Abstract
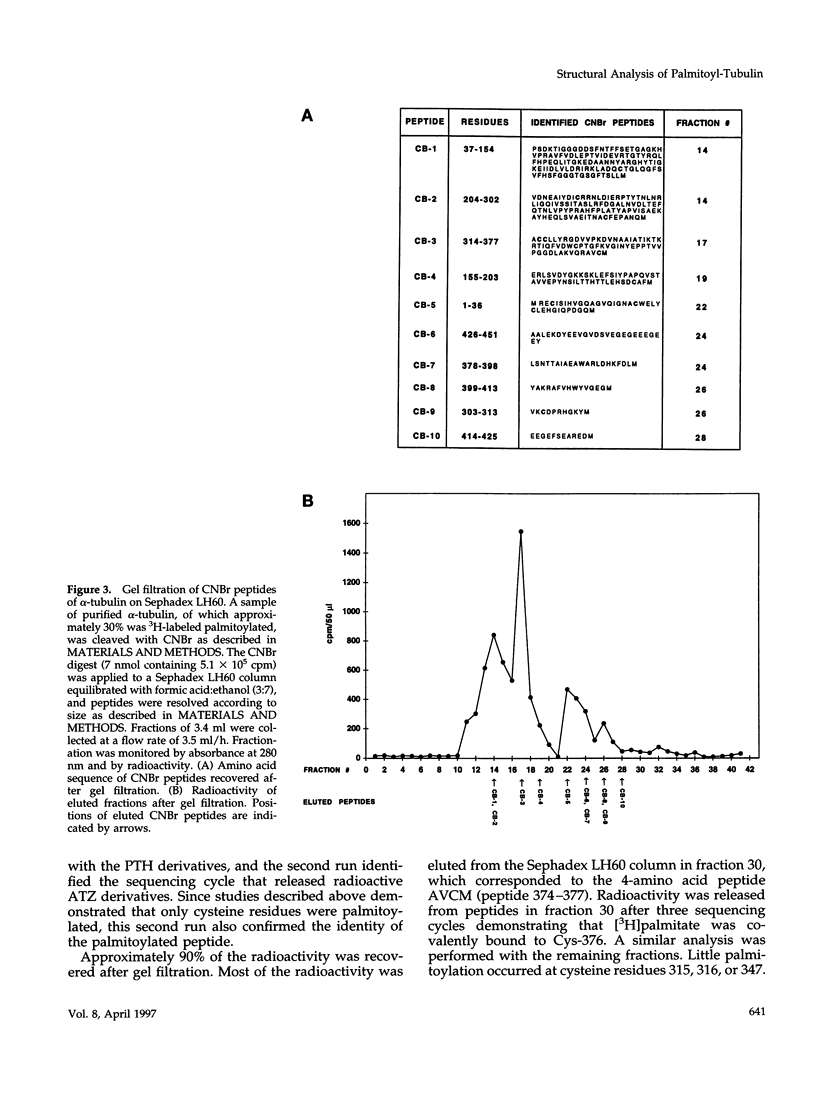
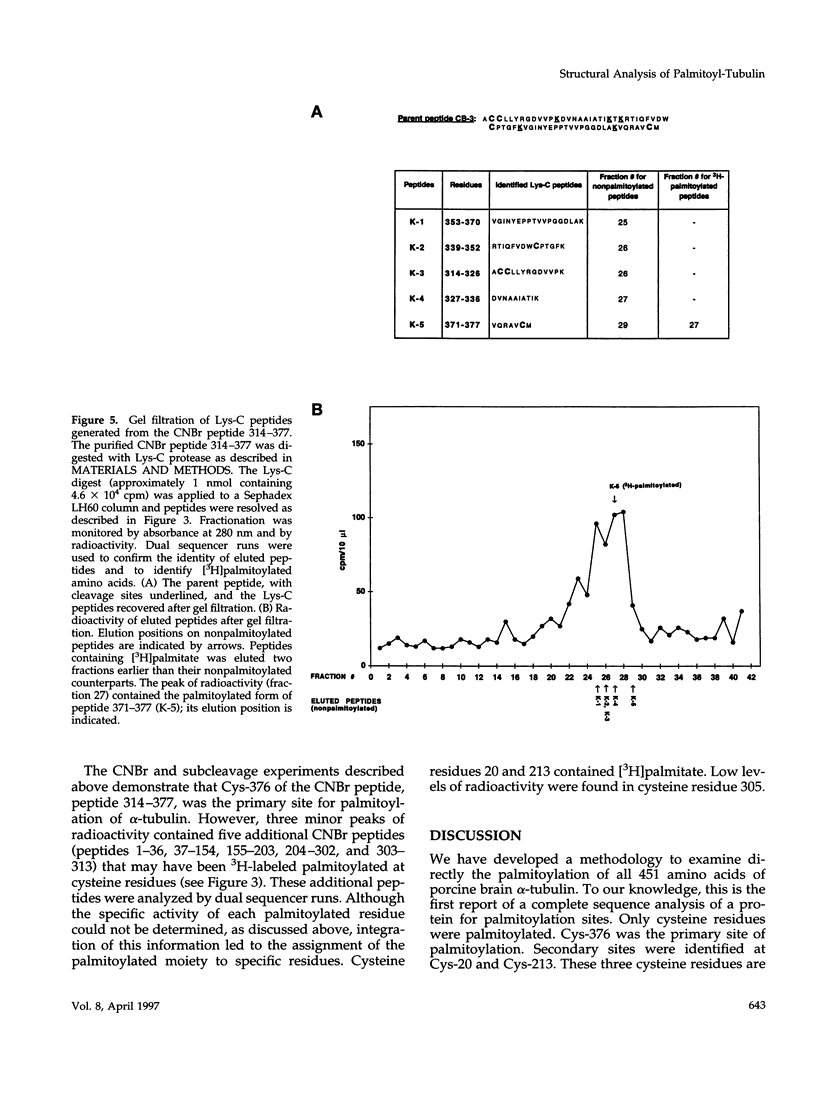
As shown in the companion article, tubulin is posttranslationally modified in vivo by palmitoylation. Our goal in this study was to identify the palmitoylation sites by protein structure analysis. To obtain quantities of palmitoylated tubulin required for this analysis, a cell-free system for enzymatic [3H]palmitoylation was developed and characterized in our companion article. We then developed a methodology to examine directly the palmitoylation of all 451 amino acids of alpha-tubulin. 3H-labeled palmitoylated alpha-tubulin was cleaved with cyanogen bromide (CNBr). The CNBr digest was resolved according to peptide size by gel filtration on Sephadex LH60 in formic acid:ethanol. The position of 3H-labeled palmitoylated amino acids in peptides could not be identified by analysis of the Edman degradation sequencer product because the palmitoylated sequencer products were lost during the final derivatization step to phenylthiohydantoin derivatives. Modification of the gas/liquid-phase sequencer to deliver the intermediate anilinothiozolinone derivative, rather than the phenylthiohydantoin derivative, identified the cycle containing the 3H-labeled palmitoylated residue. Therefore, structure analysis of peptides obtained from gel filtration necessitated dual sequencer runs of radioactive peptides, one for sequence analysis and one to identify 3H-labeled palmitoylated amino acids. Further cleavage of the CNBr peptides by trypsin and Lys-C protease, followed by gel filtration on Sephadex LH60 and dual sequencer runs, positioned the 3H-labeled palmitoylated amino acid residues in peptides. Integration of all the available structural information led to the assignment of the palmitoyl moiety to specific residues in alpha-tubulin. The palmitoylated residues in alpha-tubulin were confined to cysteine residues only. The major site for palmitoylation was cysteine residue 376.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai R., Pei X. F., Boyé O., Getahun Z., Grover S., Bekisz J., Nguyen N. Y., Brossi A., Hamel E. Identification of cysteine 354 of beta-tubulin as part of the binding site for the A ring of colchicine. J Biol Chem. 1996 May 24;271(21):12639–12645. doi: 10.1074/jbc.271.21.12639. [DOI] [PubMed] [Google Scholar]
- Bizzozero O. A., Fridal K., Pastuszyn A. Identification of the palmitoylation site in rat myelin P0 glycoprotein. J Neurochem. 1994 Mar;62(3):1163–1171. doi: 10.1046/j.1471-4159.1994.62031163.x. [DOI] [PubMed] [Google Scholar]
- Bizzozero O. A., McGarry J. F., Lees M. B. Acylation of endogenous myelin proteolipid protein with different acyl-CoAs. J Biol Chem. 1987 Feb 15;262(5):2138–2145. [PubMed] [Google Scholar]
- Bizzozero O. A., Tetzloff S. U., Bharadwaj M. Overview: protein palmitoylation in the nervous system: current views and unsolved problems. Neurochem Res. 1994 Aug;19(8):923–933. doi: 10.1007/BF00968702. [DOI] [PubMed] [Google Scholar]
- Caron J. M. Posttranslational modification of tubulin by palmitoylation: I. In vivo and cell-free studies. Mol Biol Cell. 1997 Apr;8(4):621–636. doi: 10.1091/mbc.8.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Mastrogiacomo A., Faull K., Umbach J. A. Extensive lipidation of a Torpedo cysteine string protein. J Biol Chem. 1994 Jul 29;269(30):19197–19199. [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I. Characterization of an acyltransferase acting on p21N-ras protein in a cell-free system. Biochim Biophys Acta. 1991 Jun 24;1078(2):147–154. doi: 10.1016/0167-4838(91)99003-b. [DOI] [PubMed] [Google Scholar]
- Hackett M., Guo L., Shabanowitz J., Hunt D. F., Hewlett E. L. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science. 1994 Oct 21;266(5184):433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- Huang G., Lee D. M., Singh S. Identification of the thiol ester linked lipids in apolipoprotein B. Biochemistry. 1988 Mar 8;27(5):1395–1400. doi: 10.1021/bi00405a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Fisher D. A., Storm D. R. Analysis of the palmitoylation and membrane targeting domain of neuromodulin (GAP-43) by site-specific mutagenesis. Biochemistry. 1993 Oct 12;32(40):10714–10719. doi: 10.1021/bi00091a023. [DOI] [PubMed] [Google Scholar]
- Luduena R. F., Roach M. C. Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacol Ther. 1991;49(1-2):133–152. doi: 10.1016/0163-7258(91)90027-j. [DOI] [PubMed] [Google Scholar]
- Ludueña R. F., Roach M. C. Interaction of tubulin with drugs and alkylating agents. 1. Alkylation of tubulin by iodo[14C]acetamide and N,N'-ethylenebis(iodoacetamide). Biochemistry. 1981 Jul 21;20(15):4437–4444. doi: 10.1021/bi00518a031. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Ozols J. Amino acid analysis. Methods Enzymol. 1990;182:587–601. doi: 10.1016/0076-6879(90)82046-5. [DOI] [PubMed] [Google Scholar]
- Ozols J., Carr S. A., Strittmatter P. Identification of the NH2-terminal blocking group of NADH-cytochrome b5 reductase as myristic acid and the complete amino acid sequence of the membrane-binding domain. J Biol Chem. 1984 Nov 10;259(21):13349–13354. [PubMed] [Google Scholar]
- Ozols J. Covalent structure of liver microsomal flavin-containing monooxygenase form 1. J Biol Chem. 1990 Jun 25;265(18):10289–10299. [PubMed] [Google Scholar]
- Papac D. I., Thornburg K. R., Büllesbach E. E., Crouch R. K., Knapp D. R. Palmitylation of a G-protein coupled receptor. Direct analysis by tandem mass spectrometry. J Biol Chem. 1992 Aug 25;267(24):16889–16894. [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Krauss N. E., Heerding J. M., Swindell C. S., Ringel I., Orr G. A., Horwitz S. B. 3'-(p-azidobenzamido)taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem. 1994 Feb 4;269(5):3132–3134. [PubMed] [Google Scholar]
- Rao S., Orr G. A., Chaudhary A. G., Kingston D. G., Horwitz S. B. Characterization of the taxol binding site on the microtubule. 2-(m-Azidobenzoyl)taxol photolabels a peptide (amino acids 217-231) of beta-tubulin. J Biol Chem. 1995 Sep 1;270(35):20235–20238. doi: 10.1074/jbc.270.35.20235. [DOI] [PubMed] [Google Scholar]
- Stanley P., Packman L. C., Koronakis V., Hughes C. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science. 1994 Dec 23;266(5193):1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Uppuluri S., Knipling L., Sackett D. L., Wolff J. Localization of the colchicine-binding site of tubulin. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11598–11602. doi: 10.1073/pnas.90.24.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedegaertner P. B., Bourne H. R. Activation and depalmitoylation of Gs alpha. Cell. 1994 Jul 1;77(7):1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Weimbs T., Stoffel W. Proteolipid protein (PLP) of CNS myelin: positions of free, disulfide-bonded, and fatty acid thioester-linked cysteine residues and implications for the membrane topology of PLP. Biochemistry. 1992 Dec 15;31(49):12289–12296. doi: 10.1021/bi00164a002. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]





