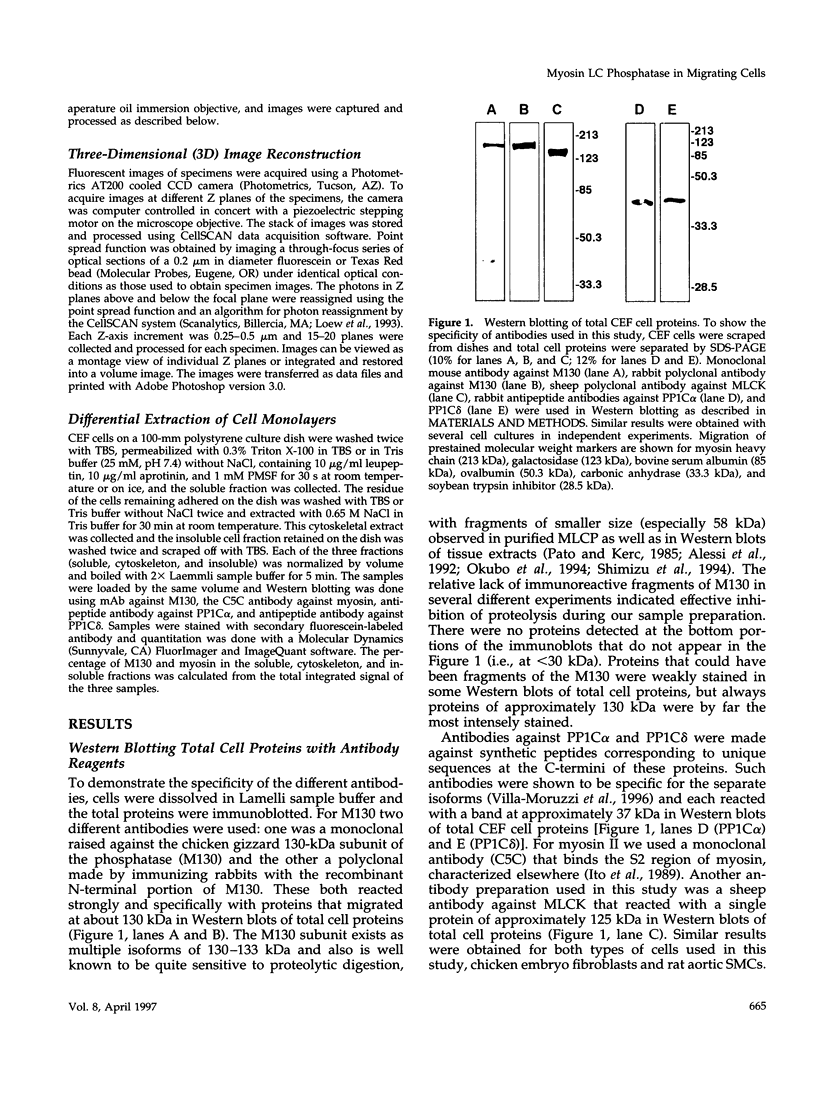
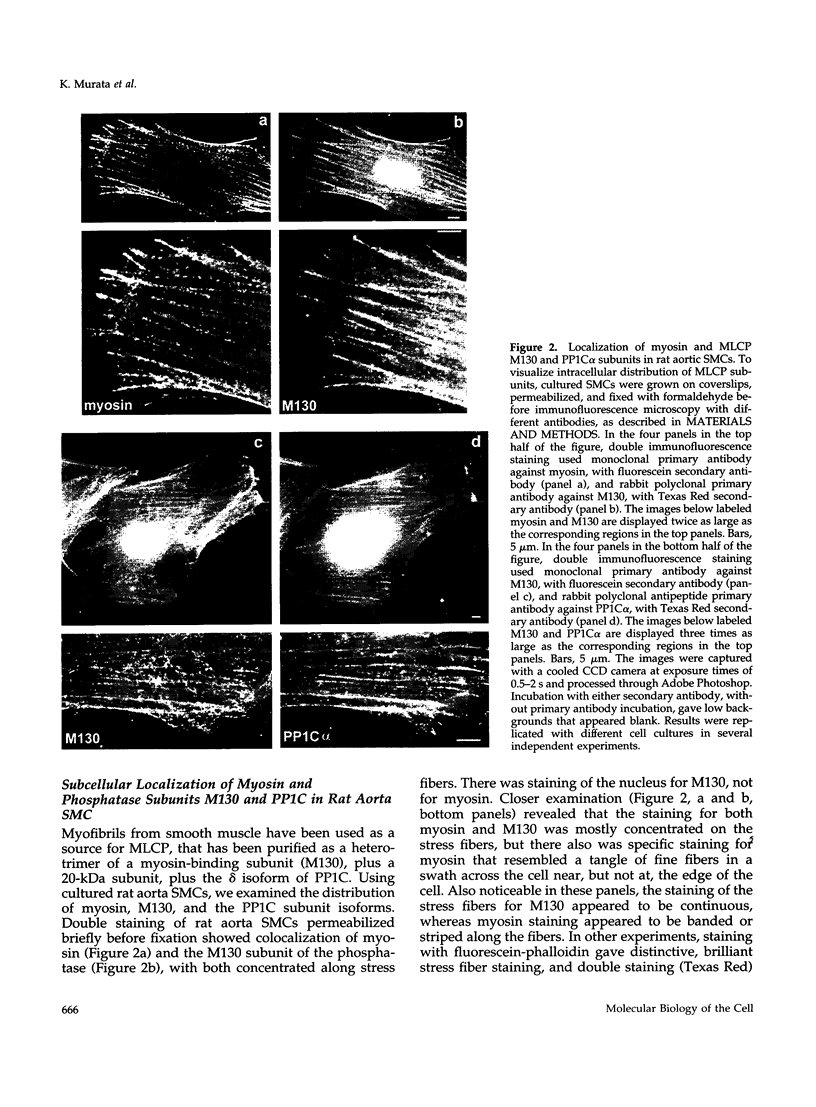
Abstract
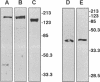
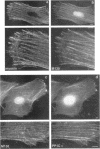
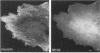
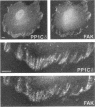
Myosin II light chains (MLC20) are phosphorylated by a Ca2+/calmodulin-activated kinase and dephosphorylated by a phosphatase that has been purified as a trimer containing the delta isoform of type 1 catalytic subunit (PP1C delta), a myosin-binding 130-kDa subunit (M130) and a 20-kDa subunit. The distribution of M130 and PP1C as well as myosin II was examined in smooth muscle cells and fibroblasts by immunofluorescence microscopy and immunoblotting after differential extraction. Myosin and M130 colocalized with actin stress fibers in permeabilized cells. However, in nonpermeabilized cells the staining for myosin and M130 was different, with myosin mostly at the periphery of the cell and the M130 appearing diffusely throughout the cytoplasm. Accordingly, most M130 was recovered in a soluble fraction during permeabilization of cells, but the conditions used affected the solubility of both M130 and myosin. The PP1C alpha isoform colocalized with M130 and also was in the nucleus, whereas the PP1C delta isoform was localized prominently in the nucleus and in focal adhesions. In migrating cells, M130 concentrated in the tailing edge and was depleted from the leading half of the cell, where double staining showed myosin II was present. Because the tailing edge of migrating cells is known to contain phosphorylated myosin, inhibition of myosin LC20 phosphatase, probably by phosphorylation of the M130 subunit, may be required for cell migration.
Full text
PDF



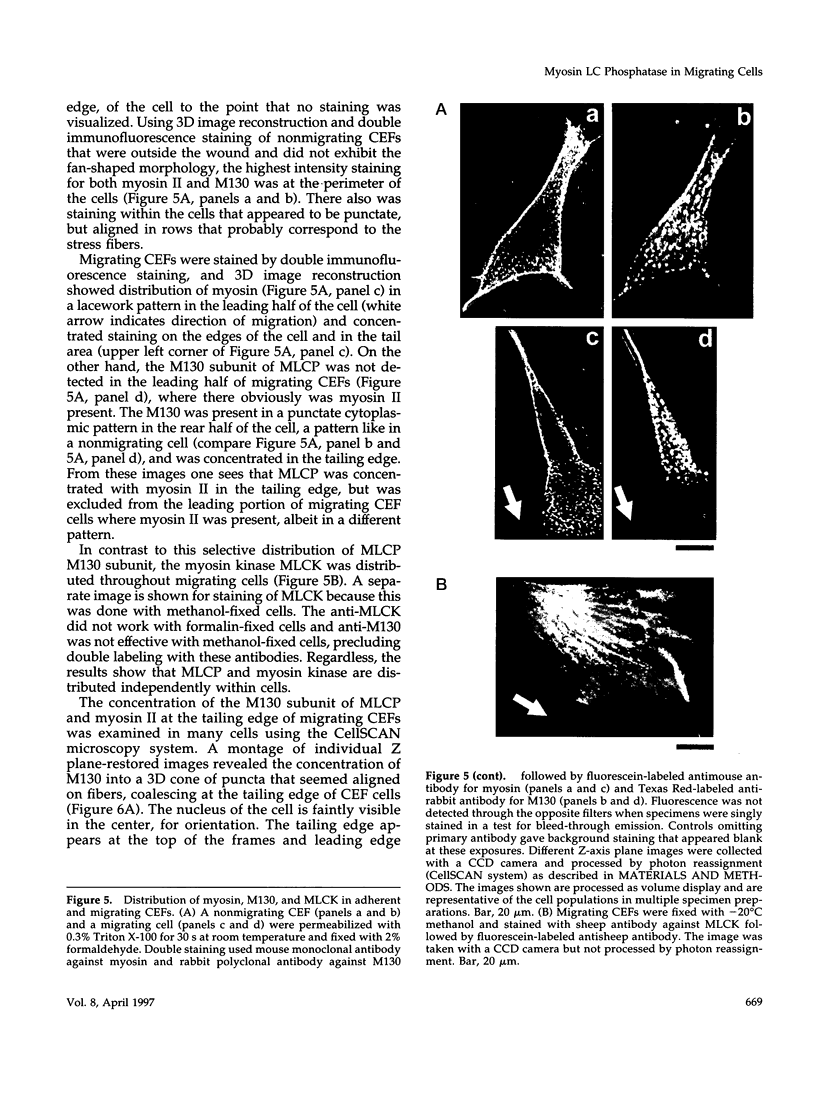
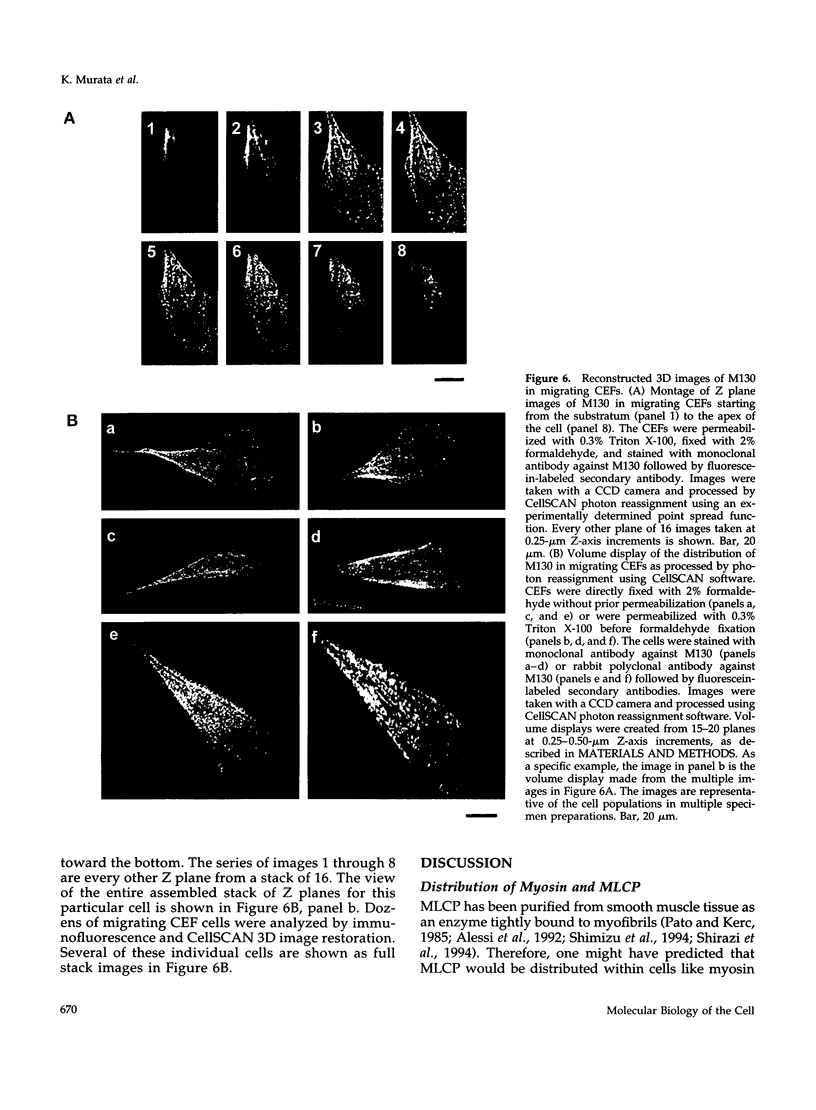



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D., MacDougall L. K., Sola M. M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur J Biochem. 1992 Dec 15;210(3):1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Chen M. X., Alessi D. R., Campbell D. G., Shanahan C., Cohen P., Cohen P. T. Molecular cloning of cDNA encoding the 110 kDa and 21 kDa regulatory subunits of smooth muscle protein phosphatase 1M. FEBS Lett. 1994 Dec 12;356(1):51–55. doi: 10.1016/0014-5793(94)01231-8. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Hansen L., Chen M. X., Bjørbaek C., Vestergaard H., Hansen T., Cohen P. T., Pedersen O. Sequence of the human glycogen-associated regulatory subunit of type 1 protein phosphatase and analysis of its coding region and mRNA level in muscle from patients with NIDDM. Diabetes. 1994 Oct;43(10):1234–1241. doi: 10.2337/diabetes.43.10.1234. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996 Jun;133(6):1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P. A., Giuliano K. A., Fisher G., Collins K., Matsudaira P. T., Taylor D. L. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol. 1993 Mar;120(6):1381–1391. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Mumby M., Lamb N. J. Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol. 1990 Jul;111(1):103–112. doi: 10.1083/jcb.111.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M. C., Fuglsang A., Alessi D., Kobayashi S., Cohen P., Somlyo A. V., Somlyo A. P. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem. 1992 Oct 25;267(30):21492–21498. [PubMed] [Google Scholar]
- Gong M. C., Iizuka K., Nixon G., Browne J. P., Hall A., Eccleston J. F., Sugai M., Kobayashi S., Somlyo A. V., Somlyo A. P. Role of guanine nucleotide-binding proteins--ras-family or trimeric proteins or both--in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hirata K., Kikuchi A., Sasaki T., Kuroda S., Kaibuchi K., Matsuura Y., Seki H., Saida K., Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992 May 5;267(13):8719–8722. [PubMed] [Google Scholar]
- Huttenlocher A., Sandborg R. R., Horwitz A. F. Adhesion in cell migration. Curr Opin Cell Biol. 1995 Oct;7(5):697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hirano K., Ito M., Tanaka J., Nakano T., Hartshorne D. J. Interactions and properties of smooth muscle myosin phosphatase. Biochemistry. 1996 May 21;35(20):6313–6320. doi: 10.1021/bi960208q. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Ito M., Hartshorne D. J. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem. 1996 Mar 1;271(9):4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- Ito M., Pierce P. R., Allen R. E., Hartshorne D. J. Effect of monoclonal antibodies on the properties of smooth muscle myosin. Biochemistry. 1989 Jun 27;28(13):5567–5572. doi: 10.1021/bi00439a034. [DOI] [PubMed] [Google Scholar]
- Jay P. Y., Pham P. A., Wong S. A., Elson E. L. A mechanical function of myosin II in cell motility. J Cell Sci. 1995 Jan;108(Pt 1):387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- Kelley C. A., Sellers J. R., Gard D. L., Bui D., Adelstein R. S., Baines I. C. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. J Cell Biol. 1996 Aug;134(3):675–687. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996 Jul 12;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Masuo M., Somlyo A. P. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J., Taylor D. L. Gradients in the concentration and assembly of myosin II in living fibroblasts during locomotion and fiber transport. Mol Biol Cell. 1993 Aug;4(8):819–836. doi: 10.1091/mbc.4.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996 Feb 9;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Tuft R. A., Carrington W., Fay F. S. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993 Dec;65(6):2396–2407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996 Aug;6(8):304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Mitsui T., Kitazawa T., Ikebe M. Correlation between high temperature dependence of smooth muscle myosin light chain phosphatase activity and muscle relaxation rate. J Biol Chem. 1994 Feb 25;269(8):5842–5848. [PubMed] [Google Scholar]
- Miura Y., Kikuchi A., Musha T., Kuroda S., Yaku H., Sasaki T., Takai Y. Regulation of morphology by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in Swiss 3T3 cells. J Biol Chem. 1993 Jan 5;268(1):510–515. [PubMed] [Google Scholar]
- Murata K., Sakon M., Kambayashi J., Okuyama M., Hase T., Mori T. Platelet talin is phosphorylated by calyculin A. J Cell Biochem. 1995 Jan;57(1):120–126. doi: 10.1002/jcb.240570112. [DOI] [PubMed] [Google Scholar]
- Noda M., Yasuda-Fukazawa C., Moriishi K., Kato T., Okuda T., Kurokawa K., Takuwa Y. Involvement of rho in GTP gamma S-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Lett. 1995 Jul 3;367(3):246–250. doi: 10.1016/0014-5793(95)00573-r. [DOI] [PubMed] [Google Scholar]
- Okubo S., Ito M., Takashiba Y., Ichikawa K., Miyahara M., Shimizu H., Konishi T., Shima H., Nagao M., Hartshorne D. J. A regulatory subunit of smooth muscle myosin bound phosphatase. Biochem Biophys Res Commun. 1994 Apr 15;200(1):429–434. doi: 10.1006/bbrc.1994.1467. [DOI] [PubMed] [Google Scholar]
- Pato M. D., Kerc E. Purification and characterization of a smooth muscle myosin phosphatase from turkey gizzards. J Biol Chem. 1985 Oct 5;260(22):12359–12366. [PubMed] [Google Scholar]
- Post P. L., DeBiasio R. L., Taylor D. L. A fluorescent protein biosensor of myosin II regulatory light chain phosphorylation reports a gradient of phosphorylated myosin II in migrating cells. Mol Biol Cell. 1995 Dec;6(12):1755–1768. doi: 10.1091/mbc.6.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Ito M., Miyahara M., Ichikawa K., Okubo S., Konishi T., Naka M., Tanaka T., Hirano K., Hartshorne D. J. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J Biol Chem. 1994 Dec 2;269(48):30407–30411. [PubMed] [Google Scholar]
- Shirazi A., Iizuka K., Fadden P., Mosse C., Somlyo A. P., Somlyo A. V., Haystead T. A. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J Biol Chem. 1994 Dec 16;269(50):31598–31606. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sasaki T., Tanaka K., Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995 Jun;20(6):227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L., Ichikawa K., Hartshorne D. J., Siegman M. J., Butler T. M. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in alpha-toxin-permeabilized smooth muscle. J Biol Chem. 1995 Aug 4;270(31):18191–18194. doi: 10.1074/jbc.270.31.18191. [DOI] [PubMed] [Google Scholar]
- Villa-Moruzzi E., Puntoni F., Marin O. Activation of protein phosphatase-1 isoforms and glycogen synthase kinase-3 beta in muscle from mdx mice. Int J Biochem Cell Biol. 1996 Jan;28(1):13–22. doi: 10.1016/1357-2725(95)00119-0. [DOI] [PubMed] [Google Scholar]