Jaffe and Hall, 2005; Ridley, 2006) as well as cell polarity (Etienne-Manneville, 2004; Macara, 2004; Seifert and Mlodzik, 2007) and cell adhesion (Kaibuchi, 1999; Jaffer and Chernoff, 2004; Gumbiner, 2005). RhoA, Rac1 and Cdc42 are the three best-characterized members of the small Rho GTPase family (Burridge and Wennerberg, 2004). In actin rearrangements, RhoA promotes the formation of stress fibers (Ridley and Hall, 1992), which are composed of contractile actin-myosin filaments. By contrast, Rac1 regulates the formation of lamellipodia (Ridley et al., 1992) and Cdc42 controls the formation of filopodia (Nobes and Hall, 1995). In the lens, Rho family GTPases have important functions at late stages of development. Expression of C3 exoenzyme (a toxin that inactivates RhoA, B and C) in the lens results in defects in the morphology of lens epithelial and fiber cells and in a changed distribution of F-actin (Maddala et al., 2004).
Filopodia are defined as dynamic cellular protrusions that range in length from 5-35 μm and are filled with bundles of parallel actin filaments (McClay, 1999; Raich et al., 1999; Passey et al., 2004; Faix and Rottner, 2006). They are usually anchored at their tips by cadherin-catenin (Raich et al., 1999; Wood et al., 2002; Partridge and Marcantonio, 2006) or integrin-dependent adhesion complexes (Partridge and Marcantonio, 2006). Broadly analyzed in fibroblasts and neurite growth cones, they are thought to probe and sense the microenvironment to guide migration and extension, respectively. Cdc42 has been found in vitro to promote filopodia formation by interacting with the effector protein N-WASP (Wasl - Mouse Genome Informatics) (Miki et al., 1998; Egile et al., 1999; Banzai et al., 2000), a Wiskott-Aldrich syndrome gene family member. N-WASP activates the Arp2/3-containing actin nucleator complex and, as a result, the fast-growing barbed-end of bundled actin filaments pushes against the plasma membrane and forms filopodia. The formation of Cdc42-stimulated filopodia in N-WASP-deficient fibroblasts revealed an alternative pathway for filopodia formation (Snapper et al., 2001). This was later found to require diaphanous-related formins (DRFs) (Peng et al., 2003), a group of actin nucleators that function downstream of Rho GTPases. A third filopodial formation pathway requires the effector protein IRSp53 (Baiap2 - Mouse Genome Informatics) interacting with either Mena (Enah) (Krugmann et al., 2001) or Eps8 (Disanza et al., 2006). IRSp53 and Eps8 are involved in signal transduction. IRSp53 is a substrate for the insulin receptor (Yeh et al., 1996) and Eps8 is a substrate for other tyrosine kinase receptors (Fazioli et al., 1993).
Events in morphogenesis require precise cell positioning and guidance that can be provided by filopodia. This function can be found in migratory tissue sheets, such as during dorsal closure in Drosophila (Martin-Blanco et al., 2000), ventral closure in C. elegans (Williams-Masson et al., 1997) and wound healing (Wood et al., 2002). Early lens morphogenesis in mammals (Chow and Lang, 2001), which begins soon after the presumptive lens makes contact with the optic vesicle, requires the same degree of precise guidance. At embryonic day 9.0 (E9.0), the lens primordium forms in response to induced Pax6 expression. This primordium then thickens to form a lens placode at E9.5 and then invaginates to form the lens pit at E10.5. Invagination of the lens pit occurs in precise coordination with invagination of the presumptive retina so that their shapes match. Genetic defects in lens induction lead to abnormal morphogenesis of the lens and retina. Examples are seen in Pax6 (Grindley et al., 1995; Smith et al., 2009) and Sox2 (Smith et al., 2009) mutants, in which the lens placode does not thicken and coordinated invagination with the optic vesicle is arrested. Similar phenotypes are observed in Frs2α2F/2F mutants (Gotoh et al., 2004), in which Fgf signaling is compromised, and in bone morphogenetic protein 7 (Bmp7) mutants (Wawersik et al., 1999). These observations imply that the lens induction signaling pathways are connected to the machinery that controls epithelial cell shape and morphogenesis.
More than 100 years ago, the anatomists Johann Lenhossek and Andre Magitot observed that cone-shaped cytoplasmic processes extend from the underside of the invaginating mammalian lens (Lenhossek, 1902; Magitot, 1910). A later light microscope study of human eye development described connections between the developing lens and optic cup as `cone-shaped' processes making contact with the optic vesicle (Mann, 1928). Subsequently, transmission electron microscopy (TEM) showed cytoplasmic processes extending from the base of the lens to the presumptive retina (McAvoy, 1980). In this study, we show that the cytoplasmic protrusions coming from the lens pit are filopodia. We make use of Cdc42 and IRSp53 mutant mice to show that these filopodia are Cdc42- and IRSp53-dependent and that they function as physical tethers that coordinate invagination of the presumptive lens and retina.
MATERIALS AND METHODS
Animal maintenance and use
Animals were housed in a pathogen-free vivarium in accordance with institutional policies. Gestational age was determined through detection of a vaginal plug. At specific gestational ages, fetuses were removed by hysterectomy after the dams had been anesthetized with isoflurane. Cdc42flox, FAKflox, E-cadflox, Rac1flox and IRSp53-/- and Le-Cre mice have been described previously (Ashery-Padan et al., 2000; Boussadia et al., 2002; Beggs et al., 2003; Gu et al., 2003; Chen et al., 2006; Sawallisch et al., 2009; Weiss et al., 2009).
Immunofluorescence
Immunofluorescence (IF) labeling of cryosections was performed as described (Smith et al., 2005). Primary antibodies were as follows: anti-β-catenin (1:1000, Santa Cruz, sc-7199), anti-Cdc42 (1:500, Abcam, ab17437), anti-collagen IV (1:5000, Abcam, ab19808), anti-E-cadherin (1:200, BD Transduction Laboratories, 6-10181), anti-laminin (1:5000, Abcam, ab30320), anti-myosin IIB (1:5000, Covance, PRB445P), anti-phospho-myosin light chain (phospho-S20) (1:2500, Genetex, GTX22480), anti-Pals (protein associated with lin-7) (1:100, Upstate, 07-708), anti-Par6 (1:250, Santa Cruz, sc-14405), anti-Pax6 (1:1000, Covance, PRB-278P), anti-Rac1 (1:200, BD Transduction Laboratories, 6-10650), anti-α-tubulin (1:1000, Santa Cruz, sc-5286) and anti-acetylated tubulin (acetyl K40) (1:1000, Genetex, GTX11323). Alexa Fluor secondary antibodies (A-11072, A-11020, A-11070, A-11017, A-12381, Molecular Probes) were used at dilutions from 1:1000 to 1:5000. For visualization of nuclei, sections were counterstained with Hoechst 33342 (Sigma, B-2261).
Quantification
Images were analyzed using ImageJ version 1.33. We chose to compare multiple wild-type and mutant tissues of the same section plane to avoid complications with variation from preparation to preparation. For F-actin quantity, average pixel intensity over a transect in the lens pit was calculated. The data were compared by one-way ANOVA analysis for the mutant and wild-type intensities. Equatorial lens diameter was measured on images of E10.5 cryosections. One-way ANOVA analysis indicated statistical significance. For analysis of lens pit curvature, coordinates were extracted from curves representing the basal surface of the lens pit using Matlab 7.1 (The MathWorks, MA, USA). The curvature was then calculated and the graph generated by Mathematica 5.0 (Wolfram Research, IL, USA). The curvature equation is:
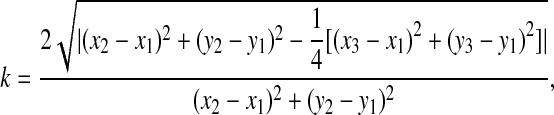 |
where (x1,y1), (x2,y2), (x3,y3) are the coordinates for three points along the curve.
RESULTS
Cytoplasmic protrusions between the lens placode and optic vesicle are filopodia
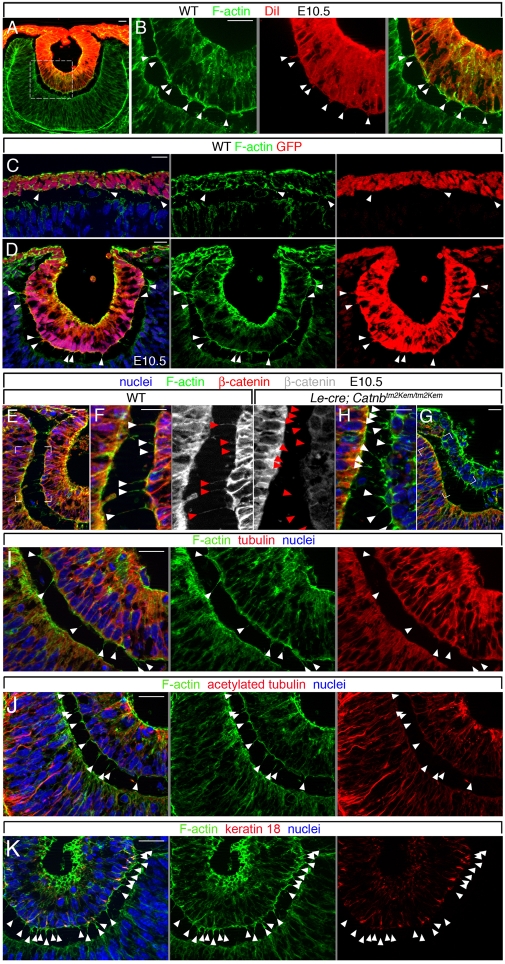
Cytoplasmic protrusions connecting presumptive lens and retina were first drawn by Ida Mann in her series on human eye development (Mann, 1950) (see Fig. 1A). A later TEM study in the rat (McAvoy, 1980) identified these processes as holding an abundance of fine filaments. To assess the nature of these fine filaments and also the phase of eye development in which these processes exist in the mouse, we performed phalloidin labeling for F-actin from E9.5 to E11.5. The processes were initially observed at ∼E9.5 (Fig. 1B,C); they were F-actin rich and crossed the epithelial interspace at various angles. They became longer and more prominent from E10.0 (Fig. 1D,E) to E10.5 (Fig. 1F,G). Counting revealed that they were transient in that they first increased from E9.5 to 10.5, and then rapidly reduced thereafter (Fig. 1H). The presence of these processes during this phase of development correlated closely with coordinated invagination of the presumptive lens and retina.
Fig. 1.
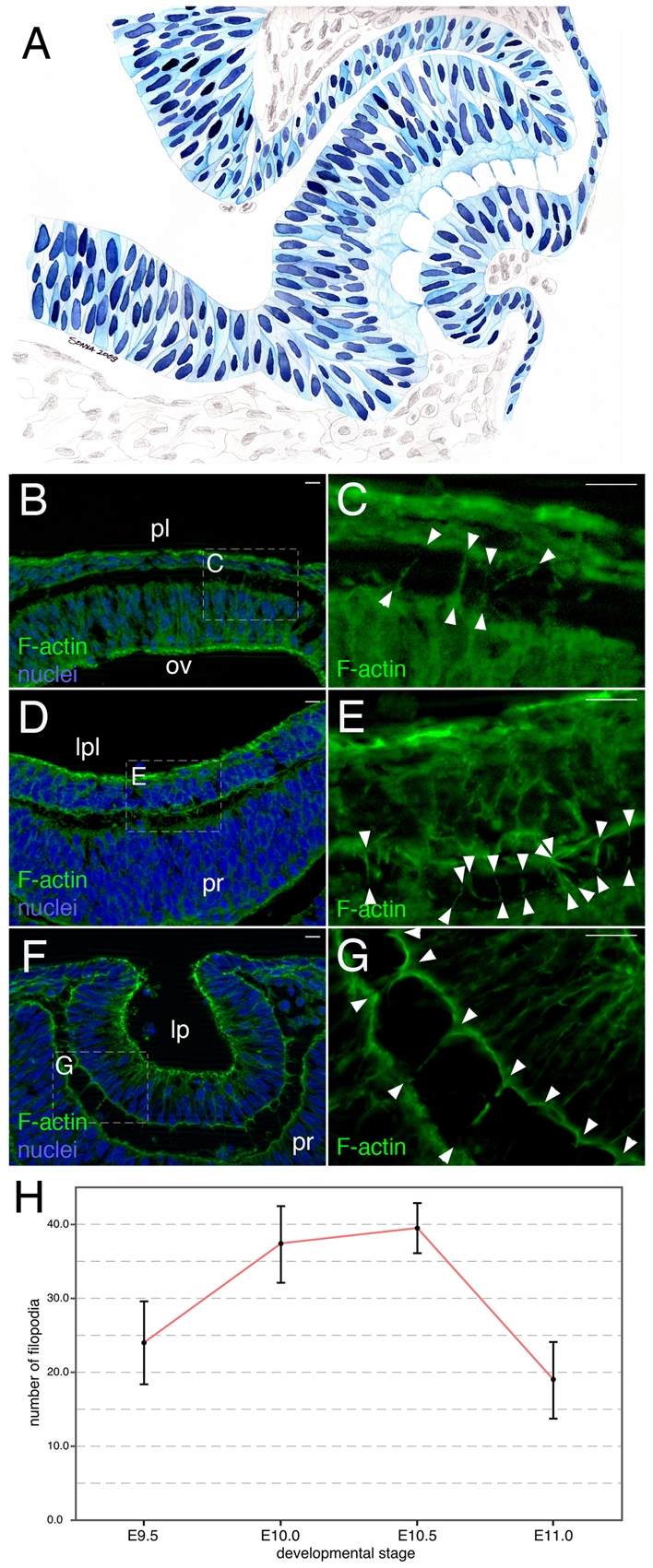
Transient, F-actin-filled processes connect lens and retina during lens pit-optic cup invagination. (A) The inter-epithelial process in the early human eye [redrawn from Mann (Mann, 1928)]. (B-G) Phalloidin (F-actin, green) and nuclear (Hoechst 33258, blue) labeling of eye region cryosections from mouse embryos of the indicated ages. Cytoplasmic protrusions containing filamentous actin are indicated by arrowheads. (H) Quantification of lens-retina inter-epithelial processes from E9.5 to E11.0. At each time-point, n=6. lp, lens pit; lpl, lens placode; ov, optic vesicle; pl, presumptive lens; pr, presumptive retina. Scale bars: 20 μm.
The Mann drawings (Mann, 1950) suggested that the connecting processes might originate in the presumptive retina (see Fig. 1A), whereas the McAvoy study (McAvoy, 1980) clearly suggested that these processes originated in the presumptive lens and made contact with the basal lamina of the presumptive retina. To determine the origin of the inter-epithelial processes, we performed three analyses. First, we used lipophilic dye (DiI) labeling as a means of tracking membrane continuity. Fixed embryos in aqueous buffer were briefly dipped into organic solvent-DiI solution to precipitate DiI onto the embryo surface and then embryos were stored at 4°C for 3-4 weeks to allow DiI membrane diffusion. This was an effective means to label the lens pit and the embryonic surface ectoderm (Fig. 2A). At higher magnification, processes that were F-actin rich according to phalloidin labeling (Fig. 2B, green) were also, in most cases, DiI labeled (Fig. 2B, red), indicating that these processes originate in the cells of the lens pit. Secondly, we took advantage of the cytoplasmic GFP expression that is restricted to the presumptive lens in Le-Cre mice and determined whether any processes could be labeled with GFP antibodies. Although the detection of positive processes was more difficult at E9.5 (Fig. 2C) than at E10.5 (Fig. 2D), at both developmental stages some processes were positive, further suggesting that they have a lens pit origin. Finally, we took advantage of a lens pit-specific deletion of β-catenin (Catnb; Ctnnb1 - Mouse Genome Informatics) (Smith et al., 2005) and determined whether this changed the β-catenin labeling of inter-epithelial processes. At E10.5 in wild-type mice (Fig. 2E), inter-epithelial processes could be labeled for F-actin with phalloidin (Fig. 2F, green) and with antibodies for β-catenin (Fig. 2F, red and gray). By contrast, in Le-Cre; Catnbtm2Kem/tm2Kem mice (Smith et al., 2005), although there were plenty of phalloidin-labeled processes (Fig. 2G,H, green), elimination of β-catenin in the lens pit (Fig. 2H, red and gray) resulted in the inability to detect β-catenin in processes (Fig. 2H, gray). We conclude that at E10.5, most inter-epithelial processes originate in the lens pit. At E9.5, when the lens placode has just formed, it remains possible that a significant proportion of inter-epithelial processes originate in the presumptive retina.
Fig. 2.
Many inter-epithelia filopodia originate in the lens pit. (A-K) Eye region cryosections from E10.5 (A,B,D-K) or E9.5 (C) mouse embryos labeled for F-actin (phalloidin, green), nuclei with Hoechst 33258 (blue) and DiI in the lens pit (A,B, red), GFP from the Le-Cre transgene (C,D, red), β-catenin (E-H, red, white), tubulin (I, red), acetylated tubulin (J, red) or keratin 18 (K, red). The position of filopodia is indicated by white arrowheads; red arrowheads indicate filopodial position according to F-actin labeling. A gray line between panels indicates that they are separated color channels of the same image. Scale bars: 20 μm.
Filopodia are defined as dynamic F-actin-based cellular protrusions, ranging in length from 5-35 μm (Nobes and Hall, 1995; McClay, 1999; Raich et al., 1999; Passey et al., 2004; Faix and Rottner, 2006). The F-actin content and length (see below) of the inter-epithelial processes fulfilled two criteria of the filopodial definition; however, these structures might have properties of other cytoskeletal filaments. To investigate this possibility, we performed labeling for tubulin, acetylated tubulin and keratin 18. Tubulin is the principal component of microtubules and a marker of cilia (Silverman and Leroux, 2009). Keratin 18 is an epithelial intermediate filament component that is expressed in the lens pit (Kasper and Viebahn, 1992). In wild-type embryos at E10.5, none of the abundant F-actin-containing processes that spanned the inter-epithelial gap labeled with antibodies to tubulin (Fig. 2I, red), acetylated tubulin (Fig. 2J, red) or keratin 18 (Fig. 2K, red). This further suggested that the inter-epithelial processes could be defined as filopodia. They will be referred to as such hereafter.
Inter-epithelial filopodia are dependent on Cdc42, IRSp53 and FAK
The mechanism of filopodium generation has been extensively investigated, primarily in cultured cells, and a handful of molecules have been implicated. Among them, the Rho family GTPase Cdc42 was the first shown to be capable of stimulating filopodia formation (Nobes and Hall, 1995). In generating filopodia, Cdc42 can function through the effector molecules WASP (Was - Mouse genome Informatics) (Wiskott-Aldrich syndrome protein) and N(neuronally enriched)-WASP (Miki and Takenawa, 2003). Each of these acts through the actin nucleator complex Arp2/3 to enhance the formation of filopodia (Machesky and Insall, 1998; Higgs and Pollard, 1999; Rohatgi et al., 1999). More recently, the effector protein IRSp53, an insulin receptor substrate, has been implicated in the generation of filopodia (Krugmann et al., 2001; Disanza et al., 2006). It has been shown in culture that filopodia can be anchored at their tips by integrin complexes that are dependent on the activity of focal adhesion kinase (FAK; Ptk2 - Mouse Genome Informatics) (Partridge and Marcantonio, 2006).
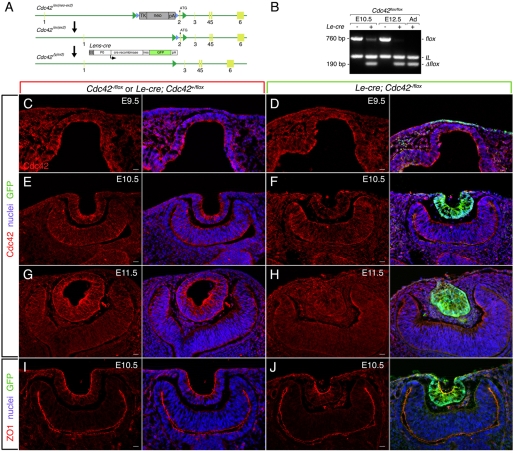
We reasoned that mouse mutants of these genes might have defects in the formation of lens pit filopodia and that a phenotypic analysis could teach us about both the function of these structures and the mechanism of their formation. To this end, we generated a germline mutant allele of IRSp53 (Sawallisch et al., 2009; Weiss et al., 2009) and loxP-flanked conditional alleles of Cdc42 (Fig. 3A, Cdc42flox) (Chen et al., 2006) and FAK (FAKflox) (Beggs et al., 2003). The conditional alleles were combined with the Le-Cre driver (Ashery-Padan et al., 2000), which is active in the lens placode and surrounding ectoderm (Smith et al., 2005).
Fig. 3.
Generation and phenotype of Cdc42flox/flox; Le-Cre mice. (A) Schematic of the Cdc42flox allele. Exons are represented by numbered light green boxes, loxP sites by green arrowheads and Frt sites by blue arrowheads. The positively selectable expression unit TK-Neo-pA is shown in gray. The Le-Cre transgene was used to delete the Cdc42flox allele in presumptive lens cells. (B) Confirmation of Cdc42flox/flox deletion by Le-Cre in dissected lens pit (E10.5), lens vesicle (E12.5) and adult lens (Ad). The undeleted Cdc42flox allele shows a 760 bp band that changes to 190 bp after recombination. IL is an internal control amplifying a region from the interleukin 1 gene. (C-J) Eye region cryosections from E9.5 (C,D), E10.5 (E,F,I,J) or E11.5 (G,H) embryos labeled for nuclei with Hoechst 33258 (blue), GFP (D,F,H,J, green), Cdc42 (C-H, red), or ZO1 (I,J, red). Scale bars: 20 μm.
We validated the Cdc42flox allele by dissecting out lens pits at E10.5, lens vesicles at E12.5 and adult mutant lenses as a positive control for the mutant allele, and performing genotyping PCR. This showed (Fig. 3B) that deletion of the Cdc42flox allele was all but complete by E10.5. We further validated Cdc42 loss-of-function by performing antibody labeling of cryosections. This showed that Le-Cre-mediated deletion of Cdc42flox/flox could be detected as reduced levels of Cdc42 immunoreactivity in the lens pit at E10.5 (Fig. 3E,F) and in the lens vesicle at E11.5 (Fig. 3G,H). Cdc42 has been proposed to regulate cell polarity (Etienne-Manneville, 2004; Macara, 2004) and we therefore determined whether the tight junction protein ZO1 (Tjp1 - Mouse Genome Informatics), a marker of the cell apex (Stevenson, 1986) was modified in distribution or level. This analysis (Fig. 3I,J) showed that ZO1 was still present at the cell apex at levels comparable to the control. Thus, this aspect of cell polarity was unchanged in the Cdc42 mutant lens pit.
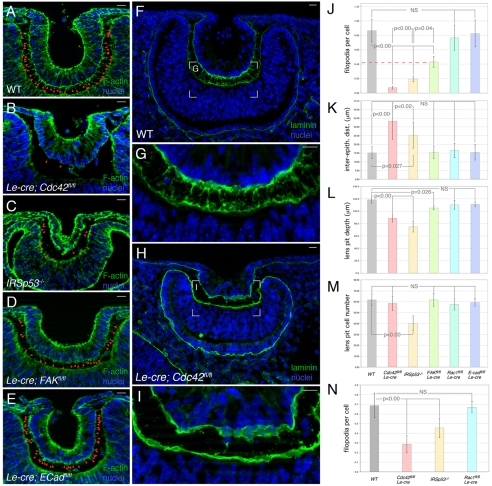
When we assessed the phenotype of the Cdc42 mutant, we used Le-Cre conditional mutants of Rac1flox/flox as a control. Since Rac1 is a Rho family GTPase with an alternate function in stimulating lamellipodia formation (Ridley et al., 1992), this was an important control for the specificity of Cdc42 in filopodiogenesis. We also assessed the Le-Cre conditional mutant for E-cadherin (Cdh1flox) as a general control for involvement of the cadherin-F-actin cytoskeleton. We performed several kinds of analysis on the above mutants. First, we assessed the number of filopodia. Quantification was performed for all the mutants and expressed as an index (filopodia per cell, Fig. 4J) that took into account significantly reduced cell number in the IRSp53-/- lens pits (Fig. 4M). This revealed that at E10.5, Le-Cre; Cdc42flox/flox embryos (Fig. 4B) had a filopodial index of 0.07 (Fig. 4J), as compared with a value of 0.85 (Fig. 4J) for control embryos (Fig. 4A). This represented a greater than 12-fold reduction in the mutant. The IRSp53 (Fig. 4C) and FAK (Fig. 4D) mutants also showed reduced filopodial numbers with indices of 0.19 and 0.42, respectively (Fig. 4J). The Cdc42, IRSp53 and FAK mutants thus represent a spectrum of filopodial deficiency (Fig. 4J). This spectrum of deficiency was similarly observed at E9.5 for the Cdc42 and IRSp53 mutants (Fig. 4N).
Fig. 4.
Filopodia are dependent on Cdc42, IRSp53 and FAK. (A-I) Eye region cryosections from E10.5 mouse embryos labeled for nuclei with Hoechst 33258 (blue), F-actin (A-E, green) or laminin (F-I, green). In A-E, the position of filopodia is indicated by red arrowheads. (J-M) Quantification of filopodial index (J), inter-epithelial distance (K), lens pit depth (L), and lens pit cell number (M) in E10.5 embryos of the indicated genotypes. (N) Quantification of filopodial index in E9.5 embryos of the indicated genotypes. All data points represent n=10. Statistical significance as indicated. Scale bars: 20 μm.
The dramatic loss of filopodia observed in the Cdc42 mutant was confirmed when basal lamina components were labeled. In a control lens pit, labeling for laminin (Fig. 4F,G) or collagen IV (data not shown) showed that filopodia are surrounded by basal lamina and appear as spikes extending from the lens pit. In many cases, these spikes extended to the basal lamina on the surface of the presumptive retina (Fig. 4G). By contrast, in the Cdc42 mutants, the basal laminae of both the lens pit and presumptive retina were relatively smooth (Fig. 4H,I), indicating that filopodia are lost. These data from mutant mice are consistent with those from the analysis of cultured cells showing that Cdc42 and IRSp53 function in the same filopodial pathway (Disanza et al., 2006; Yang et al., 2009). The loss of filopodia in the conditional FAK mutant is consistent with the suggestion that FAK is required for the adhesion of filopodia to the matrix substrate (Partridge and Marcantonio, 2006). The loss of filopodia in the lens-conditional Cdc42 and FAK mutants also confirms the lens pit origin of most filopodia.
Filopodia are contractile and regulate inter-epithelial distance and lens pit depth
One hypothesis for the function of the lens-retina inter-epithelial filopodia is that they are simple physical tethers that help to pull the lens pit down into the optic cup as invagination proceeds. If this were the case, we might expect to see changes in the inter-epithelial distance and the shape of the lens pit in the mutants with reduced numbers of filopodia. We measured inter-epithelial distance at five evenly spaced points around the lens pit of all the mutants and presented these data as an average distance (Fig. 4K). This showed that the inter-epithelial distance is, on average, nearly doubled in the Cdc42 mutant and increased by ∼65% in the IRSp53 mutant. Thus, there is a strong inverse correlation between the filopodial index and the inter-epithelial distance, consistent with the suggestion that filopodia function to position the lens pit within the optic cup. The increased inter-epithelial distance and mispositioning of the lens pit within the optic cup are obvious in some examples of the Cdc42 (Fig. 4B) and IRSp53 (Fig. 4C) mutants. FAK mutants (Fig. 4D) had about half the normal number of filopodia (Fig. 4J) but no change in inter-epithelial distance (Fig. 4K). This might suggest that half the normal number of filopodia is the threshold sufficient to constrain inter-epithelial distance. As might be expected, the increased inter-epithelial distance apparent in the Cdc42 and IRSp53 mutants corresponds to decreased lens pit depth (Fig. 4L).
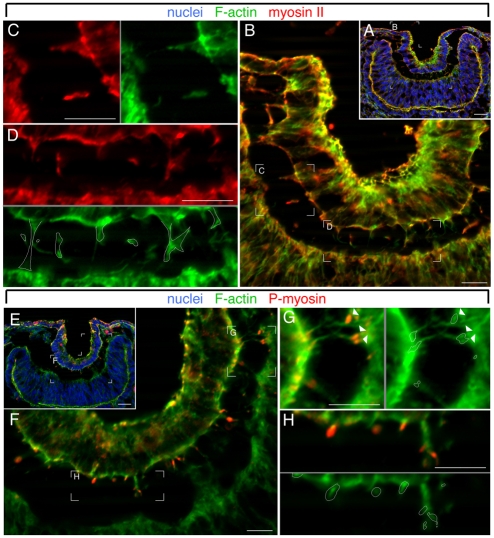
In some settings, filopodia are known to be contractile through an actin-myosin system (Iwadate and Yumura, 2008; Nemethova et al., 2008) and so we determined whether the lens-to-retina filopodia contained myosin. Antibody labeling for non-muscle myosin II showed the expected emphasis at the apices of the conical-shaped cells of the lens pit at E10.5 (Fig. 5A,B). However, in addition, filopodia had concentrations of myosin II immunoreactivity that co-localized with phalloidin-labeled F-actin (Fig. 5C,D).
Fig. 5.
Inter-epithelial filopodia contain active actin-myosin complexes. (A-H) Eye region cryosections from E10.5 mouse embryos labeled for nuclei with Hoechst 33258 (blue), F-actin (green), myosin II (A-D, red) or phospho-myosin II (E-H, red). In C,D, the position of red channel myosin labeling is indicated on the green channel (F-actin) by the white-outlined regions. In G,H, the position of red channel phospho-myosin labeling is indicated on the green channel (F-actin) by the white-outlined regions. In G, phospho-myosin labeling in filopodia that appear to originate in presumptive retina is indicated by arrowheads. A gray line between panels indicates that they are separated color channels of the same image. Scale bars: 20 μm.
The actin-myosin contraction system is dependent on the phosphorylation of myosin (Senju and Miyata, 2009; Takashima, 2009), and labeling with anti-phospho-myosin antibodies can indicate whether the actin-myosin contraction system is active. Use of anti-phospho-myosin antibodies on E10.5 lens pits showed that there were concentrations of phospho-myosin associated with the inter-epithelial filopodia (Fig. 5E-H, red). The distributions of phospho-myosin and myosin II were different, with phosphomyosin appearing in filopodial sub-domains that were typically at the base of the filopodia close to the cell body (Fig. 5G,H, red). Where patches of phospho-myosin labeling were adjacent to the retina (Fig. 5G, arrowheads), the filopodia appeared to originate in the retina.
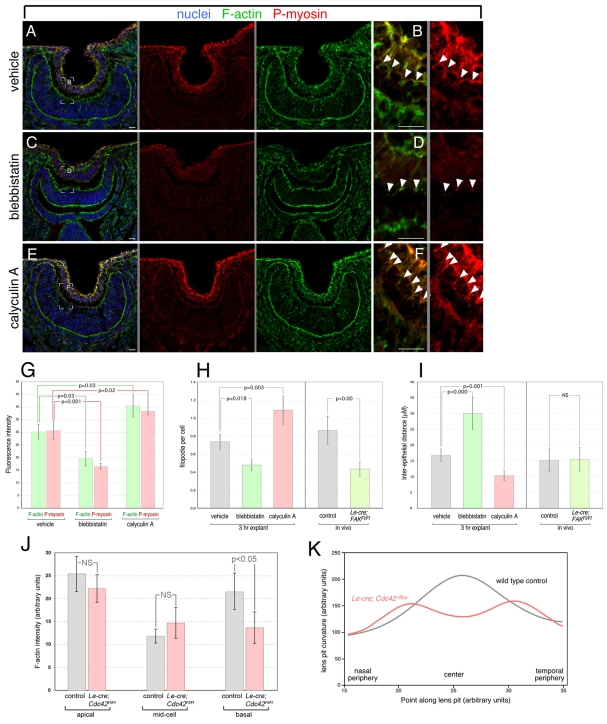
The identification of myosin II and particularly phospho-myosin in filopodia suggested that they might have a contractile function where the inter-epithelial distance might be actively regulated. To test this, we performed a series of 3-hour E10.5 embryo head explants and examined the consequences of pharmacological agents that can both inhibit and enhance myosin-mediated contraction. Blebbistatin is a high-affinity, small-molecule, non-competitive myosin II inhibitor that potently inhibits non-muscle myosins IIA and IIB at concentrations of 0.5 to 5 μM (Limouze et al., 2004). By contrast, calyculin A enhances myosin activity through suppression of the myosin light chain phosphatase (Ishihara et al., 1989).
When these agents were used to treat explants, the anticipated consequences for myosin activity were obvious once cryosections were labeled using anti-phospho-myosin antibodies. Specifically, the level of phospho-myosin was consistently reduced after blebbistatin treatment (Fig. 6C,D, red) as compared with vehicle-treated explants (Fig. 6A,B, red), but was consistently enhanced after calyculin A treatment (Fig. 6E,F, red). The change in phospho-myosin labeling in the lens pit could be quantified (Fig. 6G) and was significantly reduced (blebbistatin) or significantly enhanced (calyculin A). Furthermore, changes in phospho-myosin labeling were mimicked by changes in the levels of F-actin within the lens pit. This was observed most clearly in the apical F-actin complex as detected by phalloidin labeling (Fig. 6A,C,E, green); using F-actin labeling in the optic cup as a visual internal control, it is clear that blebbistatin reduces and calyculin A enhances the intensity of phalloidin labeling (Fig. 6A-F, green). Again, these changes are significant according to quantification (Fig. 6G). The modulation of F-actin and phosphomyosin labeling intensity by blebbistatin and calyculin A serves to validate the action of these agents in the explant.
Fig. 6.
Lens-retina filopodia control inter-epithelial distance and lens pit curvature via actin-myosin contractile activity. (A-F) Eye region cryosections from E10.5 mouse embryo heads cultured for 3 hours in the presence of vehicle (A,B), blebbistatin (C,D) or calyculin A (E,F) and labeled for nuclei with Hoechst 33258 (blue), F-actin (green), and phospho-myosin II (red). The position of phospho-myosin labeling in B, D and F is indicated by arrowheads. A gray line between panels indicates that they are separated color channels of the same image. (G-I) Quantification of lens pit F-actin (green bars) and phospho-myosin (red bars) fluorescence intensity (G), filopodial index (H) and inter-epithelial distance (I) in the eyes of explanted embryo heads after vehicle, blebbistatin or calyculin A treatment. For all data points, n=5. In H and I, data from Le-Cre; FAKflox/flox mutants is represented from Fig. 4 for comparison purposes. (J) Quantification of lens pit F-actin labeling intensity at apical, middle and basal cell positions for E10.5 control and Le-Cre; Cdc42flox/flox mutants as labeled. For all data points, n=5. (K) Quantification of basal position rate of change of curvature from the nasal to temporal side in E10.5 control and Le-Cre; Cdc42flox/flox lens pits. n=6. Scale bars: 20 μm.
Pharmacological inhibition and enhancement of myosin activity provide an opportunity to determine whether intra-filopodial myosin might regulate filopodial length and inter-epithelial distance. We counted filopodia (Fig. 6H) and measured inter-epithelial distance (Fig. 6I) in the eyes of 3-hour explants treated with vehicle, blebbistatin or calyculin A. Blebbistatin had a significant effect in reducing the number of filopodia (Fig. 6H, dark green bar), whereas calyculin A actually increased the filopodial index (Fig. 6H, red bar). These responses suggest that myosin activity is important for extension and retraction of filopodia, as might be expected from analysis of cultured cells (Brahmbhatt and Klemke, 2003; Iwabu et al., 2004). Furthermore, inter-epithelial distance was modified by these agents, being increased by blebbistatin and reduced by calyculin A treatment (Fig. 6I). This helps to confirm that filopodia control inter-epithelial distance. In the case of calyculin A-treated explants, the higher filopodial index makes interpretation equivocal as it is possible that the filopodial number, not contractility per se, is responsible for reduced inter-epithelial distance. For the blebbistatin-treated explants, however, the interpretation can be more definitive because the FAK conditional mutants serve as an excellent control. The FAK mutants had a nearly identical filopodial index to blebbistatin-treated explants (Fig. 6H), but as the FAK mutants showed no change in inter-epithelial distance (Fig. 6I, from Fig. 4) whereas blebbistatin-treated explants did (Fig. 6I), we can assign the difference to inhibition of myosin activity. These data thus suggest that myosin contractile activity regulates filopodial length and inter-epithelial distance.
To determine whether filopodia have an overall effect on lens pit curvature, we performed an analysis in which we generated coordinates from curved lines that represented the basal surface of the lens pit, averaged the curves of multiple (n=6) examples of control and Le-Cre; Cdc42 mutants and then calculated the rate of change of curvature over that line interval. Smoothing functions were applied and the data are represented in Fig. 6K. The control lens pit had an increasing curvature from the lip of the lens pit to the center (Fig. 6K, gray line), whereas in the Cdc42 mutant the increasing curvature in the central lens pit was lost (Fig. 6K, red line). This is consistent with the observation that these lens pits tend to have a flattened base (e.g. Fig. 4H). An assessment of F-actin labeling intensity at the apex, middle and base of control and Cdc42 mutant pit epithelial cells revealed a reduction in the mutant only at the base (Fig. 6J), suggesting that with the exception of the absence of filopodia, there was no dramatic redistribution in their F-actin.
DISCUSSION
In the current study we have investigated the mechanisms of the formation and function of cytoplasmic processes that extend between the presumptive lens and retina during early development of the mouse eye. Our data indicate that these structures are filopodia that tether the two epithelia as a means to coordinate a complex process of morphogenesis.
Lens-retina inter-epithelial processes are Cdc42- and IRSp53-dependent filopodia
Analysis of inter-epithelial processes in the developing eye began in 1902 (Lenhossek, 1902; Magitot, 1910) with the observation of cone-shaped processes extending from the underside of the mammalian lens. Subsequently, Mann (Mann, 1928) drew the processes in a way that suggested they might originate in the presumptive retina (see Fig. 1A). Although we find that some inter-epithelial processes may originate from the retina, both a prior TEM study (McAvoy, 1980) and the current analysis suggest that the majority of the processes originate in the presumptive lens.
The inter-epithelial processes can be defined as filopodia: dynamic, 5-35 μm cellular protrusions that are filled with bundles of parallel actin filaments (McClay, 1999; Raich et al., 1999; Passey et al., 2004; Faix and Rottner, 2006). Our analysis shows them to be rich in F-actin and dynamic in the sense that they exist only between E9 and E12 and can extend and retract when the actin-myosin pathway is manipulated. An earlier study (McAvoy, 1980) showed that equivalent processes in the rat contain F-actin-like filaments and are devoid of cytoplasmic organelles, another characteristic of filopodia. We also showed that these processes do not contain tubulin or acetylated tubulin and so appear to be unrelated to cilia, a class of cytoplasmic protrusion that can function, for example, in sensory perception (Jones and Chen, 2008; McEwen et al., 2008) and in the movement of, or within, fluids (Rosenbaum et al., 1999).
Further evidence that the inter-epithelial extensions are filopodia comes from the demonstration that they are dependent, to varying degrees, on Cdc42, IRSp53 and FAK for their formation and/or maintenance. The Rho family GTPase Cdc42 can have many functions in the cell, but is crucial for the formation of filopodia (Nobes and Hall, 1995; Ridley, 2006). In the formation of filopodia, Cdc42 can function through a variety of so-called effector proteins that include WASP and N-WASP, both members of the Wiskott-Aldrich syndrome gene family (Egile et al., 1999; Banzai et al., 2000; Miki and Takenawa, 2003). In an alternate pathway, Cdc42 can engage the effectors IRSp53 (Yeh et al., 1996) and Eps8 (Disanza et al., 2006) to form filopodia. In either case, the effectors control actin dynamics and result in the fast-growing barbed-end of bundled actin filaments pushing against the plasma membrane and forming filopodia. Since the only known common function for Cdc42 and IRSp53 is in the formation of filopodia, the observation that both Cdc42 and IRSp53 mutant mice have filopodial loss and similar defects in lens pit morphogenesis argues that it is the absence of filopodia and not some other function that is the key change. Although the loss of basal filopodia can be classified as a change in epithelial polarity, other aspects of polarity, such as the apical distribution of the tight junction marker ZO1, were unchanged. This further supports the argument that filopodial loss was the proximal cause of phenotypic change in the Cdc42 conditional mutants.
Filopodia are usually anchored at their tips by cadherin-catenin (Raich et al., 1999; Wood et al., 2002; Partridge and Marcantonio, 2006) or integrin-dependent adhesion complexes (Partridge and Marcantonio, 2006). In the setting of the lens pit, we have shown that deletion of β-catenin does not prevent filopodia from forming, suggesting that cadherin-catenin complexes are not crucial for tip attachment. However, the reduced number of lens-retina filopodia present when FAK is deleted from the lens pit suggests that integrin adhesion to an extracellular matrix (ECM) substrate is important. One possibility is that the laminin- and fibronectin-containing ECM that exists between the presumptive lens and retina is required for the formation of filopodia (Parmigiani and McAvoy, 1984). There might also be a degree of inter-dependence in that filopodia appear to be required for the localization of laminin in the inter-epithelial space. Another model is that integrins at the tip of inter-epithelial filopodia might adhere to the ECM that lies on the basal and opposing side of the presumptive retina. This latter suggestion is consistent with documentation of electron density similar to a junctional complex within retinal cells adjacent to a filopodial tip (McAvoy, 1980). It has also been observed that Cdc42 loss-of-function in cultured cells can result in reduced phosphorylation of FAK (Yang et al., 2006), perhaps suggesting a more direct connection in filopodial function.
Several signaling pathways that are required for the induction and early development of the lens have been identified. These include the Bmp7 (Wawersik et al., 1999), Bmp4 (Furuta and Hogan, 1998), Fgf (Faber et al., 2001; Gotoh et al., 2004) and Notch (Ogino et al., 2008) signaling pathways. When the Bmp4, Bmp7 and Fgf pathways are compromised in the mouse eye, phenotypes can be variable, but in their most severe form there is a complete loss of eye morphogenesis beginning with a failure of lens placode formation. Since it is well known that Rho family GTPases can be regulated by receptor kinase signaling pathways, it is likely that one or more of these pathways will be required for the generation of the filopodia in the early eye. Indeed, the timing of filopodiogenesis at E9.0-9.5 closely follows lens induction signaling that is indicated, for example, by the upregulation of placodal Pax6 (Grindley et al., 1995; Williams et al., 1998) and FoxE3 (Medina-Martinez and Jamrich, 2007) expression at E8.75. Further work will be required to determine whether Cdc42 and IRSp53 are regulated by these lens induction pathways.
Lens pit filopodia are contractile via the actin-myosin pathway
The presence of myosin II and phospho-myosin in the lens pit filopodia suggested that these processes might have an active contraction function. This was tested directly using pharmacological agents that either suppress or enhance actin-myosin contraction. When blebbistatin was used to inhibit myosin activity, lens pit filopodia became longer and the inter-epithelial distance greater. By contrast, the myosin activity enhancer calyculin A resulted in shorter filopodia. Since these assays were carried out over the course of just 3 hours, this suggested that the filopodia were able to respond rapidly to regulation of the actin-myosin pathway and that they are likely to have the capacity to be regulated dynamically in vivo.
We also observed that with blebbistatin and calyculin A treatment, rapid changes in filopodial length translated into rapid changes in inter-epithelial distance. This makes a clear case that these filopodia can generate sufficient force to control the distance between the two epithelia. Coupled to the observation that loss of filopodia in Cdc42 and IRSp53 mutants results in reduced invagination and changes in lens pit shape, the data make a strong case that one function of these processes is to transmit invagination forces between presumptive lens and retina during coordinated invagination.
In cultured cells, myosin II and filopodia have been implicated in the regulation of cell migration (Nemethova et al., 2008) and it has been proposed that F-actin bundles from filopodia provide building blocks for the contractile complex. However, in this setting, myosin II does not appear to be within filopodia, but instead is localized in lamellae some distance from the migrating cell front. In lens pit basal filopodia, myosin II and phospho-myosin are localized within the base of filopodia. Although this myosin distribution clearly equips the structures to transmit force in a dynamically regulated way, it does represent a distinction from migrating cultured cells. Further studies will be required to understand whether this distinction extends to the underlying mechanisms of contraction.
Interestingly, although we could easily detect the anticipated blebbistatin- and calyculin A-induced changes in the levels of phospho-myosin in the lens pit, there were no changes and indeed no easily detectable level of phospho-myosin in the optic cup. This suggests that the presumptive retina uses a different mechanism for invagination and might be consistent with the opposite polarity of presumptive lens and retinal epithelia. In the case of the lens pit, where the apical end of the cells faces outwards, established mechanisms of apical constriction (Haigo et al., 2003; Wallingford, 2005) are appropriate to generate the conically shaped cells that accompany invagination in the basal direction (Hendrix and Zwaan, 1974) (Fig. 7). By contrast, where invagination occurs in the apical direction, a different mechanism must be employed. Recent work in Medaka (Martinez-Morales et al., 2009) has identified a gene, ojoplano, that is required for the formation of basal junctional complexes, for basal constriction in cells of the presumptive retina and for overall morphogenesis of the optic cup. Although there are morphological distinctions between mouse and fish eye morphogenesis, it is likely that the morphogenesis pathway defined by the mouse ojoplano ortholog will function in cooperation with the Cdc42/IRSp53-dependent filopodia of the lens pit to regulate eye morphogenesis.
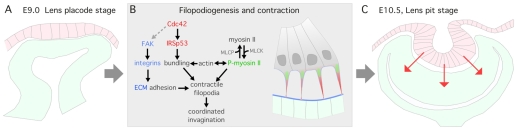
Fig. 7.
A model: Cdc42/IRSp53-dependent filopodia fine-tune coordinated invagination of presumptive lens and retina. This schematic summarizes the function, mechanism of formation and contractile activity of lens-retina inter-epithelial filopodia. (A) The filopodia form at ∼E9.0 to connect the lens placode within the embryonic surface ectoderm to the presumptive retina within the optic cup. (B) Formation of the filopodia requires Cdc42 and IRSp53 (red). We propose that phosphorylated myosin II (green) positioned in the base of the filopodia is one component of a larger actin-myosin contractile complex that regulates the inter-epithelial distance via filopodial length. We further suggest that FAK-dependent integrin adhesion might be required to anchor filopodia to the ECM (blue). (C) Based on the failure of full lens pit invagination in mutants that are deficient in filopodia and on the increased inter-epithelial distance when myosin II activity is inhibited, it is likely that the filopodia and the actin-myosin complexes within are crucial to transmitting the physical forces that partly mediate a coordinated morphogenetic process.
Contractile filopodia have been reported to be crucial for sea urchin gastrulation (Hardin and McClay, 1990; Malinda et al., 1995; Miller et al., 1995). After the thickened vegetal plate invaginates to form a short stub-like gut rudiment named the archenteron, secondary mesenchymal cells (SMCs) migrate onto the archenteron tip. These cells then extend filopodia to the apical plate. Laser ligation (Hardin, 1988) has shown that the filopodia help to pull the archenteron across the blastocoel cavity. If the filopodia of the SMCs are eliminated, about one-third of the invagination distance is lost. This represents a similar circumstance to the one we describe here, in which filopodial contraction assists invagination and coordinates morphogenesis.
We thank Paul Speeg and Albino Troilo for excellent technical assistance. We acknowledge grant support from Associazione Italiana Ricerca sul Cancro to G.S. (2006-2009 and PRIN2007), from FIRC to A.D., from the NIH to H.E.B. (EY0117379), Y.Z. (HL085362) and R.A.L. (EY15766, EY16241, EY17848, CA131270) and from the Abrahamson Pediatric Eye Institute Endowment at Children's Hospital Medical Center of Cincinnati to R.A.L. Deposited in PMC for release after 12 months.
References
- Ashery-Padan, R., Marquardt, T., Zhou, X. and Gruss, P. (2000). Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banzai, Y., Miki, H., Yamaguchi, H. and Takenawa, T. (2000). Essential role of neural Wiskott-Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J. Biol. Chem. 275, 11987-11992. [DOI] [PubMed] [Google Scholar]
- Beggs, H. E., Schahin-Reed, D., Zang, K., Goebbels, S., Nave, K. A., Gorski, J., Jones, K. R., Sretavan, D. and Reichardt, L. F. (2003). FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40, 501-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia, O., Kutsch, S., Hierholzer, A., Delmas, V. and Kemler, R. (2002). E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 115, 53-62. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt, A. A. and Klemke, R. L. (2003). ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J. Biol. Chem. 278, 13016-13025. [DOI] [PubMed] [Google Scholar]
- Burridge, K. and Wennerberg, K. (2004). Rho and Rac take center stage. Cell 116, 167-179. [DOI] [PubMed] [Google Scholar]
- Chen, L., Liao, G., Yang, L., Campbell, K., Nakafuku, M., Kuan, C. Y. and Zheng, Y. (2006). Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc. Natl. Acad. Sci. USA 103, 16520-16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, R. L. and Lang, R. A. (2001). Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 17, 255-296. [DOI] [PubMed] [Google Scholar]
- Davidson, L. A., Koehl, M. A., Keller, R. and Oster, G. F. (1995). How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development 121, 2005-2018. [DOI] [PubMed] [Google Scholar]
- Disanza, A., Mantoani, S., Hertzog, M., Gerboth, S., Frittoli, E., Steffen, A., Berhoerster, K., Kreienkamp, H. J., Milanesi, F., Di Fiore, P. P. et al. (2006). Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat. Cell Biol. 8, 1337-1347. [DOI] [PubMed] [Google Scholar]
- Egile, C., Loisel, T. P., Laurent, V., Li, R., Pantaloni, D., Sansonetti, P. J. and Carlier, M. F. (1999). Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146, 1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville, S. (2004). Cdc42-the centre of polarity. J. Cell Sci. 117, 1291-1300. [DOI] [PubMed] [Google Scholar]
- Faber, S. C., Dimanlig, P., Makarenkova, H. P., Shirke, S., Ko, K. and Lang, R. A. (2001). Fgf receptor signaling plays a role in lens induction. Development 128, 4425-4438. [DOI] [PubMed] [Google Scholar]
- Faix, J. and Rottner, K. (2006). The making of filopodia. Curr. Opin. Cell Biol. 18, 18-25. [DOI] [PubMed] [Google Scholar]
- Fazioli, F., Minichiello, L., Matoska, V., Castagnino, P., Miki, T., Wong, W. T. and Di Fiore, P. P. (1993). Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. EMBO J. 12, 3799-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta, Y. and Hogan, B. L. M. (1998). BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12, 3764-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, N., Ito, M., Yamamoto, S., Yoshino, I., Song, N., Wang, Y., Lax, I., Schlessinger, J., Shibuya, M. and Lang, R. A. (2004). Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc. Natl. Acad. Sci. USA 101, 17144-17149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley, J. C., Davidson, D. R. and Hill, R. E. (1995). The role of Pax-6 in eye and nasal development. Development 121, 1433-1442. [DOI] [PubMed] [Google Scholar]
- Gu, Y., Filippi, M. D., Cancelas, J. A., Siefring, J. E., Williams, E. P., Jasti, A. C., Harris, C. E., Lee, A. W., Prabhakar, R., Atkinson, S. J. et al. (2003). Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302, 445-449. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622-634. [DOI] [PubMed] [Google Scholar]
- Haigo, S. L., Hildebrand, J. D., Harland, R. M. and Wallingford, J. B. (2003). Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr. Biol. 13, 2125-2137. [DOI] [PubMed] [Google Scholar]
- Hardin, J. (1988). The role of secondary mesenchyme cells during sea urchin gastrulation studied by laser ablation. Development 103, 317-324. [DOI] [PubMed] [Google Scholar]
- Hardin, J. and Keller, R. (1988). The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development 103, 211-230. [DOI] [PubMed] [Google Scholar]
- Hardin, J. and McClay, D. R. (1990). Target recognition by the archenteron during sea urchin gastrulation. Dev. Biol. 142, 86-102. [DOI] [PubMed] [Google Scholar]
- Hendrix, R. W. and Zwaan, J. (1974). Cell shape regulation and cell cycle in embryonic lens cells. Nature 247, 145-147. [DOI] [PubMed] [Google Scholar]
- Higgs, H. N. and Pollard, T. D. (1999). Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J. Biol. Chem. 274, 32531-32534. [DOI] [PubMed] [Google Scholar]
- Ishihara, H., Martin, B. L., Brautigan, D. L., Karaki, H., Ozaki, H., Kato, Y., Fusetani, N., Watabe, S., Hashimoto, K., Uemura, D. et al. (1989). Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 159, 871-877. [DOI] [PubMed] [Google Scholar]
- Iwabu, A., Smith, K., Allen, F. D., Lauffenburger, D. A. and Wells, A. (2004). Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J. Biol. Chem. 279, 14551-14560. [DOI] [PubMed] [Google Scholar]
- Iwadate, Y. and Yumura, S. (2008). Molecular dynamics and forces of a motile cell simultaneously visualized by TIRF and force microscopies. Biotechniques 44, 739-750. [DOI] [PubMed] [Google Scholar]
- Jaffe, A. B. and Hall, A. (2005). Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247-269. [DOI] [PubMed] [Google Scholar]
- Jaffer, Z. M. and Chernoff, J. (2004). The cross-Rho'ds of cell-cell adhesion. J. Biol. Chem. 279, 35123-35126. [DOI] [PubMed] [Google Scholar]
- Jones, C. and Chen, P. (2008). Primary cilia in planar cell polarity regulation of the inner ear. Curr. Top. Dev. Biol. 85, 197-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi, K. (1999). Regulation of cytoskeleton and cell adhesion by Rho targets. Prog. Mol. Subcell. Biol. 22, 23-38. [DOI] [PubMed] [Google Scholar]
- Kasper, M. and Viebahn, C. (1992). Cytokeratin expression and early lens development. Anat. Embryol. (Berl.) 186, 285-290. [DOI] [PubMed] [Google Scholar]
- Kimberly, E. L. and Hardin, J. (1998). Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev. Biol. 204, 235-250. [DOI] [PubMed] [Google Scholar]
- Krugmann, S., Jordens, I., Gevaert, K., Driessens, M., Vandekerckhove, J. and Hall, A. (2001). Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr. Biol. 11, 1645-1655. [DOI] [PubMed] [Google Scholar]
- Lecuit, T. and Lenne, P. F. (2007). Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633-644. [DOI] [PubMed] [Google Scholar]
- Lenhossek. (1902). Die Entwicklung des Glaskorpers Vortr. Kgl. Akad. Wissensch. Budapest.
- Leptin, M. and Grunewald, B. (1990). Cell shape changes during gastrulation in Drosophila. Development 110, 73-84. [DOI] [PubMed] [Google Scholar]
- Limouze, J., Straight, A. F., Mitchison, T. and Sellers, J. R. (2004). Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 25, 337-341. [DOI] [PubMed] [Google Scholar]
- Macara, I. G. (2004). Parsing the polarity code. Nat. Rev. Mol. Cell Biol. 5, 220-231. [DOI] [PubMed] [Google Scholar]
- Machesky, L. M. and Insall, R. H. (1998). Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347-1356. [DOI] [PubMed] [Google Scholar]
- Maddala, R., Deng, P. F., Costello, J. M., Wawrousek, E. F., Zigler, J. S. and Rao, V. P. (2004). Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab. Invest. 84, 679-692. [DOI] [PubMed] [Google Scholar]
- Magitot, A. (1910). Etude sur le developpement de la retine humaine. Ann. Ocul. 143, 241-282. [Google Scholar]
- Malinda, K. M., Fisher, G. W. and Ettensohn, C. A. (1995). Four-dimensional microscopic analysis of the filopodial behavior of primary mesenchyme cells during gastrulation in the sea urchin embryo. Dev. Biol. 172, 552-566. [DOI] [PubMed] [Google Scholar]
- Mann, I. (1928). The Development of the Human Eye. New York: Grune and Stratton.
- Mann, I. (1950). The Development of the Human Eye. New York: Grune and Stratton.
- Martin-Blanco, E., Pastor-Pareja, J. C. and Garcia-Bellido, A. (2000). JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. USA 97, 7888-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales, J. R., Rembold, M., Greger, K., Simpson, J. C., Brown, K. E., Quiring, R., Pepperkok, R., Martin-Bermudo, M. D., Himmelbauer, H. and Wittbrodt, J. (2009). ojoplano-mediated basal constriction is essential for optic cup morphogenesis. Development 136, 2165-2175. [DOI] [PubMed] [Google Scholar]
- McAvoy, J. W. (1980). Cytoplasmic processes interconnect lens placode and optic vesicle during eye morphogenesis. Exp. Eye Res. 31, 527-534. [DOI] [PubMed] [Google Scholar]
- McClay, D. R. (1999). The role of thin filopodia in motility and morphogenesis. Exp. Cell Res. 253, 296-301. [DOI] [PubMed] [Google Scholar]
- McEwen, D. P., Jenkins, P. M. and Martens, J. R. (2008). Olfactory cilia: our direct neuronal connection to the external world. Curr. Top. Dev. Biol. 85, 333-370. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez, O. and Jamrich, M. (2007). Foxe view of lens development and disease. Development 134, 1455-1463. [DOI] [PubMed] [Google Scholar]
- Miki, H. and Takenawa, T. (2003). Regulation of actin dynamics by WASP family proteins. J. Biochem. (Tokyo) 134, 309-313. [DOI] [PubMed] [Google Scholar]
- Miki, H., Sasaki, T., Takai, Y. and Takenawa, T. (1998). Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391, 93-96. [DOI] [PubMed] [Google Scholar]
- Miller, J., Fraser, S. E. and McClay, D. (1995). Dynamics of thin filopodia during sea urchin gastrulation. Development 121, 2501-2511. [DOI] [PubMed] [Google Scholar]
- Nemethova, M., Auinger, S. and Small, J. V. (2008). Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella. J. Cell Biol. 180, 1233-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C. D. and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53-62. [DOI] [PubMed] [Google Scholar]
- Odell, G. M., Oster, G., Alberch, P. and Burnside, B. (1981). The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev. Biol. 85, 446-462. [DOI] [PubMed] [Google Scholar]
- Ogino, H., Fisher, M. and Grainger, R. M. (2008). Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development 135, 249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani, C. and McAvoy, J. W. (1984). Localisation of laminin and fibronectin during rat lens morphogenesis. Differentiation 28, 53-61. [DOI] [PubMed] [Google Scholar]
- Partridge, M. A. and Marcantonio, E. E. (2006). Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol. Biol. Cell 17, 4237-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passey, S., Pellegrin, S. and Mellor, H. (2004). What is in a filopodium? Starfish versus hedgehogs. Biochem. Soc. Trans. 32, 1115-1117. [DOI] [PubMed] [Google Scholar]
- Peng, J., Wallar, B. J., Flanders, A., Swiatek, P. J. and Alberts, A. S. (2003). Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr. Biol. 13, 534-545. [DOI] [PubMed] [Google Scholar]
- Raich, W. B., Agbunag, C. and Hardin, J. (1999). Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr. Biol. 9, 1139-1146. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J. (2006). Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522-529. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J. and Hall, A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389-399. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. and Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401-410. [DOI] [PubMed] [Google Scholar]
- Rohatgi, R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T. and Kirschner, M. W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221-231. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, J. L., Cole, D. G. and Diener, D. R. (1999). Intraflagellar transport: the eyes have it. J. Cell Biol. 144, 385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawallisch, C., Berhorster, K., Disanza, A., Mantoani, S., Kintscher, M., Stoenica, L., Dityatev, A., Sieber, S., Kindler, S., Morellini, F. et al. (2009). The insulin receptor substrate of 53 kDa (IRSp53) limits hippocampal synaptic plasticity. J. Biol. Chem. 284, 9225-9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, T. E. (1970). Neurulation in Xenopus laevis: an analysis and model based upon light and electron microscopy. J. Embryol. Exp. Morphol. 23, 427-462. [PubMed] [Google Scholar]
- Seifert, J. R. and Mlodzik, M. (2007). Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126-138. [DOI] [PubMed] [Google Scholar]
- Senju, Y. and Miyata, H. (2009). The role of actomyosin contractility in the formation and dynamics of actin bundles during fibroblast spreading. J. Biochem. 145, 137-150. [DOI] [PubMed] [Google Scholar]
- Silverman, M. A. and Leroux, M. R. (2009). Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 19, 306-316. [DOI] [PubMed] [Google Scholar]
- Smith, A. N., Miller, L. A., Song, N., Taketo, M. M. and Lang, R. A. (2005). The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev. Biol. 285, 477-489. [DOI] [PubMed] [Google Scholar]
- Smith, A. N., Miller, L. A., Radice, G., Ashery-Padan, R. and Lang, R. A. (2009). Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development 136, 2977-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper, S. B., Takeshima, F., Anton, I., Liu, C. H., Thomas, S. M., Nguyen, D., Dudley, D., Fraser, H., Purich, D., Lopez-Ilasaca, M. et al. (2001). N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat. Cell Biol. 3, 897-904. [DOI] [PubMed] [Google Scholar]
- Stevenson, B. R., Siliciano, J. D., Mooseker, M. S. and Goodenough, D. A. (1986). Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103, 755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima, S. (2009). Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction. Circ. J. 73, 208-213. [DOI] [PubMed] [Google Scholar]
- Wallingford, J. B. (2005). Neural tube closure and neural tube defects: studies in animal models reveal known knowns and known unknowns. Am. J. Med. Genet. C Semin. Med. Genet. 135C, 59-68. [DOI] [PubMed] [Google Scholar]
- Wawersik, S., Purcell, P., Rauchman, M., Dudley, A. T., Robertson, E. J. and Maas, R. (1999). BMP7 Acts in Murine Lens Placode Development. Dev. Biol. 207, 176-188. [DOI] [PubMed] [Google Scholar]
- Weiss, S. M., Ladwein, M., Schmidt, D., Ehinger, J., Lommel, S., Stading, K., Beutling, U., Disanza, A., Frank, R., Jansch, L. et al. (2009). IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe 5, 244-258. [DOI] [PubMed] [Google Scholar]
- Williams, S. C., Altmann, C. R., Chow, R. L., Hemmati-Brivanlou, A. and Lang, R. A. (1998). A highly conserved lens transcriptional control element from the Pax-6 gene. Mech. Dev. 73, 225-229. [DOI] [PubMed] [Google Scholar]
- Williams-Masson, E. M., Malik, A. N. and Hardin, J. (1997). An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 124, 2889-2901. [DOI] [PubMed] [Google Scholar]
- Wood, W., Jacinto, A., Grose, R., Woolner, S., Gale, J., Wilson, C. and Martin, P. (2002). Wound healing recapitulates morphogenesis in Drosophila embryos. Nat. Cell Biol. 4, 907-912. [DOI] [PubMed] [Google Scholar]
- Yang, C., Hoelzle, M., Disanza, A., Scita, G. and Svitkina, T. (2009). Coordination of membrane and actin cytoskeleton dynamics during filopodia protrusion. PLoS ONE 4, e5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L., Wang, L. and Zheng, Y. (2006). Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol. Biol. Cell 17, 4675-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, T. C., Ogawa, W., Danielsen, A. G. and Roth, R. A. (1996). Characterization and cloning of a 58/53-kDa substrate of the insulin receptor tyrosine kinase. J. Biol. Chem. 271, 2921-2928. [DOI] [PubMed] [Google Scholar]