Abstract
Background
Burn injury is exacerbated by inhalation injury, causing higher morbidity and mortality rates compared to those with a comparable burn injury alone. The complex pathophysiology of inhalation injury is well described, but analysis of treatment is a mammoth task and requires individual focus on a number of components of management. In this case, the focus of the review is treatment of inhalation injury using pharmacological means. It provides a concise overview of the disease process and a summary of the evidence for specific manipulation of various disease pathways.
Methods
A literature search through PubMed was completed and all links and bibliography reference articles were explored.
Results
A total of 47 papers matched the search terms. Of these, one was a comparative study with historical controls, 2 were retrospective case series, 2 studies reported a single human case series, 34 were examinations in animals, and 8 were expert opinion or reviews.
Conclusion
The literature illustrates the complicated immunobiochemical pathways that have conflicting roles and importance, complicating integrated understanding. Secondly, there is an almost complete absence of high quality data from humans. Clinical use of pharmaco-therapies for inhalation injuries is further limited by the lack of commercial availability.
Keywords: inhalation injury, smoke, burns, inhalation, medication
Introduction
In the United States, of the estimated 2 million burns per year, 100,000 require hospitalization, and 5000 result in death (Herndon and Spies 2001). Burns are exacerbated by inhalation injury, and associated pulmonary complications (Murakami and Traber 2003). Prevalence rates vary but authors concur that those with combined burn and inhalation injury have higher morbidity and mortality rates than those with comparable burn injury alone (Tasaki et al 2002; Cox et al 2003; Endorf and Gamelli 2007). Data from the Royal Perth Hospital (RPH) Western Australia, confirms that inhalation injury causes more deaths and increased bed days and resource usage than burns alone (Table 1). Further, it is proposed that acute lung injury (ALI) and resultant acute respiratory distress syndrome (ARDS) account for up to 75% of the deaths related to fire (PatientPlus 2007).
Table 1.
Burn patient data from RPH, comparing inhalation injury statistics with burns of comparable TBSA and all burns admitted between 2004–Oct 2006
| Mean burn patient data | Inhalation injury (n = 32) | Significant burns (n = 79) (>20%TBSA*) | All burns admitted (n = 717) |
|---|---|---|---|
| Age (yrs) (SD) | 41.9 (19.8) | 41.3 (16.8) | 38.9 (17.7) |
| TBSA (%) (SD) | 20.6 (24.1) | 34.5 (17.5) | 8.4 (11.2) |
| ICU LOS (dys) (SD) | 4.7 (9.9) | 3.1 (8.5) | 0.43 (3.0) |
| Total LOS (dys) (SD) | 27.5 (36.0) | 27.1 (29.5) | 10.9 (15.5) |
| Operations# (SD) | 1.4 (2.2) | 1.8 (1.8) | 0.6 (1.0) |
| Mortality Rate (%) | 6.3 | 3.8 | 1.0 |
TBSA – Total burn surface area.
Number of surgical skin reconstruction procedures.
(SD) – Standard deviation of measure.
The pathophysiology of inhalation injury is well described in the literature, though the vast majority of studies have been completed in the animal model (Table 2) (Enkhbaatar and Traber 2004; Lee and Mellins 2006; Cancio et al 2007). In brief, inhalation injury is the result of thermal or chemical injury to the airways and alveoli from exposure to superheated gases, steam, hot liquids and, or toxic products of incomplete combustion (Herndon et al 1986). Dry heat does not easily penetrate to the lower respiratory tract, and hence true thermal damage of the lung parenchyma is rare (Cox et al 2003). Tissue damage above the vocal cords is often the result of direct thermal exposure while below the cords, injury is generally caused by chemical toxins and particulate matter (Mlcak et al 2007). The by products of combustion; namely hydrogen cyanide, aldehydes, hydrochloric acid, acrolein, ammonia, chlorine, sulphur dioxide, and nitrogen dioxide, that are chiefly responsible for initial pulmonary tissue insult (Haponik et al 1988).
Table 2.
Pathophysiological processes that have been linked to the etiology acute lung injury post smoke inhalation
| Airways oxidative stress | Beta 2 – agonists |
| Airways cast formation | Nitric oxide |
| Loss of pulmonary surfactants | Eicosanoids – Prostaglandins |
| Airways bronchoconstriction | – Thromboxane |
| Neutrophil activation/chemotaxis | – Leukotrienes |
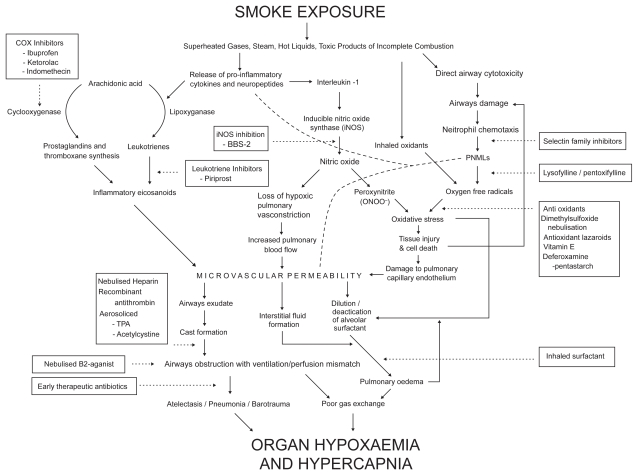
Armed with the data and the knowledge of acute lung injury pathophysiology (Figure 1), the multi-disciplinary burns team (MBT) wished to examine ways of improving outcomes in the inhalation injury group. The first step towards implementing evidenced-based practice is to review the relevant literature (Eccles and Grimshaw 2004).
Figure 1.
Flow diagram describing the pathophysiological pathways involved in the body’s response to inhalation injury (and burns).
Aims
Due to the complexity of the body’s response to inhalation injury, the RPH MBT decided to focus initially on pharmacological management. The aims of this review are therefore to:
Determine the pharmacological options for management of inhalation injury; and
Examine the levels of evidence to support various pharmacological interventions.
Methods
An on-line literature search through PubMed was completed using the following search terms: “inhalation injury”, “burn*”, “pharmacomanagement”, and “medication*”. Where possible, all links and bibliography reference articles were explored.
Results
A total of 47 papers including 1 internet citation matched the search terms. Of the papers, one was a human case series with historical controls (n = 90), 2 were retrospective descriptive case series (n = 80, n = 98), 2 studies reported an overlapping human case series (n = 6), 34 were examinations in animals, 8 were expert opinion or reviews and the remainder were from non-reliable or public relations sources.
Table 3 presents the results of the review according to the etiology of the negative effects of inhalation injury.
Table 3.
Stratified list of researched pharmaco-intervention to the etiology of inhalation injury
| Promising experimental results | |
| Anti-oxidants | Beta 2 – agonists |
| Leukotriene inhibitors | Aerosolised tissue plasminogen activator |
| Nebulised heparin ± Acetylcystine | Recombinant anti-thrombin |
| Nitric oxide inhibition | Nebulised dimethylsulfoxide |
| Deferoxamine-pentastarch aerosol | Antioxidant lazaroids |
| Vitamin E | NSAIDs; ketorolac |
| Mixed experimental results | |
| Parenteral heparin | Inhaled detergent/surfactant |
| Anti-adhesion (selectin) molecules | NSAIDs (indomethacin, ibuprofen) |
| Experimental results showing no benefit or adverse outcomes | |
| Corticosteroids | Allopurinol |
| Manganese superoxide dismutase | |
Discussion
PubMed is a well recognized first contact database for high profile journals and high impact medical literature. Warranted, this paper does not provide an exhaustive literature search. but the likelihood is that the results represent the current medical practice in pharmaco-management of inhalation injury.
The primary aim of this study was to determine the level of evidence in order to determine best practice. In summary, according to the Australian National Health and Medical Research Council (NH&MRC) guidelines (1999), at best this review suggests, level III-3 evidence (comparative study, historical controls) for the clinical use of alternating nebulized heparin alternating with the mucolytic agent, acetylcystine. Secondly, the review reveals level IV (case series) evidence promotes short term positive effects from aerosolized surfactant and negative outcomes associated with prophylactic antibiotics and corticosteroid use to ameliorate acute inhalation injury.
The first aim of this review was to examine the options for manipulation of the inflammatory pathways using medication. The findings are discussed in detail below with reference to the pathophysiological pathways in Table 2.
Nitric oxide (NO)
Increased inducible nitric oxide synthase (iNOS) and resultant NO levels have been isolated in lung tissue as a result of inhalation injury (Enkhbaatar et al 2003). Up-regulation of cytokines, primarily interleukin-1, and endotoxin release are implicated as causes for NO presence as both are found in increased amounts after burn and inhalation injury. Increased levels of NO lead to loss of hypoxic vasoconstriction. Murakami and Traber (2003) reported the ensuing vasodilation leads to an increase in bronchial blood flow by up to 800%. Additionally, loss of hypoxic vasoconstriction allows poorly ventilated lung segments to receive increased blood flow (Enkhbaatar and Traber 2004). This creates ventilation/perfusion mismatch, increases the pulmonary shunt fraction, and overall results in lower oxygen saturations in the blood.
Furthermore, Enkhbaatar and Traber (2004) comment that increased respiratory compromise is created by the oxidizing and nitrating action of high concentrations of NO. Nitrous oxide becomes cytotoxic and a pro-inflammatory agent by reacting with O2− to form peroxynitrate (ONOO−). Peroxynitrate causes oxidation and nitration, producing further tissue damage and lung inflammation (Enkhbaatar and Traber 2004).
To reduce nitric oxide production, Enkhbaatar and colleagues (2003) examined the use of an iNOS inhibitor, BBS-2, on sheep. BBS-2 showed beneficial effects on pulmonary gas exchange and shunt fraction, lung lymph flow, lung water content, airways obstruction, airways blood flow and airways pressures.
Cyclooxyganase (COX) pathway
Elevated prostacyclin and thromboxane A2 are significantly elevated post burn and smoke inhalation (Kimura, Traber et al 1988). Both are produced by the action of the enzyme COX. A number of studies consequently look at nonsteroidal anti-inflammatory (NSAID) medications.
Ibuprofen was shown by Stewart and colleagues (1990) to reduce early-onset lung water after smoke inhalation injury alone, or in combination with a thermal injury in adult rabbits. Sakurai and colleagues (1998) demonstrated that ibuprofen impoved intestinal organ blood flow during the acute hypovolemic period. Finally, Kimura and colleagues (1988) found that ibuprofen attenuated the elevation of lung lymph flow, microvascular permeability and pulmonary edema in an ovine model.
The advantage of the nonselective COX inhibitor, ketorolac, is its availability as a parenteral preparation. Enkhbaatar and colleagues (2003) showed in sheep that it slowed the decline in the PaO2/FiO2 ratio, the increase in lung lymph flow and significantly lowered bronchial obstruction and airway pressures. They found that ketorolac had multiple favorable pharmacodynamic attributes including significantly less cardiac myocardial contractility depression, hemo-concentration, and bleeding tendencies.
Conversely, Abdi and colleagues (1995) iterated concern regarding the use of ibuprofen. They found that myocardial depression seen post inhalation injury is worse in animals treated with ibuprofen. This was not seen with ketorolac by Enkhbaatar and colleagues (2003).
Other than a strong association, the role of eicosanoids in inhalation injury is less well understood. Prostacyclins prevent increase in microvascular permeability. Kimura and colleagues (1988) propose that the COX inhibition stabilizes lysosomal membranes, inhibits leukocyte migration and prevents the release of oxygen free radicals. Additionally, COX inhibitors are reported to lower plasma NO levels by down regulating iNOS (Enkhbaatar et al 2003).
Lipoxygenase pathway
Several studies have implicated leukotrienes in the etiology of edema after smoke exposure. Witten and colleagues (1990) demonstrated and increase in leukotriene B4 in bronchoalveolar lavage (BAL) after inhalation injury in rabbits. They further demonstrated that piriprost, a leukotriene synthesis inhibitor, given 1 hour prior to smoke exposure resulted in attenuation of the swelling in type 1 alveolar cell epithelium, BAL leukotriene B4, prostaglandin E2 and thromboxane B2 levels at 1 hour post smoke exposure. Hales and Musto (1995) compared a combined COX and lipoxygenase inhibitor (BW-755C) to the NSAID, indomethacin. They found that BW-755C prevented edema, whereas indomethacin did not.
Finally, Janssens and colleagues (1994) compared three eicosanoids by inhibiting them in isolation. They gave sheep either BW-755C, U-63557A (a specific thromboxane synthetase inhibitor), or indomethacin. They found that neither indomethacin nor U-63557A prevented the increase in lymph flow or reduced the lung wet-to-dry weight ratio. However, these did blunt and delay the rise in airway pressure and the rises in pulmonary arterial pressure and pulmonary vascular resistance. They concluded that leukotriene B4 may be the main oedematogenic eicosanoid. This was echoed by Abdi and colleagues (1995) who looked at the effects of ibuprofen on bronchial blood flow in the ovine model. They concluded that vasodilatory prostaglandins do not play a major role in the bronchial vascular response to smoke inhalation injury. This last comment conflicts with the above mentioned COX annotations.
B2 agonists/bronchodilators
These are successful in the management of airways pathology for conditions such as asthma, causing bronchodilation and subsequent increase in airway caliber (Palmieri et al 2006). A controlled experiment by Palmieri and colelagues (2006) looked at continually nebulized albuterol in sheep. Results were promising; with comparison to the control group, those given the albuterol had lower pause and peak inspiratory pressures, decreased pulmonary trans-vascular fluid flux, a significantly higher PaO2/FiO2 ratio and decreased shunt fraction.
Oxidative stress
There is increased oxygen-free radical activity after smoke inhalation (Maybauer et al 2005). This arises from both direct inhalation of oxidizing agents, as well as from a by-product of cellular activity. Both the peroxide and hydroxyl ions have been implicated, and as noted, peroxynitrate has been identified in oxidization. Oxidization results in damage to the pulmonary capillary endothelium, evoking inflammation, increased microvascular permeability, and edema formation (Stothert et al 1990).
Endogenous production of oxidizing agents is mostly released from marginating polymorphonuclear leukocytes (Brown et al 1988). In an ovine experiment, Murakami and Traber (2003) used nitrogen mustard to suppress bone marrow. They found, in the leukocyte-depleted sheep, that smoke-inhalation pulmonary edema and vascular permeability were prevented. There was also significantly less lipid peroxidation and cellular oxidation (Murakami and Traber 2003).
There are a number of methods proposed to curb damage associated with oxygen-free radicals. A number of papers look at the use of anti-oxidant agents. Brown et al looked at the use of dimethylsulfoxide, an O2-free radical scavenger (Brown et al 1988). Their trial had four arms; control sham group, sheep treated by dimethylsulfoxide or heparin, and sheep given both. They found that dimethylsulfoxide significantly diminished the pulmonary lymphatic response, concluding this was a result of attenuated rise in microvascular permeability.
Several other studies showed promising results with the administration of various anti-oxidant agents. La Londe and colleagues (1994) found that aerosol delivery of deferoxamine-pentastarch complex prevented progression to pulmonary edema. Three studies by Wang and colleagues (1996, 1997, 1999) found that lazaroid analogue U75412E, a free radical scavenger, ameliorated interstitial edema. Vitamin E (alpha-tocopheral) is another example of an oxygen superoxide scavenger. Depleted vitamin E levels have found to occur after combined burn and inhalation injury (Morita, Shimoda et al 2006). Morita and colleagues administered aerosolized vitamin E and demonstrated decreased pulmonary oedema and significant improvement in pulmonary gas exchange in sheep with burn and smoke inhalation (Morita, Shimoda et al 2006; Morita, Traber et al 2006). In contrast, the use of manganese superoxide dismutase, an enzyme that converts the superoxide radical to peroxide, was looked at by Maybauer and colleagues (2005). They found that nebulized delivery to smoke exposed sheep failed to improve lung edema or pulmonary gas exchange. Another unsuccessful agent is allopurinol. This is proposed to decrease O2− by its inhibition of xanthine oxidase. A study on sheep by Ahn and colleagues (1990) looked at the effects of allopurinol on sheep. They found no significant difference in those managed with or without allopurinol.
There are several papers looking at approaches to attenuate neutrophil response. The selectin family of anti-adhesion molecules mediate neutrophil migration by allowing endothelial cell contact (Katahira et al 2002). In sheep, Katahira and colleagues (2002) found that pre-treatment with the antibody for L-selectin called leukocyte adhesion molecule (LAM) 1 hour after smoke exposure decreased the lung lymph flow and pulmonary permeability. Similarly, several authors reported that the oligosaccharide Sulfo lewis C, a putative ligand of E-selectins, attenuated lung injury after smoke exposure on sheep (Tasaki et al 1998; Mandel and Hales 2007). In a comparable experiment, Chandra and colleagues (2003) gave an anti-P-selectin antibody. This did not protect against lung injury in sheep exposed to smoke (Chandra et al 2003).
Inflammation and airways cast formation
Pathological lung specimens, after inhalation of smoke, demonstrate the presence of obstructive casts in the airways. In sheep, Cox and Burke (2003) found that casts formed at all three studied airways levels (bronchial, bronchiolar and terminal bronchiolar). Obstructive changes were maximal at 24 hours in large airways, and rose continually to the cut of time of 72 hours at the bronchiolar level. Casts are composed of epithelial cells, neutrophils, mucus and fibrin. Mucosal hyperemia, increased microvascular permeability, acute inflammatory cell influx, exfoliation of epithelial lining, and mucous secretion are the causative factors (Cox et al 2003). Airway obstruction leads to further ventilation/perfusion mismatch, and complications such as atelectasis and pneumonia. Barotrauma may ensue from overstretching of alveoli supplied by the remaining patent airways (PatientPlus 2007).
One method to reduce cast formation is the attenuation of the inflammatory response. The use of anti-inflammatories and anti-adhesion molecules may be indicated for this reason. In their experiment with dimethylsulfoxide, Brown and colleagues (1988) found the administration of this O2-free radical scavenger also reduced airways cast formation (presumably as a succeeding effect).
An alternate approach to over-coming airways obstruction is the use of heparin. The main action of heparin is potentiating anti-thrombin III mediated inactivation of thrombin. Heparin may also act as a free radical scavenger (Tasaki et al 2002). There have been multiple experiments looking at both parenteral and nebulized heparin. Cox and colleagues (1993) found that in sheep given a heparin infusion, there was significant improvement in oxygenation, with less barotrauma and pulmonary edema. At necropsy, those treated with heparin had significantly fewer tracheo-bronchial casts.
Murakami and colleagues (2002) found that high dose heparin attenuated lung dysfunction after combined smoke and septic lung injury. They found in histological specimens, decreased lung cellular infiltrates, lung edema, congesting, and cast formation. Their study differed from Cox and colleagues (1993), in that the sheep were also exposed to Pseudomonas aeruginosa and heparin was administered by nebulization. Conversely, Murakami and colleagues (2002, 2003) in an analogous experiment to one previously reported from the same setting, found that high dose heparin did not prevent lung dysfunction after combined smoke and septic lung injury. In this experiment the heparin was given via intravenous infusion. Nebulized heparin was effective in the applicable arm in the controlled experiment by Brown and colleagues (1988).
More importantly, in a comparative case series with historical controls by Desai and colleagues (1998), the effect of giving a regime of aerosolized heparin alternating with a solution of 20% acetylcystine was performed on a pediatric human population. Acetylcystine is a mucolytic agent, and hence should diminish airway cast formation. The study included 90 patients with a mean age of 8 years, suffering from inhalation injury, diagnosed by bronchoscopy. They found a significantly decreased mortality, incidence of atelectasis, and re-intubation rate in the treatment group.
Another approach to overcoming airways cast obstruction is the administration of antithrombin. It should have comparable effects to heparin. This was confirmed by Murakami and colleagues (2002) in sheep exposed to smoke and septic injury an infusion of human recombinant antithrombin.
Lastly, as casts are also composed of fibrin, Enkhbaatar and colleagues (2004) experimented with aerosolised tissue plasminogen activator (TPA) in sheep. TPA lyses fibrin clots. They found that TPA, attenuated airways obstruction as expected.
Aerosolized surfactant
Within the lung alveoli, there is a delicate balance between negative intrathoracic pressure, alveolar wall surface tension and tissue elasticity. The presence of surfactant lining the alveolar walls results in lower alveolar surface tension and stabilizes the fluid balance in the lung. The removal of which allows increased microvascular permeability and subsequent lung edema formation. This has been demonstrated in multiple animal experiments (Brendenberg et al 1983; Nieman and Brendenberg 1985; Nieman et al 1990; Wang et al 1993). Pulmonary surfactant also assists with removal of inorganic dust particles, opsonization of bacteria and activation of alveolar macrophages (Pallua et al 1998).
The loss of surfactant is thought to arise from: direct deactivation or inhibition of the alveolar surfactant by oxidants, proteases, fibrin and protein rich exudate; incorporation of hyaline membranes in fibrin polymers; lifting of the surfactant away from the alveolar wall by edema; and disturbed synthesis resultant of damage to the type II pneumatocytes (Wang et al 1993).
The benefit of surfactant therapy has been demonstrated in the context of ARDS and infant respiratory distress syndrome (Wang et al 1993). However, there is a dearth of literature regarding it application in the setting of burns. Pallua and colleagues (1997, 1998) provides two case series of 4 and 2 patients respectively, with severe burns and poor prognosis that benefited from surfactant therapy. After the limits of mechanical ventilation had been reached, they introduced intrabronchial surfactant via bronchoscopy. They subsequently found that there was temporary improved gas exchange, an increase in arterial oxygenation (PaO2), a reduction in required inspiratory O2 concentration (FiO2), and improved lung compliance (Wang et al 1993; Pallua et al 1997).
Steroids
Controlled experiments thus far looking at the usefulness of steroids in burn and inhalation injuries have been disappointing. No direct data support their use in smoke inhalation and this was confirmed by a recent disaster case series report (Herndon et al 1988; Cha et al 2007). In fact, due to their immunosuppressive effects, steroids are likely to lead to the increased risk of infection and delayed wound healing. Nieman and colleagues (1991) found in sheep, that methylprednisolone did not protect the lung from the acute physiological consequences of inhalation injury.
Prophylactic antibiotics
Pulmonary infection is a sequela of inhalation injury, and hence antibiotic prophylaxis is not considered here in depth. Patients with pulmonary damage from inhalation injury are at increased risk for secondary bacterial infection, commonly Staphylococcus aureus and P. aeruginosa. Though appealing in concept, usage of antibiotics is recommended on clinical grounds of definitive microbiologic evidence of infection only (Herndon et al 1987). Administration of prophylactic antibiotics increases the risk of resistant organism emergence.
Clinical implications and future studies
The research on inhalation injuries thus far has given important insight into the etiology and potential pharmaco-interventions. The amalgamated algorithm outlining the key pathophysiological steps also highlights the various points at which interventions have been proposed (Figure 1).
Of note, there are several parallel pathways that eventually result in organ hypoxemia, suggesting that multiple medications may be warranted in for meaningful intervention. This was illustrated in the study by Brown and colleagues (1988) who looked at the use of heparin and dimethylsulfoxide. Both of the groups treated with either of these performed favorably but those given both outperformed those given either preparation alone.
The physical management of pulmonary edema, airway obstruction by cast formation and sputum, ventilation/perfusion mismatch and atelectasis was not within the scope of this review. Ventilation strategies as well as physical (or respiratory) therapy are obvious adjuncts to pharmaco-therapy and must be considered as part of a multi-disciplinary approach to improving inhalation injury outcomes. The recent review paper by Cancio and colleagues (2007) provides an excellent summary of ventilation and artificial lung strategies for management of inhalation injury. Finally, cell replacement therapies delivered into the lungs remain a largely unexplored but promising area with respect to ‘surgical’ management of tissue damage in the lungs after inhalation injury (Duncan et al 2005).
Conclusion
There are several striking limitations of the literature. Firstly, the discussion illustrates the complicated immunobiochemical pathways that have conflicting roles and importance throughout the journal papers, complicating integrated understanding. The experiments and hypotheses generally only consider one process in isolation. Secondly, there is a complete absence of high quality data collected from humans. At best, human case series data supports short term positive effect from aerosolized surfactant and, negative outcomes from prophylactic antibiotics and corticosteroid use.
It is fair to comment, within the ‘ABC’ paradigm of burns care, with satisfactory airways and circulatory management strategies, the ability to control the inflammatory response of the lungs with medication lags in comparison. The advances in the management of inhalation injuries may well herald the next significant step forward in severe burn management. However, thus far, the clinical use of pharmaco-therapies for inhalation injuries is limited by the lack of commercial availability, dearth of human trials and confounding experimental results.
Footnotes
Disclosure
All authors warrant that there has been no financial or personal support involved in the preparation of this paper, no affiliations with organisations interested in the subject matter and there are no actual or potential conflicts of interest to be noted.
References
- Abdi S, Traber LD, Herndon DN, et al. Effects of ibuprofen on airway vascular response to cotton smoke injury. Eur J Pharmacol. 1995;293:475–81. doi: 10.1016/0926-6917(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Ahn SY, Sugi K, Talke P, et al. Effects of allopurinol on smoke inhalation in the ovine model. J Appl Physiol. 1990;68:228–34. doi: 10.1152/jappl.1990.68.1.228. [DOI] [PubMed] [Google Scholar]
- Barrett A, Ragg M, Cockburn J, et al. NH&MRC : How to prepare and present evidence based information for consumers of health services: A literature review. Canberra: Commonwealth of Australia; 1999. How to review evidence: systematic identification and review of the scientific literature; p. 56. [Google Scholar]
- Brendenberg C, Paskanik A, Nieman J. High surface tension pulmonary edema. J Surg Res. 1983;34:515–23. doi: 10.1016/0022-4804(83)90104-x. [DOI] [PubMed] [Google Scholar]
- Brown M, Desai M, Traber LD, et al. Dimethylsulfoxide with heparin in the treatment of smoke inhalation injury. J Burn Care Rehabil. 1988;9:22–5. doi: 10.1097/00004630-198801000-00007. [DOI] [PubMed] [Google Scholar]
- Cancio LC, Batchinsky AI, Dubick MA, et al. Inhalation injury: Pathophysiology and clinical care. Proceedings of a symposium conducted at the Trauma Institute of San Antonio, San Antonio, TX, USA on 28 March 2006. Burns. 2007;33:681–92. doi: 10.1016/j.burns.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Cha SI, Kim CH, Lee JH, et al. Isolated smoke inhalation injuries: Acute respiratory dysfunction, clinical outcomes, and short-term evolution of pulmonary functions with the effects of steroids. Burns. 2007;33:200–8. doi: 10.1016/j.burns.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Chandra A, Katahira J, Schmalstieg FC, et al. P-selectin blockade fails to improve acute lung injury in sheep. Clin Sci (Lond) 2003;104:313–21. doi: 10.1042/CS20020244. [DOI] [PubMed] [Google Scholar]
- Cox CS, Jr, Zwischenberger JB, Traber DL, et al. Heparin improves oxygenation and minimizes barotrauma after severe smoke inhalation in an ovine model. Surg Gynecol Obstet. 1993;176:339–49. [PubMed] [Google Scholar]
- Cox RA, Burke AS, Soejima K, et al. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- Desai MH, Mlack R, Richardson J, et al. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/acetylcystine therapy. J Burn Care Rehab. 1998;19:210–12. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- Duncan CO, Shelton RM, Navsaria H, et al. In vitro transfer of keratinocytes: Comparison of transfer from fibrin membrane and delivery by aerosol spray. J Biomed Mater Res. 2005;73B(Part B: Applied Biomaterials):221–8. doi: 10.1002/jbm.b.30198. [DOI] [PubMed] [Google Scholar]
- Eccles M, Grimshaw J. Selecting, presenting and delivering clinical guidelines: are there any magic bullets? Med J Am. 2004;180(Supp):S52–S54. doi: 10.5694/j.1326-5377.2004.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Endorf F, Gamelli R. Inhalation injury, pulmonary perturbations and fluid resuscitation. J Burn Care Res. 2007;28:80–3. doi: 10.1097/BCR.0B013E31802C889F. [DOI] [PubMed] [Google Scholar]
- Enkhbaatar P, Murakami K, Cox C, et al. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22:70–5. doi: 10.1097/01.shk.0000129201.38588.85. [DOI] [PubMed] [Google Scholar]
- Enkhbaatar P, Murakami K, Shimoda K, et al. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am J Physiol Heart Circ Physiol. 2003;285:H2430–6. doi: 10.1152/ajpheart.00055.2003. [DOI] [PubMed] [Google Scholar]
- Enkhbaatar P, Murakami K, Shimoda K, et al. Ketorolac attenuates cardiopulmonary derangements in sheep with combined burn and smoke inhalation injury. Clin Sci (Lond) 2003;105:621–8. doi: 10.1042/CS20030180. [DOI] [PubMed] [Google Scholar]
- Enkhbaatar P, Traber D. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci. 2004;107:137–43. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- Hales CA, Musto S, Hutchison WG, et al. BW-755C diminishes smoke-induced pulmonary edema. J Appl Physiol. 1995;78:64–9. doi: 10.1152/jappl.1995.78.1.64. [DOI] [PubMed] [Google Scholar]
- Haponik EF, Crapo RO, Herndon DN, et al. Smoke inhalation. Am Rev Respir Dis. 1988;138:1060–3. [PubMed] [Google Scholar]
- Herndon DN, Spies M. Modern burn care. Semin Pediatr Surg. 2001;10:28–31. doi: 10.1053/spsu.2001.19389. [DOI] [PubMed] [Google Scholar]
- Herndon DN, Barrow RE, Linares HA, et al. Inhalation injury in burned patients: effects and treatment. Burns Incl Therm Inj. 1988;14:349–56. doi: 10.1016/0305-4179(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Herndon DN, Langner F, Thompson P, et al. Pulmonary injury in burned patients. Surg Clin North Am. 1987;67:31–46. doi: 10.1016/s0039-6109(16)44131-9. [DOI] [PubMed] [Google Scholar]
- Herndon DN, Traber LD, Linares H, et al. Etiology of the pulmonary pathophysiology associated with inhalation injury. Resuscitation. 1986;14(1–2):43–59. doi: 10.1016/0300-9572(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Janssens SP, Musto SW, Hutchison WG, et al. Cyclooxygenase and lipoxygenase inhibition by BW-755C reduces acrolein smoke-induced acute lung injury. J Appl Physiol. 1994;77:888–95. doi: 10.1152/jappl.1994.77.2.888. [DOI] [PubMed] [Google Scholar]
- Katahira J, Murakami K, Schmalstieg FC, et al. Role of anti-L-selectin antibody in burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1043–50. doi: 10.1152/ajplung.00305.2001. [DOI] [PubMed] [Google Scholar]
- Kimura R, Traber L, Herndon D, et al. Ibuprofen reduces the lung lymph flow changes associated with inhalation injury. Circ Shock. 1988;24:183–91. [PubMed] [Google Scholar]
- LaLonde C, Ikegami K, Demling R. Aerosolized deferoxamine prevents lung and systemic injury caused by smoke inhalation. J Appl Physiol. 1994;77:2057–64. doi: 10.1152/jappl.1994.77.5.2057. [DOI] [PubMed] [Google Scholar]
- Lee A, Mellins R. Lung injury from smoke inhalation. Paediatr Respir Rev. 2006;7:123–8. doi: 10.1016/j.prrv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Mandel J, Hales A. Smoke inhalation: Up to date [online] 2007. [Accessed on Jun 27, 2007]. URL: http://patients.uptodate.com/topic.asp?file=cc_medi/11903.
- Maybauer M, Kikuchi Y, Westphal M, et al. Effects of manganese superoxide dismutase nebulisation on pulmonary function in an ovine model of acute lung injury. Shock. 2005;23:138–43. doi: 10.1097/01.shk.0000150777.39484.b0. [DOI] [PubMed] [Google Scholar]
- Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33:2–13. doi: 10.1016/j.burns.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Morita N, Shimoda K, Westphal M, et al. Vitamin E attenuates acute lung injury in sheep with burn and smoke injury. Redox Rep. 2006;11:61–70. doi: 10.1179/135100006X101020. [DOI] [PubMed] [Google Scholar]
- Morita N, Traber M, Westphal PE, et al. Aerosolised alpha-tocopherol ameliorates acute lung injury following combined burn and smoke inhalation injury in sheep. Shock. 2006;25:277–82. doi: 10.1097/01.shk.0000208805.23182.a7. [DOI] [PubMed] [Google Scholar]
- Murakami K, Enkhbaatar P, Shimoda K, et al. High-dose heparin fails to improve acute lung injury following smoke inhalation in sheep. Clin Sci (Lond) 2003;104:349–56. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Murakami K, McGuire R, Cox RA, et al. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock. 2002;18:236–41. doi: 10.1097/00024382-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci. 2003;18:125–9. doi: 10.1152/nips.01427.2002. [DOI] [PubMed] [Google Scholar]
- Nieman G, Clark WTH. Methylprednisolone does not protect the lung from inhalation injury. Burns. 1991;17:384–90. doi: 10.1016/s0305-4179(05)80071-3. [DOI] [PubMed] [Google Scholar]
- Nieman J, Brendenberg C. High surface tension pulmonary edema induced by detergent aerosol. J Applied Physiol. 1985;58:129–36. doi: 10.1152/jappl.1985.58.1.129. [DOI] [PubMed] [Google Scholar]
- Nieman J, Goyette F, Paskani A, et al. Surfactant displacement by plasma lavage results in pulmonary edema. Surgery St Louis. 1990;107:677–83. [PubMed] [Google Scholar]
- Pallua N, Warbanow K, Machens HG, et al. 1997Intrabronchial surfactant application in postburn smoke inhalation injury and adult respiratory distress syndrome. First cases Unfallchirurg 100363–70.9297244 [Google Scholar]
- Pallua N, Warbanow K, Noah EM, et al. Intrabronchial surfactant application in cases of inhalation injury: First results from patients with severe burns and ARDS. Burns. 1998;24:197–206. doi: 10.1016/s0305-4179(97)00112-5. [DOI] [PubMed] [Google Scholar]
- Palmieri T, Enkhbaatar P, Bayliss R, et al. Continuous nebulised albuterol attenuates acute lung injury in an ovine model of combined burn and smoke inhalation. Crit Care Med. 2006;34:1719–24. doi: 10.1097/01.CCM.0000217215.82821.C5. [DOI] [PubMed] [Google Scholar]
- PatientPlus. Inhalation injury [online] 2007. [Accessed on Oct 20, 2007]. URL: http://www.patient.co.uk/showdoc/40001199/
- Sakurai H, Traber L, Traber D. Altered systemic organ blood flow after combined injury with burn and smoke inhalation. Shock. 1998;9:369–74. doi: 10.1097/00024382-199805000-00010. [DOI] [PubMed] [Google Scholar]
- Stewart R, Yamaguchi K, Knost P, et al. Effects of ibuprofen on pulmonary oedema in an animal smoke inhalation model. Burns. 1990;16:409–13. doi: 10.1016/0305-4179(90)90067-7. [DOI] [PubMed] [Google Scholar]
- Stothert JC, Jr, Basadre JO, Gbaanador GB, et al. Airway aspiration of hydrochloric acid in sheep. Circ Shock. 1990;30:237–54. [PubMed] [Google Scholar]
- Tasaki O, Mozingo D, Dubick M, et al. Effects of heparin and lisofyl-line on pulmonary function after smoke inhalation injury in an ovine model. Crit Care Med. 2002;30:637–43. doi: 10.1097/00003246-200203000-00024. [DOI] [PubMed] [Google Scholar]
- Tasaki O, Mozingo D, Ishihara S, et al. Effect of Sulfo Lewis C on smoke inhalation injury in an ovine model. Crit Care Med. 1998;26:1159. doi: 10.1097/00003246-199807000-00028. [DOI] [PubMed] [Google Scholar]
- Wang C, Barrow C, Cox C, et al. Influence of detergent aerosol on lung microvasculature permeability. J Appl Physiol. 1993;74:1016–23. doi: 10.1152/jappl.1993.74.3.1016. [DOI] [PubMed] [Google Scholar]
- Wang S, Lantz R, Rider E, et al. A free radical scavenger (Lazaroid U75412E) attenuates tumor necrosis factor-alpha generation in rabbit model of smoke inhalation lung injury. Respiration. 1997;64:358–63. doi: 10.1159/000196704. [DOI] [PubMed] [Google Scholar]
- Wang S, Lantz R, Vermeulen M, et al. Functional alterations of alveolar macrophages subjected to smoke exposure and antioxidant lazaroids. Toxicol Ind Health. 1999;15:464–9. doi: 10.1177/074823379901500501. [DOI] [PubMed] [Google Scholar]
- Wang S, Lantz RC, Chen GJ, et al. The prophylactic effects of U75412E pretreatment in a smoke-induced lung injury rabbit model. Pharmacol Toxicol. 1996;79:231–7. doi: 10.1111/j.1600-0773.1996.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Witten M, Grad R, Quan S, et al. Piriprost pre-treatment attenuates the smoke-induced increase in 99mTcDTPA lung clearance. Exp Lung Res. 1990;16:339–53. doi: 10.3109/01902149009108849. [DOI] [PubMed] [Google Scholar]