Abstract
Plasmalemmal vesicles (PVs) or caveolae are plasma membrane invaginations and associated vesicles of regular size and shape found in most mammalian cell types. They are particularly numerous in the continuous endothelium of certain microvascular beds (e.g., heart, lung, and muscles) in which they have been identified as transcytotic vesicular carriers. Their chemistry and function have been extensively studied in the last years by various means, including several attempts to isolate them by cell fractionation from different cell types. The methods so far used rely on nonspecific physical parameters of the caveolae and their membrane (e.g., size-specific gravity and solubility in detergents) which do not rule out contamination from other membrane sources, especially the plasmalemma proper. We report here a different method for the isolation of PVs from plasmalemmal fragments obtained by a silica-coating procedure from the rat lung vasculature. The method includes sonication and flotation of a mixed vesicle fraction, as the first step, followed by specific immunoisolation of PVs on anticaveolin-coated magnetic microspheres, as the second step. The mixed vesicle fraction, is thereby resolved into a bound subfraction (B), which consists primarily of PVs or caveolae, and a nonbound subfraction (NB) enriched in vesicles derived from the plasmalemma proper. The results so far obtained indicate that some specific endothelial membrane proteins (e.g., thrombomodulin, functional thrombin receptor) are distributed about evenly between the B and NB subfractions, whereas others are restricted to the NB subfraction (e.g., angiotensin converting enzyme, podocalyxin). Glycoproteins distribute unevenly between the two subfractions and antigens involved in signal transduction [e.g., annexin II, protein kinase C alpha, the G alpha subunits of heterotrimeric G proteins (alpha s, alpha q, alpha i2, alpha i3), small GTP-binding proteins, endothelial nitric oxide synthase, and nonreceptor protein kinase c-src] are concentrated in the NB (plasmalemma proper-enriched) subfraction rather than in the caveolae of the B subfraction. Additional work should show whether discrepancies between our findings and those already recorded in the literature represent inadequate fractionation techniques or are accounted for by chemical differentiation of caveolae from one cell type to another.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. R., Palade G. E. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968 May;37(2):244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. J., Ying Y. S., Rothberg K. G., Hooper N. M., Turner A. J., Gambliel H. A., De Gunzburg J., Mumby S. M., Gilman A. G., Anderson R. G. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994 Jul;126(1):127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P., Parton R. G., Raposo G., Kurzchalia T. V., Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993 Apr;12(4):1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A. M., Williamson E., Simons K., Parton R. G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994 Dec 9;269(49):30745–30748. [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cardeña G., Oh P., Liu J., Schnitzer J. E., Sessa W. C. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Bendayan M. Transendothelial transport of serum albumin: a quantitative immunocytochemical study. J Cell Biol. 1992 May;117(4):745–755. doi: 10.1083/jcb.117.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Galis Z., Simionescu M., Simionescu N. Differentiated uptake and transcytosis of albumin in successive vascular segments. J Submicrosc Cytol Pathol. 1988 Oct;20(4):657–669. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodinsky A., Harris D. A. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995 May;129(3):619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat R., Hovorka A., Dekan G., Poczewski H., Kerjaschki D. Endothelial cell membranes contain podocalyxin--the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986 Feb;102(2):484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat R., Palade G. E. The functional thrombin receptor is associated with the plasmalemma and a large endosomal network in cultured human umbilical vein endothelial cells. J Cell Sci. 1995 Mar;108(Pt 3):1155–1164. doi: 10.1242/jcs.108.3.1155. [DOI] [PubMed] [Google Scholar]
- Horvat R., Palade G. E. Thrombomodulin and thrombin localization on the vascular endothelium; their internalization and transcytosis by plasmalemmal vesicles. Eur J Cell Biol. 1993 Aug;61(2):299–313. [PubMed] [Google Scholar]
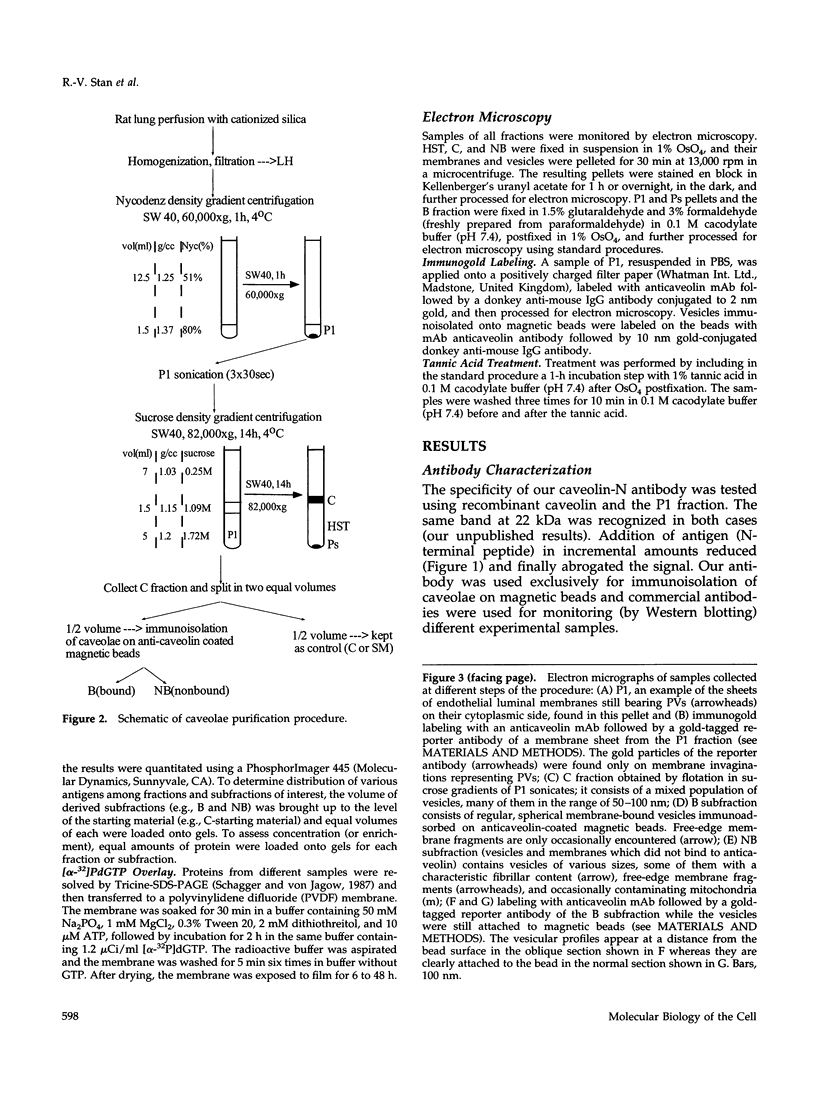
- Jacobson B. S., Schnitzer J. E., McCaffery M., Palade G. E. Isolation and partial characterization of the luminal plasmalemma of microvascular endothelium from rat lungs. Eur J Cell Biol. 1992 Aug;58(2):296–306. [PubMed] [Google Scholar]
- Kurzchalia T. V., Dupree P., Parton R. G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992 Sep;118(5):1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F., Simons K., Ikonen E. Dissecting the molecular mechanisms of polarized membrane traffic: reconstitution of three transport steps in epithelial cells using streptolysin-O permeabilization. Cold Spring Harb Symp Quant Biol. 1995;60:753–762. doi: 10.1101/sqb.1995.060.01.081. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994 Jul;126(1):111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Tang Z., Scherer P. E., Sargiacomo M. Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 1995;250:655–668. doi: 10.1016/0076-6879(95)50103-7. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994 Feb;42(2):155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
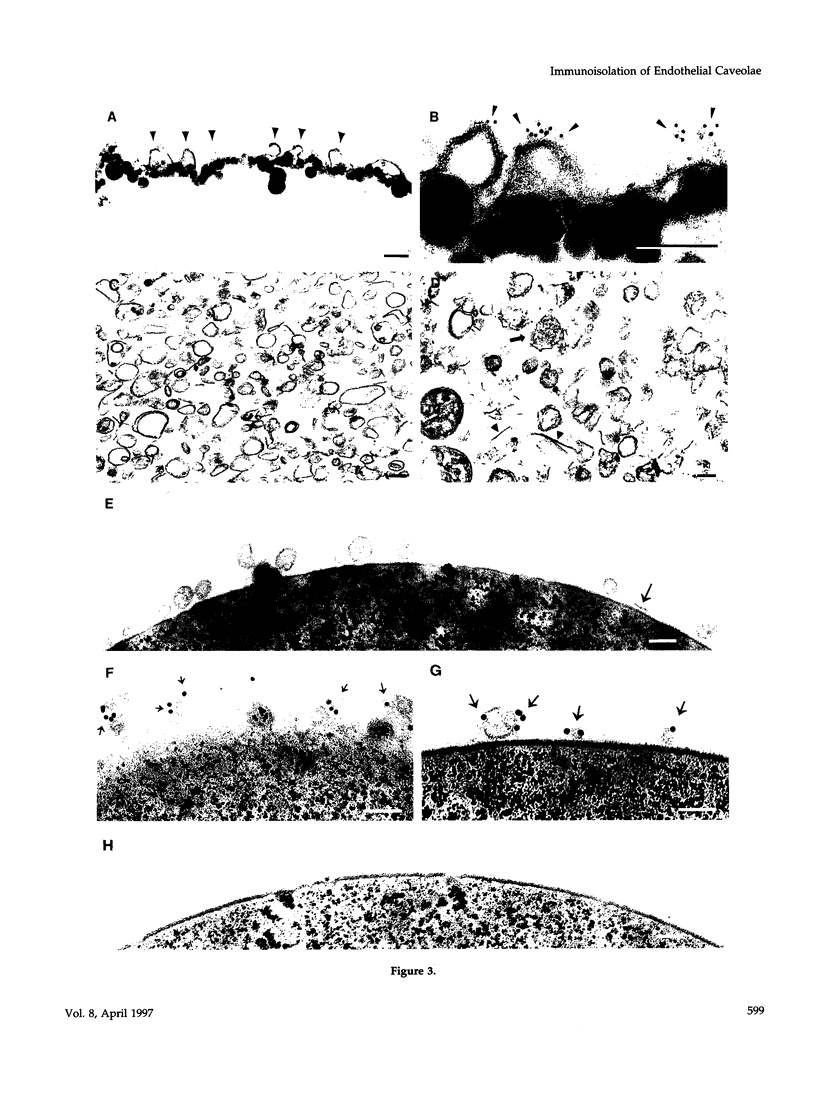
- Peters K. R., Carley W. W., Palade G. E. Endothelial plasmalemmal vesicles have a characteristic striped bipolar surface structure. J Cell Biol. 1985 Dec;101(6):2233–2238. doi: 10.1083/jcb.101.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu D., Horvat R., Predescu S., Palade G. E. Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3014–3018. doi: 10.1073/pnas.91.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S., Schultz D. R., Whitaker C., Chung A. Subcellular localization of pulmonary antiotensin-converting enzyme (kininase II). Biochem J. 1975 Feb;146(2):497–499. doi: 10.1042/bj1460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucan L., Palade G. E. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicular carriers. J Cell Biol. 1994 May;125(4):733–741. doi: 10.1083/jcb.125.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
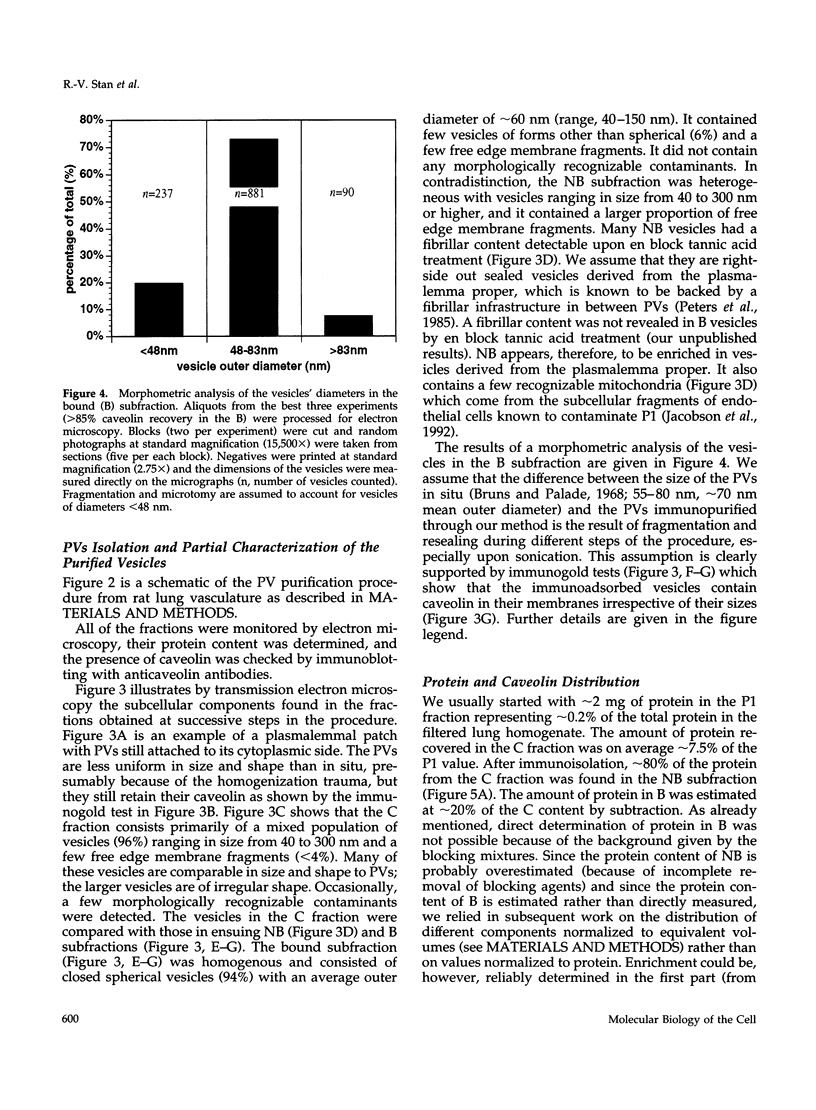
- Scherer P. E., Lisanti M. P., Baldini G., Sargiacomo M., Mastick C. C., Lodish H. F. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994 Dec;127(5):1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Liu J., Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995 Jun 16;270(24):14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., McIntosh D. P., Dvorak A. M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995 Sep 8;269(5229):1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
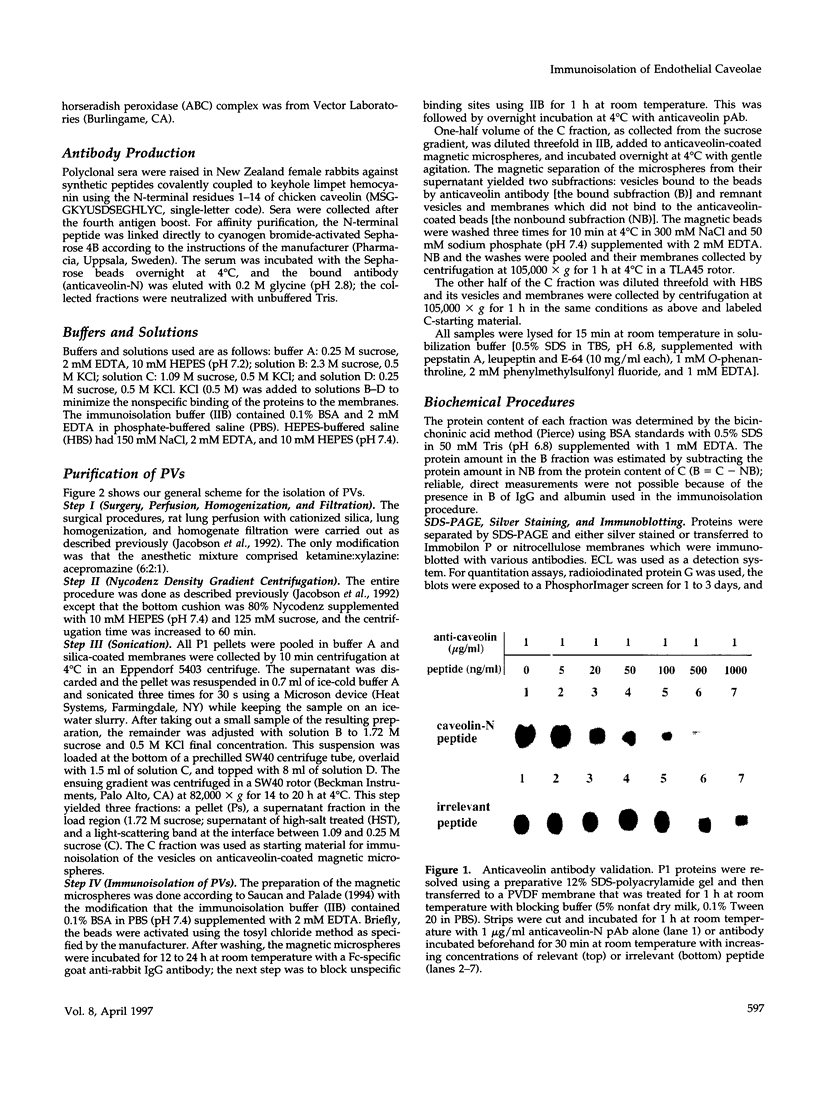
- Schnitzer J. E., Oh P., Jacobson B. S., Dvorak A. M. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1759–1763. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
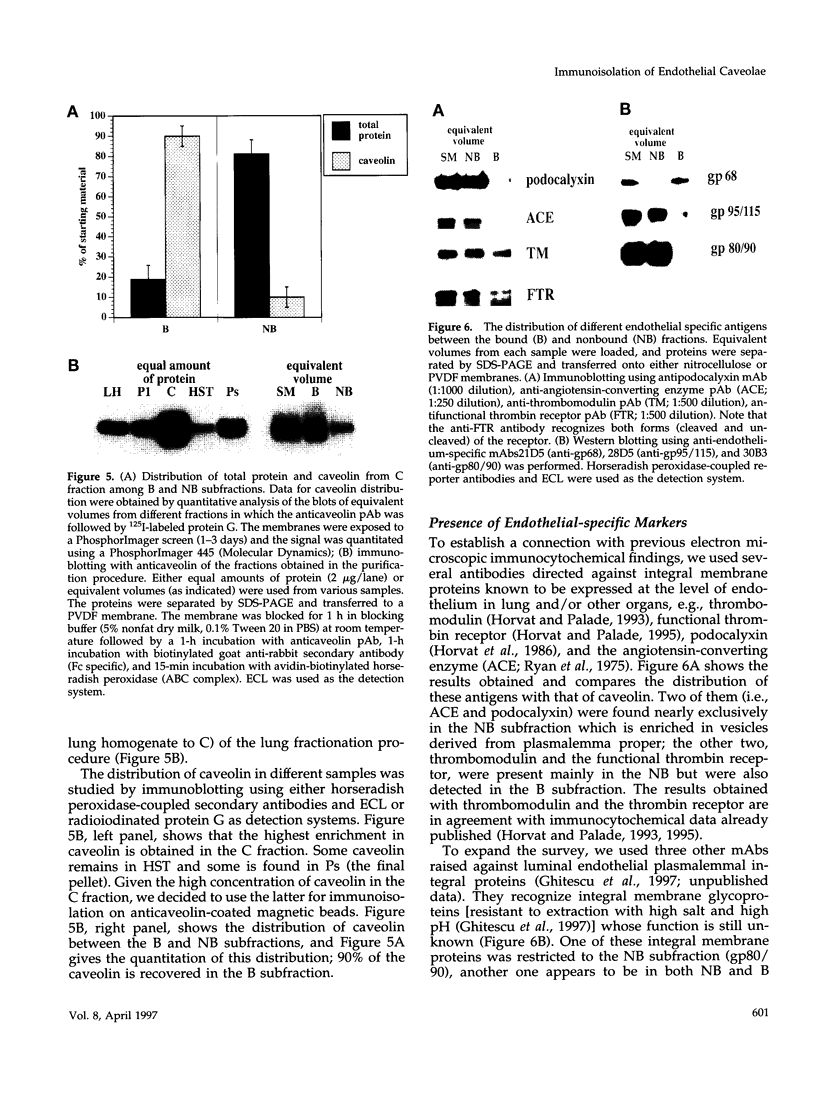
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996 Mar 15;271(11):6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Dietzen D. J., Kwong J., Link D. C., Lublin D. M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994 Jul;126(2):353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Mineo C., Anderson R. G. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A., Mueller B. M. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995 Apr;129(2):335–344. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955 Sep 25;1(5):445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]