Abstract
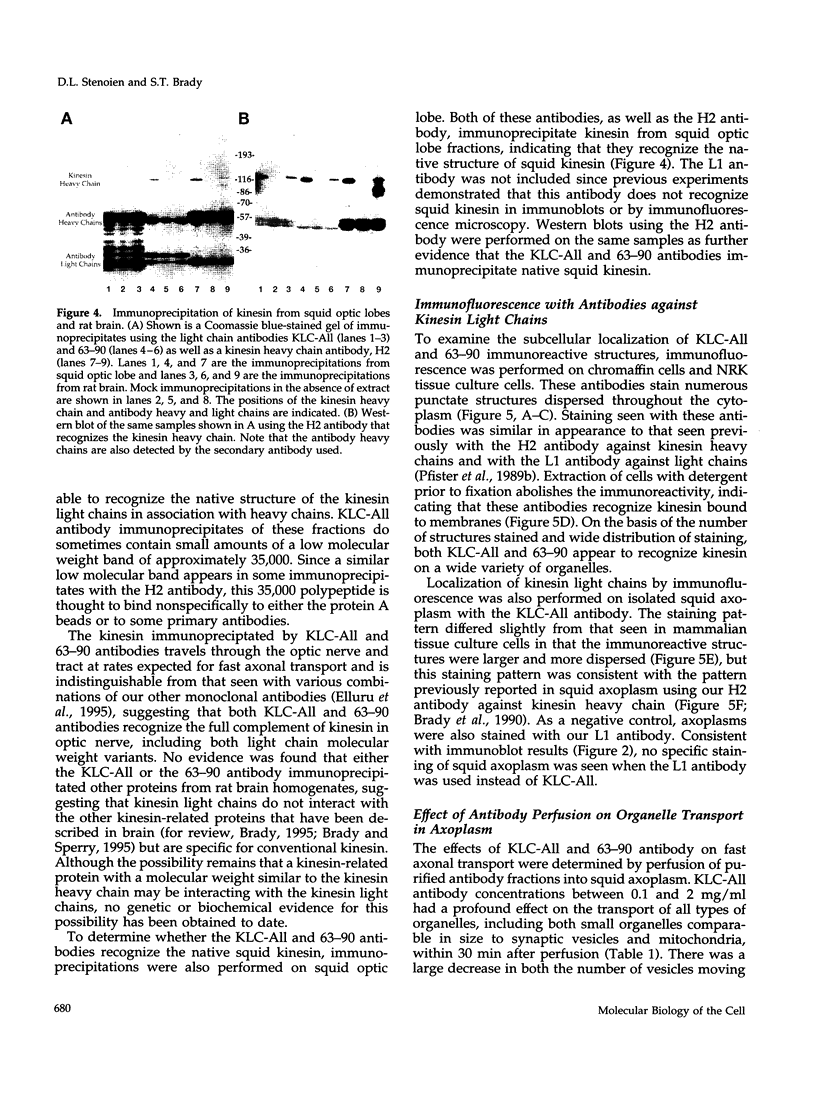
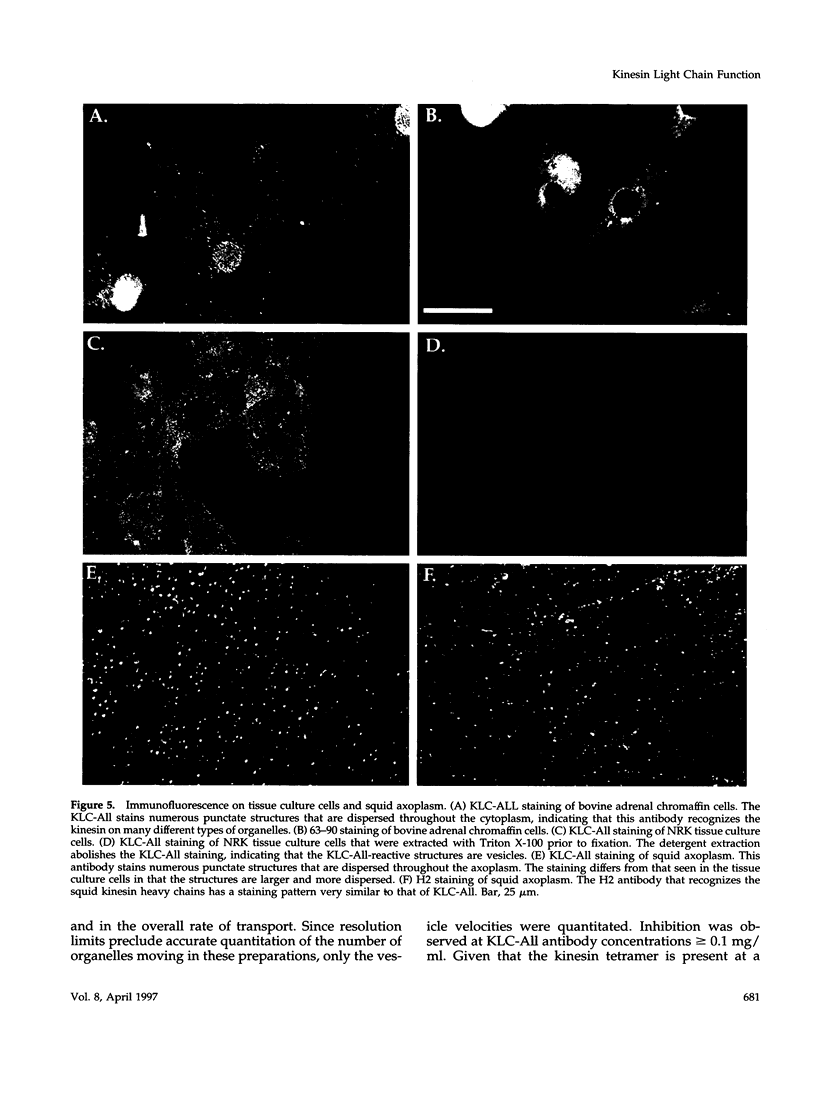
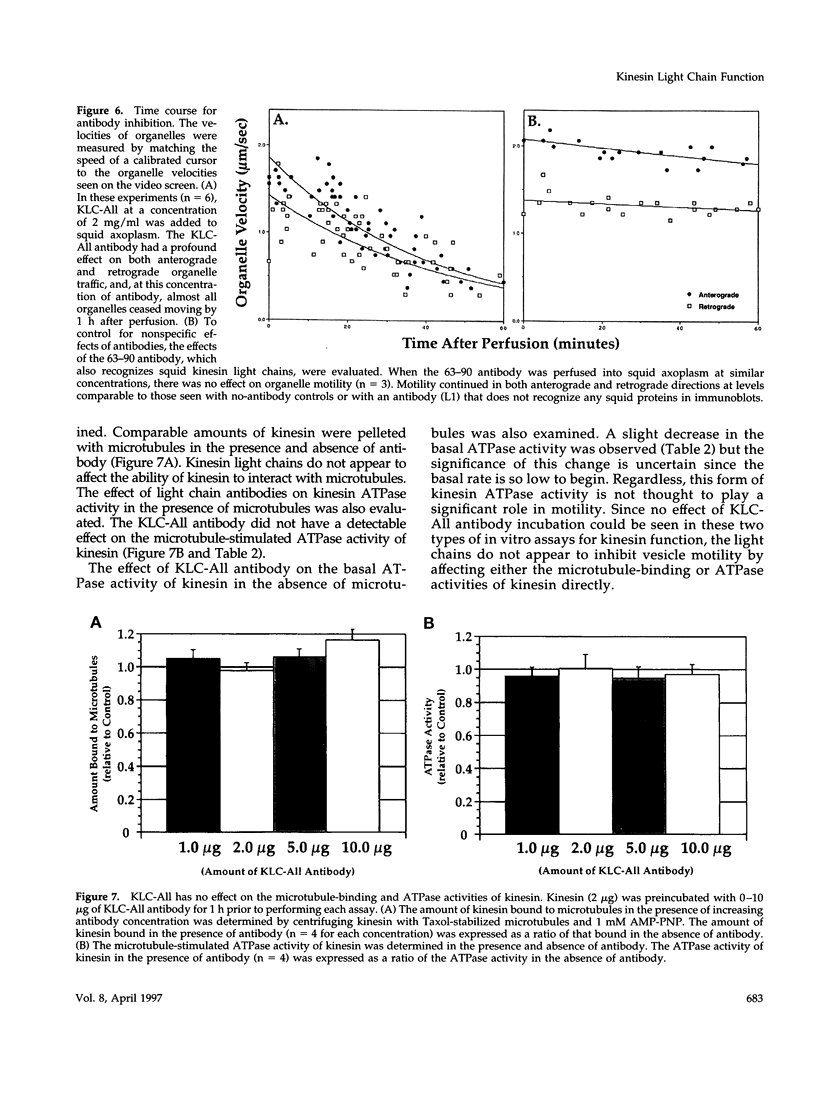
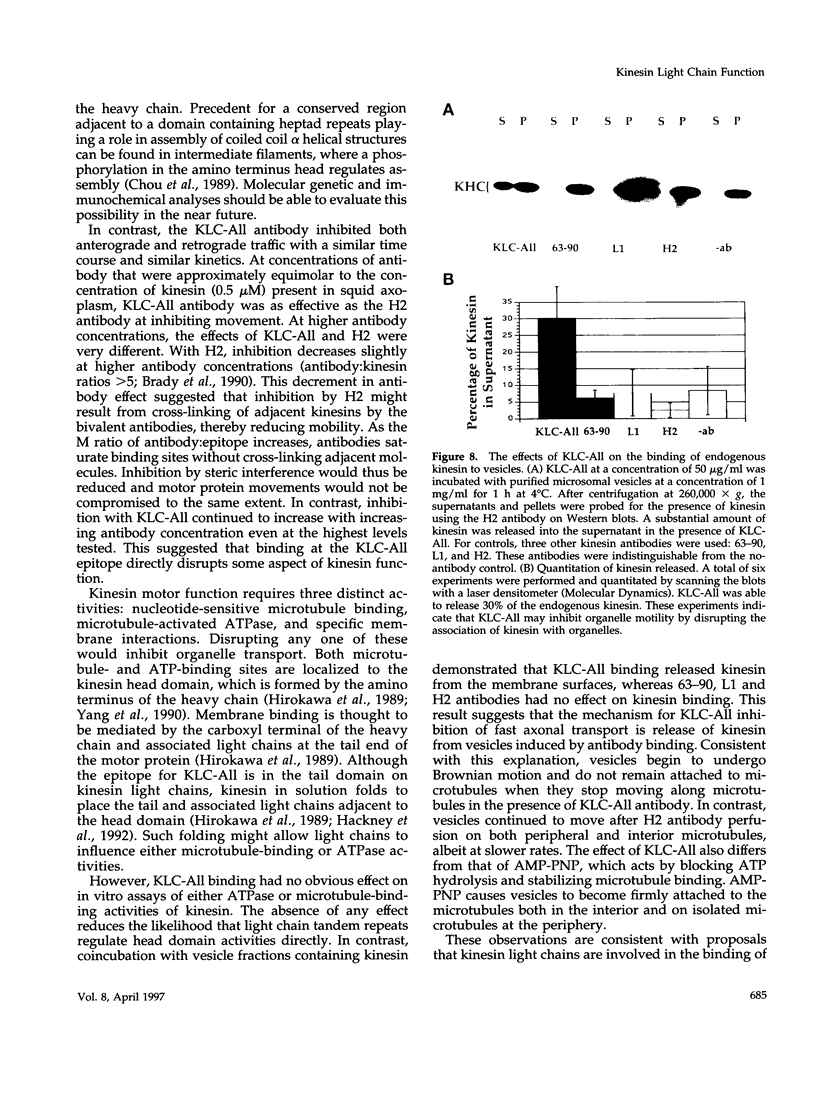
The kinesin heterotetramer consists of two heavy and two light chains. Kinesin light chains have been proposed to act in binding motor protein to cargo, but evidence for this has been indirect. A library of monoclonal antibodies directed against conserved epitopes throughout the kinesin light chain sequence were used to map light chain functional architecture and to assess physiological functions of these domains. Immunocytochemistry with all antibodies showed a punctate pattern that was detergent soluble. A monoclonal antibody (KLC-All) made against a highly conserved epitope in the tandem repeat domain of light chains inhibited fast axonal transport in isolated axoplasm by decreasing both the number and velocity of vesicles moving, whereas an antibody against a conserved amino terminus epitope had no effect. KLC-All was equally effective at inhibiting both anterograde and retrograde transport. Neither antibody inhibited microtubule-binding or ATPase activity in vitro. KLC-All was unique among antibodies tested in releasing kinesin from purified membrane vesicles, suggesting a mechanism of action for inhibition of axonal transport. These results provide further evidence that conventional kinesin is a motor for fast axonal transport and demonstrate that kinesin light chains play an important role in kinesin interaction with membranes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Sekine Y., Takemura R., Zhang Z., Nangaku M., Hirokawa N. Kinesin family in murine central nervous system. J Cell Biol. 1992 Dec;119(5):1287–1296. doi: 10.1083/jcb.119.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga A., Morin P. J., Kosik K. S., Fine R. E. Inhibition of kinesin synthesis and rapid anterograde axonal transport in vivo by an antisense oligonucleotide. J Biol Chem. 1993 Aug 15;268(23):17427–17430. [PubMed] [Google Scholar]
- Bearer E. L., DeGiorgis J. A., Bodner R. A., Kao A. W., Reese T. S. Evidence for myosin motors on organelles in squid axoplasm. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11252–11256. doi: 10.1073/pnas.90.23.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beushausen S., Kladakis A., Jaffe H. Kinesin light chains: identification and characterization of a family of proteins from the optic lobe of the squid Loligo pealii. DNA Cell Biol. 1993 Dec;12(10):901–909. doi: 10.1089/dna.1993.12.901. [DOI] [PubMed] [Google Scholar]
- Bloom G. S., Endow S. A. Motor proteins 1: kinesins. Protein Profile. 1995;2(10):1105–1171. [PubMed] [Google Scholar]
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988 May 3;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A kinesin medley: biochemical and functional heterogeneity. Trends Cell Biol. 1995 Apr;5(4):159–164. doi: 10.1016/s0962-8924(00)88980-1. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982 Dec 10;218(4577):1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 1985;5(2):81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K., Bloom G. S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K. Kinesin interactions with membrane bounded organelles in vivo and in vitro. J Cell Sci Suppl. 1991;14:103–108. doi: 10.1242/jcs.1991.supplement_14.21. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Sperry A. O. Biochemical and functional diversity of microtubule motors in the nervous system. Curr Opin Neurobiol. 1995 Oct;5(5):551–558. doi: 10.1016/0959-4388(95)80058-1. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr J. L., Pfister K. K., Bloom G. S., Slaughter C. A., Brady S. T. Molecular genetics of kinesin light chains: generation of isoforms by alternative splicing. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10114–10118. doi: 10.1073/pnas.88.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A. B., Pfister K. K., Brady S. T. The axonal transport motor 'kinesin' is bound to anterogradely transported organelles: quantitative cytofluorimetric studies of fast axonal transport in the rat. Acta Physiol Scand. 1991 Apr;141(4):469–476. doi: 10.1111/j.1748-1716.1991.tb09107.x. [DOI] [PubMed] [Google Scholar]
- Elluru R. G., Bloom G. S., Brady S. T. Fast axonal transport of kinesin in the rat visual system: functionality of kinesin heavy chain isoforms. Mol Biol Cell. 1995 Jan;6(1):21–40. doi: 10.1091/mbc.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Amos L. A. Kinesin light chain isoforms in Caenorhabditis elegans. J Mol Biol. 1994 Jul 29;240(5):507–512. doi: 10.1006/jmbi.1994.1465. [DOI] [PubMed] [Google Scholar]
- Feiguin F., Ferreira A., Kosik K. S., Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulations. J Cell Biol. 1994 Nov;127(4):1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Niclas J., Vale R. D., Banker G., Kosik K. S. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992 May;117(3):595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger A. K., Goldstein L. S. The Drosophila kinesin light chain. Primary structure and interaction with kinesin heavy chain. J Biol Chem. 1993 Jun 25;268(18):13657–13666. [PubMed] [Google Scholar]
- Gho M., McDonald K., Ganetzky B., Saxton W. M. Effects of kinesin mutations on neuronal functions. Science. 1992 Oct 9;258(5080):313–316. doi: 10.1126/science.1384131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Hudspeth A. J. Chemiluminescence detection of proteins from single cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2563–2567. doi: 10.1073/pnas.88.6.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D., Levitt J. D., Suhan J. Kinesin undergoes a 9 S to 6 S conformational transition. J Biol Chem. 1992 Apr 25;267(12):8696–8701. [PubMed] [Google Scholar]
- Hackney D. D., Levitt J. D., Wagner D. D. Characterization of alpha 2 beta 2 and alpha 2 forms of kinesin. Biochem Biophys Res Commun. 1991 Jan 31;174(2):810–815. doi: 10.1016/0006-291x(91)91490-4. [DOI] [PubMed] [Google Scholar]
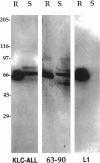
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
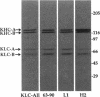
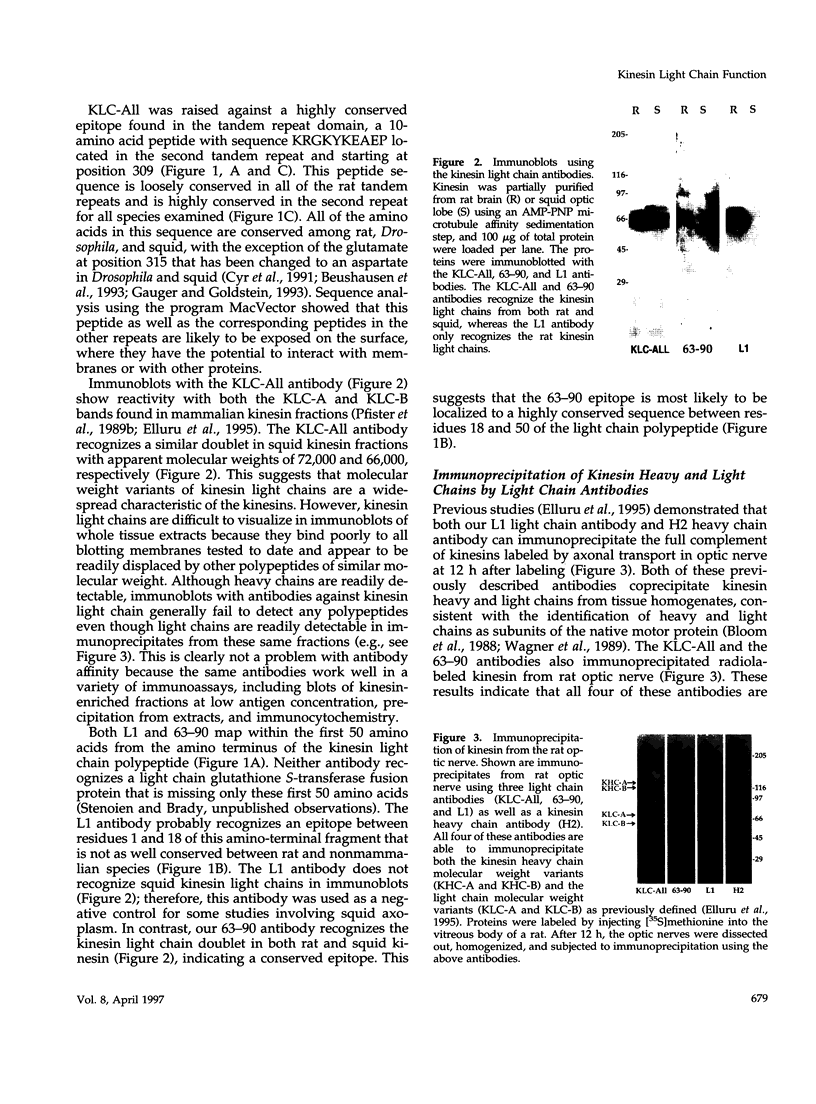
- Hirokawa N., Sato-Yoshitake R., Kobayashi N., Pfister K. K., Bloom G. S., Brady S. T. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991 Jul;114(2):295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Sato-Yoshitake R., Yoshida T., Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990 Sep;111(3):1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck P. J., Swanson J. A. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature. 1990 Aug 30;346(6287):864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J. The distribution, abundance and subcellular localization of kinesin. J Cell Biol. 1989 Jun;108(6):2335–2342. doi: 10.1083/jcb.108.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J., Vale R. D. Movement of microtubules by single kinesin molecules. Nature. 1989 Nov 9;342(6246):154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Johnston K. M., Brady S. T., van der Kooy D., Connolly J. A. A unique tubulin antibody which disrupts particle movement in squid axoplasm. Cell Motil Cytoskeleton. 1987;7(2):110–115. doi: 10.1002/cm.970070203. [DOI] [PubMed] [Google Scholar]
- Kondo S., Sato-Yoshitake R., Noda Y., Aizawa H., Nakata T., Matsuura Y., Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 1994 Jun;125(5):1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg E. A., Shanina N. A., Magretova N. N., Chernyak V. Y., Gelfand V. I. The quaternary structure of bovine brain kinesin. EMBO J. 1988 Feb;7(2):353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg Y. A., Rothwell S. W., Murphy D. B., Gelfand V. I. Isolation of a 45-kDa fragment from the kinesin heavy chain with enhanced ATPase and microtubule-binding activities. J Biol Chem. 1989 Jan 5;264(1):589–595. [PubMed] [Google Scholar]
- Langford G. M., Kuznetsov S. A., Johnson D., Cohen D. L., Weiss D. G. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: evidence for a barbed-end-directed organelle motor. J Cell Sci. 1994 Aug;107(Pt 8):2291–2298. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985 Aug 15;316(6029):645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leopold P. L., McDowall A. W., Pfister K. K., Bloom G. S., Brady S. T. Association of kinesin with characterized membrane-bounded organelles. Cell Motil Cytoskeleton. 1992;23(1):19–33. doi: 10.1002/cm.970230104. [DOI] [PubMed] [Google Scholar]
- Nangaku M., Sato-Yoshitake R., Okada Y., Noda Y., Takemura R., Yamazaki H., Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994 Dec 30;79(7):1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Noda Y., Sato-Yoshitake R., Kondo S., Nangaku M., Hirokawa N. KIF2 is a new microtubule-based anterograde motor that transports membranous organelles distinct from those carried by kinesin heavy chain or KIF3A/B. J Cell Biol. 1995 Apr;129(1):157–167. doi: 10.1083/jcb.129.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Shpetner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987 Sep;105(3):1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Vallee R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987 Nov 12;330(6144):181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Bloom G. S., Brady S. T. Modification of the microtubule-binding and ATPase activities of kinesin by N-ethylmaleimide (NEM) suggests a role for sulfhydryls in fast axonal transport. Biochemistry. 1989 Nov 14;28(23):9006–9012. doi: 10.1021/bi00449a008. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. E., Scholey J. M., Stemple D. L., Vigers G. P., Vale R. D., Sheetz M. P., McIntosh J. R. Characterization of the microtubule movement produced by sea urchin egg kinesin. J Biol Chem. 1987 Feb 25;262(6):2794–2802. [PubMed] [Google Scholar]
- Saxton W. M., Hicks J., Goldstein L. S., Raff E. C. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991 Mar 22;64(6):1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S., Bechtold R. Kinesin is bound with high affinity to squid axon organelles that move to the plus-end of microtubules. J Cell Biol. 1992 Oct;119(2):389–399. doi: 10.1083/jcb.119.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y., Okada Y., Noda Y., Kondo S., Aizawa H., Takemura R., Hirokawa N. A novel microtubule-based motor protein (KIF4) for organelle transports, whose expression is regulated developmentally. J Cell Biol. 1994 Oct;127(1):187–201. doi: 10.1083/jcb.127.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias D. A., Cole D. G., Wedaman K. P., Scholey J. M. The carboxyl-terminal domain of kinesin heavy chain is important for membrane binding. J Biol Chem. 1994 Jan 14;269(2):1477–1485. [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Mitchison T., Steuer E., Reese T. S., Sheetz M. P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell. 1985 Dec;43(3 Pt 2):623–632. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Wagner M. C., Pfister K. K., Bloom G. S., Brady S. T. Copurification of kinesin polypeptides with microtubule-stimulated Mg-ATPase activity and kinetic analysis of enzymatic properties. Cell Motil Cytoskeleton. 1989;12(4):195–215. doi: 10.1002/cm.970120403. [DOI] [PubMed] [Google Scholar]
- Wedaman K. P., Knight A. E., Kendrick-Jones J., Scholey J. M. Sequences of sea urchin kinesin light chain isoforms. J Mol Biol. 1993 May 5;231(1):155–158. doi: 10.1006/jmbi.1993.1267. [DOI] [PubMed] [Google Scholar]
- Wright B. D., Terasaki M., Scholey J. M. Roles of kinesin and kinesin-like proteins in sea urchin embryonic cell division: evaluation using antibody microinjection. J Cell Biol. 1993 Nov;123(3):681–689. doi: 10.1083/jcb.123.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Nakata T., Okada Y., Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995 Sep;130(6):1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Saxton W. M., Stewart R. J., Raff E. C., Goldstein L. S. Evidence that the head of kinesin is sufficient for force generation and motility in vitro. Science. 1990 Jul 6;249(4964):42–47. doi: 10.1126/science.2142332. [DOI] [PubMed] [Google Scholar]