Abstract
Unlike most other effector cells of the innate, as well as the adaptive immune systems, the neutrophil is a relatively undiscerning aggressor with scant regard for damage limitation. Although this highly combative, professional phagocyte has become increasingly implicated in the immunopathogenesis of many acute and chronic inflammatory disorders, of both infective and noninfective origin, effective pharmacological strategies to counter neutrophilaggression have remained elusive. Activation of neutrophils results in rapid mobilization of both stored and extracellular Ca2+, resulting in abrupt, usually transient increases in cytosolic Ca2+, which precede, and are a prerequisite for activation of the Ca2+-dependent pro-inflammatory activities of these cells. Mobilization of Ca2+ by, and restoration of Ca2+ homeostasis to activated neutrophils are multistep processes which present a number of potential targets, some well recognized and others noveland unconventional, for the pharmacological control of neutrophil-mediated inflammation. Uncovering these targets represents the primary focus of this review.
Keywords: calcium, cyclic AMP, NADPH oxidase, neutrophils, sodium-calcium exchanger
Introduction
Polymorphonuclear leukocytes, of which the neutrophil granulocyte is the most abundant, are key components of the innate immune system. These cells are activated and recruited to sites of infection by inflammatory stimuli generated by microbial pathogens, or by the interaction of these with pattern recognition molecules present on/in neighboring tissue cells such as epithelial cells, mast cells and tissue macrophages. Notwithstanding their abruptly mobilizable arsenal of antimicrobialagents, which include antimicrobial peptides/proteins, enzymes, bioactive lipids, and reactive oxidant species (ROS) (Anderson et al 1998; Theron et al 2002; Hatanaka et al 2004), these cells also have biosynthetic capability, albeit limited (Cassatella 1999; Witko-Sarsat et al 2000), which enables them to produce chemokines/cytokines, especially interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α). Acting in concert, TNF-α and IL-8 amplify neutrophil-mediated inflammatory responses by promoting extravasation and accumulation of neutrophils at sites of tissue injury and infection through induction and upregulation of expression of endothelialadhesion molecules and chemotaxis, respectively. Neutrophil migration and localization are further potentiated by leukotriene B4 (LTB4), an endogenously generated bioactive lipid with potent chemoattractant activity (Chen et al 2006).
Although extremely effective in eradicating microbial pathogens, the neutrophil, with its arsenal of indiscriminate oxidants and proteases, poses a potential threat to bystander host cells and tissues in the vicinity of the inflammatory reaction. Accordingly, neutrophil influx must be effcient, protective and promptly downregulated. Nevertheless, there is increasing awareness of the involvement of inappropriate activation of neutrophils in the etiology of many acute/hyper acute and chronic inflammatory disorders of both infective and noninfective origin, important examples of which are shown in Figure 1, with the airways and cardiovascular system being particularly vulnerable.
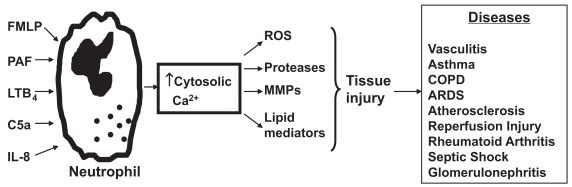
Figure 1.
Activation of neutrophils by chemoattractants such as FMLP, PAF, C5a, and LTB4 increases cytosolic Ca2+ concentrations with resultant generation of toxic reactive oxygen species (ROS) and release of proteases, matrix metalloproteinases (MMPs) and lipid mediators. The tissue injury that may be associated with release of these harmful molecules into the vicinity of innocent bystander host tissues contributes to the pathogenesis of numerous diseases, including chronic obstructive pulmonary disease (COPD) and the acute respiratory distress syndrome (ARDS).
Abbreviations: FMLP, N-formylated peptides/polypeptides; LTB4, leukotriene B4; PAF, platelet-activating factor.
Responsiveness to corticosteroids
Few currently available therapeutic agents, including corticosteroids, effectively control the harmful pro-inflammatory activities of neutrophils. Indeed, insensitivity to corticosteroids appears to be a feature of those disorders in which the neutrophil is the predominant offender.
The apparent insensitivity of neutrophils is attributable to the coexistence of several different resistance mechanisms in these cells. Firstly, in contradistinction to other types of immune and inflammatory cells, glucocorticoids delay neutrophil apoptosis. This antiapoptotic effect of glucocorticoids in neutrophils is achieved by a mechanism which involves sustained expression of the antiapoptotic Bcl-2 family protein, Mcl-1L (Sivertson et al 2007). Secondly, neutrophils contain high levels of the functionally inactive beta isoform of the glucocorticoid receptor (GR), the synthesis of which is further upregulated on exposure of the cells to IL-8 (Strickland et al 2001), rendering them even less sensitive to corticosteroids. Although not yet described in neutrophils, the activity of the enzyme histone deacetylase, which is recruited by activated GRs as a mechanism of repression of expression of multiple inflammatory genes, is decreased in macrophages and circulating mononuclear leukocytes of patients with COPD and severe asthma respectively (reviewed in Barnes 2007). Thirdly, many of the proinflammatory activities of neutrophils, including generation of ROS, release of granule proteases, and generation of prostanoids, eicosanoids, and platelet-activating factor (PAF) occur within seconds of receptor-mediated activation of these cells and are independent of de novo protein synthesis.
Clearly the identification of novel targets for effective neutrophil-directed anti-inflammatory chemotherapy is a priority.
Calcium and neutrophils
Interaction of neutrophil membrane receptors with chemoattractants, opsonized particles, or endothelialadhesion molecules, results in abrupt, transient increases in cytosolic Ca2+ which precede, and are a prerequisite, for initiation of the proinflammatory activities of these cells. Ca2+-activatible inflammatory functions include generation of superoxide by the membrane-associated electron transporting NADPH oxidase, adhesion to vascular endothelium, degranulation, activation of cytosolic phospholipase A2 and 5-lipoxygenase, as well as synthesis of IL-8. Because of this critical dependence of activation of the proinflammatory activities of neutrophils on Ca2+, the mechanisms utilized by these cells to mobilize and dispose of the cation represent attractive, and in several cases, novel potential targets for neutrophil-directed anti-inflammatory chemotherapy.
Calcium handling by activated neutrophils
A model of calcium mobilization and restoration of calcium homeostasis in activated human neutrophils is presented in Figure 2 and discussed below. Release of Ca2+ from intracellular stores following receptor-mediated activation of neutrophils with various stimuli, including chemoattractants such as N-formylated peptides/polypeptides (FMLP), C5a, leukotriene B4 (LTB4), PAF, or IL-8, occurs rapidly, reaching peak values within several seconds of ligand-receptor binding which are 5–10-fold above the basal value of about 100 nM (Favre et al 1996). The receptors for the aforementioned chemoattractants belong to the 7-transmembrane, G-protein-coupled family of receptors. Occupation of these receptors, which are regulated by various Gα and Gβγ subunits, results in activation of the β isoforms of phospholipase C (PLC), which in turn mediate production of inositol-1,4,5-triphosphate (IP3) by hydrolysis of phosphatidylinositol–4,5,-biphosphate (Aliet al 1998; Yue et al 1998). IP3 interacts with Ca2+-mobilizing receptors on intracellular storage vesicles, resulting in discharge of stored Ca2+ into the cytosol. Only modest increases in IP3 of around 15% of maximal are required to mobilize the total pool of stored Ca2+. The duration of the peak increase in cytosolic Ca2+ varies according to the type and concentration of the chemoattractant, but is usually brief, being followed by a progressive decline in the concentration of cytosolic Ca2+ with a return to basal values within several minutes. The duration of the peak cytosolic Ca2+ response, as wellas the rate of decline in the concentration of cytosolic Ca2+, are determined by at least 4 mechanisms. These are: i) shuttling of Ca2+ between the stores and the cytosol (ie, repetitive bouts of release from, and resequestration of Ca2+ into stores (Anderson et al 2005); ii) activation of a secondary wave of Ca2+ influx due to endogenously-generated LTB4 by chemoattractant-activated neutrophils (Steel et al 2007); iii) the efficiency of the systems which promote clearance of Ca2+ from the cytosol (Anderson et al 1998; Steeland Anderson 2002); and iv) the efficiency of the systems which regulate the time of onset, rate and magnitude of influx of extracellular cation (Tintinger et al 2001a).
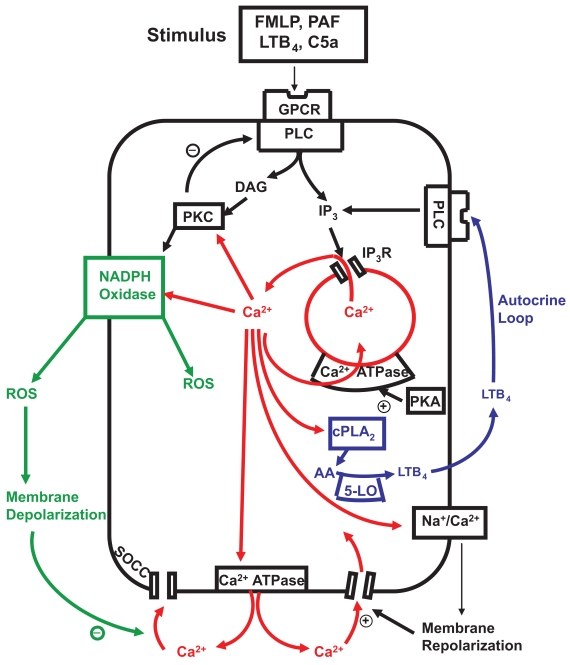
Figure 2.
Calcium-mobilizing stimuli interact with membrane G-protein coupled receptors (GPCR) to activate phospholipase C (PLC) generating inositol triphosphate (IP3) which interacts with IP3 receptors (IP3R) releasing Ca2+ from storage vesicles. Cytosolic Ca2+ phospholipase A2 (cPLA2) Which mobilizes arachidonic acid (AA) for the 5-lipoxygenase (5-LO) pathway. The AA metabolite leukotriene B4 (LTB4) is actively transported to the cell exterior where it binds to its receptor to activate PLC, completing a positive feedback autocrine loop. Ca2+ released into the cytosol is rapidly extruded from the cell by the plasma membrane Ca2+ ATPase and resequested into storage vesicles by the protein kinase A (PKA)-sensitive endomembrane Ca2+ ATPase. Protein kinase C (PKC) activated by Ca2+ and diacylglycerol (DAG) facilitates assembly and activation of NADPH oxidase on the outer membrane which generates reactive oxygen species (ROS) with concomitant membrane depolarization. The depolarized membrane potential delays Ca2+ entry through store operated channels (SOCCs) until the Ca2+-activatible Na+/Ca2+ exchanger, operating in reverse mode, mediates recovery of the membrane potential promoting Ca2+ reuptake via SOCCs. PKC down-regulates PLC as part of a negative feedback loop to terminate IP3 production
Restoration of Ca2+ homeostasis
Rapid and efficient removal of Ca2+ from the cytosol of activated neutrophils ensures that activation of the cells is brief, thereby avoiding Ca2+ overload and hyperreactivity. This is achieved primarily by removal of Ca2+ from the cytosol by two adenosine triphosphate (ATP)-driven Ca2+ pumps operating in unison. These are the plasma membrane Ca2+-ATPase and the endomembrane Ca2+-ATPase, which mediate Ca2+ efflux and resequestration respectively. These two Ca2+ pumps appear to contribute equally to the removal of Ca2+ from the cytosol of activated neutrophils (Anderson and Goolam Mahomed 1997; Pettit and Hallett 2000).
The plasma membrane Ca2+-ATPase of neutrophils is upregulated by calmodulin, acidic phospholipids, and polyunsaturated fatty acids, all of which shift the pump to a higher affinity for Ca2+, resulting in enhanced maximal velocity (Carafoliet al 1992).
Apart from causing activation of PLC and release of stored Ca2+, activation of neutrophils with chemoattractants such as FMLP is also accompanied by transient activation of adenylate cyclase (Iannone et al 1989; Theron et al 2002). This results from the interaction of adenosine, generated by dephosphorylation of adenylates, presumably by ecto-5′nucleotidase, with G-protein/adenylyl cyclase-coupled adenosine receptors (AR) of the A2A subtype on the neutrophil membrane (Iannone et al 1989; Theron et al 2002), resulting in activation of adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent protein kinase A (PKA). Phospholamban, a polypeptide regulator of the endomembrane Ca2+-ATPase, undergoes PKA-mediated phosphorylation which results in up-regulation of the Ca2+ sequestering/resequestering activity of the pump (Chu et al 2000).
Importantly, efficient clearance of Ca2+ by the plasma membrane and endomembrane Ca2+-ATPases is facilitated by the membrane depolarizing action of NADPH oxidase which restricts the influx of extracellular Ca2+. NADPH oxidase undergoes Ca2+-dependent activation on exposure of neutrophils to chemoattractants such as C5a, FMLP, and LTB4, but not PAF or IL-8 (Steeland Anderson 2002; Guichard et al 2007), as well as to opsonized antigens. Activation of NADPH oxidase is accompanied by an abrupt and steep decrease in membrane potential which results primarily from the electrogenic properties of the oxidase (Tintinger et al 2001a). When the cells are depolarized, the driving force for entry of Ca2+ is abolished because the electrical component of the electrochemical gradient promoting Ca2+ entry is markedly reduced. Consequently, NADPH oxidase-mediated membrane depolarization enables the plasma membrane and endomembrane Ca2+-ATPases to mediate clearance of Ca2+ from the cytosol of activated neutrophils, unhindered by influx of extracellular Ca2+.
Influx of Ca2+
Depletion of intracellular Ca2+ stores following receptor-mediated activation is followed by refilling of the stores, a process known as store-operated Ca2+ influx, or capacitative Ca2+ influx (Parekh and Penner 1997). In neutrophils, the time of onset and rate of store-operated Ca+ influx are determined primarily by the duration and intensity of activity of NADPH oxidase. In the case of cells activated with Ca2+-mobilizing stimuli which are inefficient activators of the oxidase, such as PAF (Steeland Anderson 2002), influx of Ca2+occurs rapidly, overwhelming the Ca2+-ATPases, resulting in prolonged elevations in peak cytosolic Ca2+ concentrations. On the other hand, exposure of the cells to Ca2+-mobilizing activators of the oxidase, such as FMLP, is accompanied by efficient clearance of store-derived cytosolic Ca2+, in the setting of a gradual influx of Ca2+, the rate of which is super-imposable on that of membrane repolarization (Tintinger and Anderson 2004).
While the magnitude and duration of the maximal membrane depolarization responses of neutrophils activated with FMLP are determined by the intensity and duration of activation of NADPH oxidase, as wellas by the counteracting effects of an efflux of protons from the cells (Schrenzel et al 1998; Bánfi et al 2000), until relatively recently less was known about the mechanisms which contribute to membrane repolarization. We have identified a role for the electrogenic Na+/Ca2+ exchanger operating in reverse mode in mediating membrane repolarization in activated neutrophils (Tintinger and Anderson 2004).
As opposed to being a major transporter of extracellular Ca2+ for store refilling, the primary role of the exchanger when operating in reverse mode is to mediate recovery of the membrane potential which is necessary to drive the influx of Ca2+ through store-operated Ca2+ channels (Tintinger and Anderson 2004). However, the Na+/Ca2+ exchanger is vulnerable to oxidative inactivation, being sensitive to the phagocyte-derived oxidants, hydrogen peroxide, and especially hypochlorous acid (Coetzee et al 1994; Tintinger et al 2007). The efficiency of the exchanger in activated neutrophils is therefore dependent on its level of exposure to phagocyte-derived oxidants.
Store-operated Ca2+ channels
Until very recently, the precise molecular identity of the store-operated Ca2+ channels operative in human neutrophils and other cell types had not been conclusively established. One particular family of nonvoltage-activated Ca2+ channels which had attracted considerable attention and interest was the family of transient receptor potential (TRP) channels (Elliot 2001; Liet al 2002). Although two members of this family, LTFP2 and TRP6, have been described in neutrophils (Liet al 2002; Heiner et al 2003), the characteristics of these channels are not entirely compatible with those of putative, prototype store-operated Ca2+ channels. However, in the past two years the identities of the major components of store-operated Ca2+ channels have been elucidated. These are the proteins Stim1 (and possibly Stim2), and Orai1 (and possibly Orai2 and 3), which function as the Ca2+ sensing and channel-forming proteins, respectively (Spassova et al 2006). Stim1 is located in the endoplasmic reticulum where it binds reversibly to Ca2+. Following store depletion, dissociation of Ca2+ causes Stim1 to redistribute within the endoplasmic reticulum to areas which are in close proximity to Orai1 within the plasma membrane. Stim1 then activates the Ca2+-selective Oraichannels by a mechanism which remains to be elucidated (reviewed by Putney 2007).
Neutrophil-directed anti-inflammatory strategies
Notwithstanding the increasing awareness of the anti-inflammatory potential of macrolide antimicrobialagents (Simpson et al 2008), several other categories of anti-inflammatory agents have recently been described which have the potential to target neutrophils. These agents, described in detail elsewhere (Barnes 2007), include: i) antagonists of receptors for chemoattractants such as C5a, IL-8, and LTB4; ii) antagonists of endothelialadhesion molecules; iii) inhibitors of pro-inflammatory enzymes and transcription factors such as phosphoinositide 3-kinase, 38 mitogen-activated protein kinase, and nuclear factor kappa B; and iv) activators of histone deacetylase 2 which is recruited by activated glucocorticoid receptors to switch off multiple proinflammatory genes.
In addition to these, recent insights into the mechanisms utilized by activated neutrophils to mobilize both intracellular and extracellular Ca2+, as wellas to restore Ca2+ homeostasis to the cells present a number of novel targets which are amenable to pharmacological control. In some cases target enhancement of activity improves the efficiency of clearance of Ca2+ from the cytosol, while in others this is achieved by target inhibition. Targets in the former category include endomembrane Ca2+ ATP-ases, NADPH oxidase, myeloperoxidase, and protein kinase C, while in the latter category, phospholipase C, the Na2+/Ca2+ exchanger, 5′-lipoxygenase, and store-operated Ca2+ channels represent attractive targets.
Ca2+ mobilization and restoration of Ca2+ homeostasis as targets for neutrophil-directed anti-inflammatory chemotherapy
Inhibitors of phospholipase C
The importance of PLC as a potential target is underscored by the critical involvement of IP3, not only in mediating the release of Ca2+ from intracellular stores, but also in sustaining elevations in cytosolic Ca2+ by promoting shuttling of the cation between the stores and the cytosol (Anderson et al 2005) and possibly by initiating influx of Ca2+ (Ma 2000; Bolotina 2004). Furthermore, diacylglycerol (DAG) generated by PLC activates protein kinase C (PKC), which in turn promotes the assembly and activation of NADPH oxidase (Tauber 1987).
Although pharmacological inhibitors of PLC and antagonists of IP3 receptors such as U73122 and 2-aminoethoxydiphenyl borate respectively, are effective in experimental systems, no such inhibitors are available for clinicaluse. However, PLC activity is inhibited in various cell types such as vascular endothelial cells (Avdonin and Ryan 2000) and platelets (Murphy et al 1991) by a negative feedback loop involving PKC. This feedback inhibition on PLC by PKC appears to be operative in human neutrophils as we have demonstrated recently that in the presence of PKC inhibitors, such as GF109203X, IP3, and cytosolic Ca2+ concentrations in chemoattractant-activated neutrophils reach higher peaks and remain elevated longer than those measured in untreated cells (unpublished observations). This finding is important as it provides insight into the physiological mechanisms that down-regulate PLC in activated neutrophils and has implications for the design of pharmacological strategies targeting PKC, as inhibitors of this enzyme may paradoxically enhance the Ca2+-dependent proinflammatory activities of phagocytes. In contradistinction to the paucity of clinically useful PLC or PKC inhibitors, the endomembrane Ca2+ ATPase is considerably more amenable to pharmacologic interventions.
Upregulation of endomembrane Ca2+ ATP-ases
The activity of the Ca2+ resequestering endomembrane Ca2+ ATP-ases can be upregulated by the cAMP-sensitive enzyme, protein kinase A (PKA) (Chu et al 2000). PKA-mediated enhancement of the activity of the endomembrane Ca2+ ATP-ase markedly accelerates the clearance of cytosolic Ca2+ following release of the cation from storage vesicles (Anderson et al 1998; Tintinger et al 2001b). The apparent sensitivity of PKA to cAMP has been exploited with the introduction of a wide range of pharmacologic agents, all of which share the ability to elevate intracellular cAMP concentrations. This can be achieved by several mechanisms, including activation of adenylate cyclase, or inhibition of phosphodiesterase enzymes (PDEs) responsible for the metabolism of cAMP (Moore and Willoughby 1995). Adenylate cyclase is activated via G-protein-coupled receptors linked to membrane associated β-adrenergic, adenosine (A2A) and prostaglandin E2 receptors. PDE isoenzymes of various classes have been identified in neutrophils and PDE 4B2 is probably the most abundant of these (Wang et al 1999). The relative tissue specificity of PDE 4 isoenzymes for inflammatory cells has enabled some degree of pharmacological targeting of neutrophils by new generation agents such as roflimulast (Sanz et al 2007) and cilomilast (Baumer et al 2007).
The validity of this pharmacologic approach has been confirmed in vitro as cAMP-elevating agents markedly suppress a range of Ca2+-dependent proinflammatory activities of neutrophils, including adhesion to vascular endothelium (Bloemen et al 1997), oxidant production (Tintinger et al 2000), as wellas release of proteases, eicosanoids, and cytokines (Moore and Willoughby 1995; Tintinger et al 2000). Furthermore, numerous clinical trials are ongoing or have been completed in which the efficacy of these agents has been verified in vivo (Pauwels et al 1997; Sullivan et al 2001; Vignola 2004). Less well documented, however, is the role of cGMP during the restoration of Ca2+ homeostasis in activated neutrophils. Intracellular cGMP concentrations can be increased by activation of guanylate cyclase, or by inhibition of PDEs that metabolize cGMP, predominantly PDEs 3 and 5 (Torphy 1998). The endomembrane Ca2+-ATPase has been identified as a putative target of cGMP acting via protein kinase G (Ay et al 2006), although other mechanisms may exist whereby cGMP accelerates the clearance of cytosolic Ca2+. In this regard, cGMP may elevate intracellular cAMP levels by effectively acting as a competitive antagonist of PDE 3-mediated hydrolysis of cAMP (PDE 3 hydrolyzes both cyclic nucleotides) (Boswell-Smith et al 2006). The selective PDE 3 inhibitor, cilostazol, significantly attenuated FMLP-mediated adhesion of neutrophils to human umbilical endothelial cells in vitro (Yang et al 2006), and suppressed the cough threshold in elderly asthmatics (Ishiura et al 2005).
Inhibitors of 5-Lipoxygenase
Leukotrienes, such as LTB4, are produced by activated neutrophils consequent to upregulation of the 5-lipoxygenase (5-LO) pathway (Peters-Golden and Henderson 2007). Phospholipase A2 hydrolyzes membrane phospholipids liberating arachidonic acid, the substrate for 5-LO, and in the presence of calcium and 5-lipoxygenase-activating protein (FLAP), activated 5-LO generates leukotriene A4, which in neutrophils is converted to LTB4. The diverse role of LTB4 in promoting inflammation includes recruitment of inflammatory cells (De Caterina and Zampolli 2004), activation of cytosolic PLA2 and degranulation (Boyce 2007), as well as the release of cytokines and matrix metalloproteinases, thus playing a role in immunoregulation (Rola-Pleszczynkiet al 1986; Ford-Hutchinson 1990; Leppert et al 1995). In addition to the physiological responses above, LTB4 also participates in a positive feedback autocrine loop which activates phospholipase C and triggers sustained elevations of cytosolic Ca2+ in PAF-activated neutrophils (McDonald et al 1994; Steel et al 2007). Intracellular LTB4 derived from 5-LO is actively transported to the cell exterior where it interacts with high affinity LTB4 receptors (BLT1) linked to PLC on the neutrophil plasma membrane. This interaction induces a secondary pulse of IP3 which mediates sustained release of Ca2+ into the cytosoland this in turn potentiates Ca2+-dependent pro-inflammatory responses (Surette et al 1999; Steel et al 2007). The validity of pharmacological anti-inflammatory strategies targeting LTB4 is underscored by recent evidence for the involvement of this leukotriene in the pathogenesis of bronchialasthma (De Caterina and Zampolli 2004), COPD (Marian et al 2006), and inflammatory arthritis (Chen et al 2006). Importantly, the imidazole antimycotic, itraconazole, antagonizes the effects of LTB4 in PAF-activated neutrophils by inhibiting 5-LO (Steel et al 2007). The concentrations of itraconazole required to inhibit 5-LO are similar to those achieved in the plasma of patients receiving this antimycotic (Kageyama et al 1999). Interestingly, other agents in this class, fluconazole and flutrimazole, may also possess anti-inflammatory properties as the former decreased mortality in critically ill patients by a mechanism unrelated to its antifungal properties (Jacobs et al 2003), while the latter inhibited arachidonic acid-induced ear edema in a murine model (Merlos et al 1996). Other 5-LO inhibitors such as zileuton, which inhibits production of both LTB4 and the cysteinyl leukotrienes, LTC4 and LTD4, by activated eosinophils, have shown efficacy in clinical trials of patients with bronchialasthma (Peters-Golden and Henderson 2007).
This is in keeping with the well established pathogenetic role of cysteinyl leukotrienes in inflammatory airway diseases (Arm 2004), and although neutrophils do not generate LTC4, or LTD4, receptors for cysteinyl leukotrienes, namely CysLT1 and CysLT2 are present on the plasma membrane of these cells. The physiological significance of these receptors is unknown, but we have found recently that cysteinyl leukotrienes act as receptor-mediated priming agents for human neutrophils in vitro. Pretreatment of neutrophils with these agents augments the subsequent responses to FMLP with marked increases in oxidant production and elastase release compared with cells activated with the chemoattractant alone (unpublished observations). This finding is likely to be of importance given the widespread use of CysLT1 receptor antagonists such as montelukast, pranlukast and zafirlukast, all of which may modulate cysteinyl leukotriene-mediated priming of neutrophils in a clinical setting.
NADPH oxidase
The membrane-associated NADPH oxidase complex is assembled on the plasma membrane and becomes incorporated into the phagocytic vacuole following phagocytosis of microbial pathogens. When activated, the oxidase generates highly ROS by transferring electrons from NADPH to molecular oxygen. This electrogenic transfer of electrons results in significant depolarization of the resting membrane potential. The oxygen radicals formed during this process include superoxide anions, hydroxyl radicals, hydrogen peroxide and hypochlorous acid (Nagata 2005), all of which may participate in the killing of microbial pathogens. However, these toxic molecules have also been implicated in the pathogenesis of numerous diseases as their release to the extracellular environment may damage innocent bystander host tissues (Moraes et al 2006). Although inhibition of the oxidase may abolish the generation of toxic ROS, this also abrogates the membrane depolarization response and dissipates the electrical gradient required to restrain Ca2+ entry into leukocytes. Veritably, neutrophils lacking a functional oxidase such as those of patients with chronic granulomatous disease (CGD), are inherently predisposed to Ca2+ overload (Tintinger et al 2001a; Rada et al 2003) with consequent exaggeration of Ca2+-dependent proinflammatory activity, including increased release of proteolytic enzymes (Tintinger et al 2001a) and cytokines (Bylund et al 2007). NADPH oxidase thus fulfi lls dual roles as destroyer of microbial pathogens and in protecting the cell from Ca2+ flooding of the cytosol mediated by its important membrane depolarizing action. Therefore, somewhat paradoxically, enhancing the activity of the oxidase may uncover unforeseen anti-inflammatory properties (Hallett 2003; Hultqvist et al 2006).
In addition to mediating membrane depolarization, recent evidence suggests that the oxidase also indirectly modulates the rate of neutrophil membrane repolarization (Tintinger et al 2007). In this regard, myeloperoxidase (MPO) and hypochlorous acid have emerged as important regulators of the neutrophil membrane potential following activation of NADPH oxidase.
Myeloperoxidase and hypochlorous acid
Myeloperoxidase is stored in tertiary granules in the neutrophil cytosol and following the formation of phagocytic vacuoles is released into the phagosome, together with ROS generated from the concomitant activation of NADPH oxidase. Hydrogen peroxide thus formed acts as a substrate for MPO which catalyzes its conversion to the highly reactive oxidant, hypochlorous acid (HOCl). In the presence of MPO inhibitors such as sodium azide or 4-aminobenzoyl hydrazide (ABAH), the magnitude of neutrophil membrane depolarization in response to activating stimuli such as FMLP is not altered. However, the rate of recovery of the membrane potential is significantly accelerated (Tintinger et al 2007) and this is associated with an increased rate and magnitude of Ca2+ reuptake. Thus, inhibition of HOCl generation promotes rapid recovery of the membrane potentialand accelerates the rate of capacitative Ca2+ entry, underscoring the regulatory roles of MPO and HOCl during these events. This may paradoxically represent a physiologicalanti-inflammatory activity of HOCl given the calcium requirements of numerous pro-inflammatory neutrophil functions. Lending support to this contention, exaggerated inflammatory responses have been observed in the skin and lungs of MPO-deficient mice exposed to injurious ultraviolet light and irradiation, respectively (Milla et al 2004; Komatsu et al 2006).
Inhibition of store-operated calcium channels
The IP3-mediated release of Ca2+ from neutrophil storage vesicles is associated with activation of Ca2+ entry channels on the plasma membrane of these cells in order to facilitate store refilling. The mechanism of activation of these store-operated channels (SOCCs) remains controversial, although recent evidence suggests a role for proteins Stim 1 and Orai 1 (Spassova et al 2006). Given the elusive nature of the structure and mechanism/s of activation of these channels, it has been difficult to design pharmacologic inhibitors of SOCCs. However, the recent advances in our understanding of these channels, notably Stim 1 and Orai 1 (Putney 2007), may pave the way for the development of agents that antagonize this mechanism of Ca2+ reuptake. Regardless of the precise identity of, or coupling mechanisms that activate SOCCs, the rate of Ca2+ entry through these channels is regulated by the electrochemical gradient for Ca2+ across the plasma membrane (Geiszt et al 1997). Accordingly, at depolarized potentials, Ca2+ reuptake is opposed by the electrical gradient and is only initiated during the recovery phase of the membrane potential. Crucially, the rate of membrane repolarization is modulated by the activity of the plasma membrane Na+/Ca2+ exchanger.
Inhibition of the Na+/Ca2+ exchanger
The reverse mode of the Na+/Ca2+ exchanger is activated by the rapid rise in cytosolic Ca2+ consequent to release of the cation from stores. When operating in reverse mode, the exchanger extrudes 3 Na+ ions for each Ca2+ ion entering the cell (Blaustein and Lederer 1999), with a resultant net loss of positive charge. Although proton efflux from the cell is the primary charge compensating mechanism (Schrenzel et al 1998; Bánfi et al 1999), the electrogenic Na+/Ca2+ exchanger contributes to recovery of the membrane potential (Tintinger and Anderson 2004) and represents a target amenable to pharmacologic intervention. Inhibitors of the reverse mode of the Na+/Ca2+ exchanger such as KB-R7943 markedly retard the rate of membrane repolarization with consequent attenuation of the rate and magnitude of Ca2+ reuptake (Tintinger and Anderson 2004). It is likely that delayed Ca2+ entry with incomplete refilling of Ca2+ storage vesicles mediated by inhibitors of the exchanger may down-regulate the proinflammatory activity of neutrophils. Agents that inhibit the reverse mode of the Na+/Ca2+ exchanger including KB-R7943 and SN-6 have shown promise in animal models of ischemia-reperfusion injury (Iwamato 2007), a condition in which activated neutrophils are considered to play a central pathogenetic role (Buras and Reenstra 2007). Interestingly, the expression of Na+/Ca2+ exchanger genes is upregulated in leukocytes from patients with cardiac failure (Seiler et al 2004).
Conclusions
Advances in our understanding of the physiologic mechanisms pertaining to calcium handling by activated neutrophils have facilitated recognition of novel pharmacologic strategies designed to suppress the pro-inflammatory activities of these cells. The distinct potential for agents such as imidazole anti-mycotics, antagonists of cysteinyl leukotriene receptors, inhibitors of the Na+/Ca2+ exchanger, long-acting beta-adrenergic receptor agonists and phosphodiesterase inhibitors to modulate neutrophil-mediated inflammation in vivo, creates exciting new opportunities for researchers in this important field.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- Ali H, Sozzani S, Fisher I, et al. Differential regulation of formyl peptide and platelet-activating factor receptors: role of phospholipase Cβ, phosphorylation by protein kinase A. J Biol Chem. 1998;273:11012–16. doi: 10.1074/jbc.273.18.11012. [DOI] [PubMed] [Google Scholar]
- Anderson R, Goolam Mahomed A, Theron AJ, et al. Effect of rolipram and dibutyryl cyclic AMP on resequestration of cytosolic calcium in FMLP-activated human neutrophils. Br J Pharmacol. 1998;124:547–55. doi: 10.1038/sj.bjp.0701849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Goolam Mahomed A. Calcium efflux and influx in f-met-leu-phe (fMLP)-activated human neutrophils are chronologically distinct events. Clin Exp Immunol. 1997;110:132–38. doi: 10.1046/j.1365-2249.1997.5051403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Steel HC, Tintinger GR. Inositol 1,4,5-triphosphate-mediated shuttling between intracellular stores and the cytosol contributes to the sustained elevation in cytosolic calcium in FMLP-activated human neutrophils. Biochem Pharmacol. 2005;69:1567–75. doi: 10.1016/j.bcp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Arm JP. Leukotriene generation and clinical implications. Allergy Asthma Proc. 2004;25:37–42. [PubMed] [Google Scholar]
- Avdonin P, Ryan US. Receptor-dependent regulation of [Ca2+]; and phospholipase C in vascular endothelial cells. J Recept Signal Transduct Res. 2000;20:235–54. doi: 10.3109/10799890009150646. [DOI] [PubMed] [Google Scholar]
- Ay B, Iyanoye A, Sieck GC, et al. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Cell Mol Physiol. 2006;290:L278–83. doi: 10.1152/ajplung.00188.2005. [DOI] [PubMed] [Google Scholar]
- Bánfi B, Maturana A, Jaconi S, et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–42. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol. 2007;119:1055–62. doi: 10.1016/j.jaci.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Baumer W, Hoppmann J, Rundfeldt C, et al. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets. 2007;6:17–26. doi: 10.2174/187152807780077318. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/Calcium exchange: Its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Bloemen PGM, Van den Tweel MC, Hendricks PAJ, et al. Increased cAMP levels in stimulated neutrophils inhibit their adhesion to human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1997;16:L580–7. doi: 10.1152/ajplung.1997.272.4.L580. [DOI] [PubMed] [Google Scholar]
- Bolotina VM. Store-operated channels: diversity and activation mechanisms [online] Sci STKE: Signal Transduction Knowledge Environment 2004. 2004;(24):pe34. doi: 10.1126/stke.2432004pe34. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147:S252–7. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras JA, Reenstra WR. Endothelial-neutrophil interactions during ischemia and reperfusion injury: basic mechanisms of hyperbaric oxygen. Neurol Res. 2007;29:127–31. doi: 10.1179/016164107X174147. [DOI] [PubMed] [Google Scholar]
- Bylund J, Macdonald KL, Brown KL, et al. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-dependent activation of NF-kappa B. Eur J Immunol. 2007;37:1087–96. doi: 10.1002/eji.200636651. [DOI] [PubMed] [Google Scholar]
- Carafolie E, Kessler F, Falchetto R, et al. The molecular basis of the modulation of the plasma membrane calcium pump by calmodulin. Ann NY Acad Sci. 1992;671:58–68. doi: 10.1111/j.1749-6632.1992.tb43784.x. [DOI] [PubMed] [Google Scholar]
- Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- Chen M, Lam BK, Kanaoka Y, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthiris. J Exp Med. 2006;203:837–42. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu GX, Lester JW, Young KB, et al. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta-agonists. J Biol Chem. 2000;275:38938–43. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Ichikawa H, Hearse DJ. Oxidant stress inhibits Na-Ca-exchange current in cardiac myocytes: mediation by sulphydryl groups? Am J Physiol Heart Circ Physiol. 1994;266:H909–19. doi: 10.1152/ajpheart.1994.266.3.H909. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Zampolli A. From asthma to atherosclerosis – 5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350:4–7. doi: 10.1056/NEJMp038190. [DOI] [PubMed] [Google Scholar]
- Elliott AC. Recent developments in non-excitble cell calcium entry. Cell Calcium. 2001;30:73–93. doi: 10.1054/ceca.2001.0215. [DOI] [PubMed] [Google Scholar]
- Favre CJ, Nüsse O, Lew DP, et al. Store-operated calcium influx: what is the message from the stores to the membrane? J Lab Clin Med. 1996;128:19–26. doi: 10.1016/s0022-2143(96)90110-9. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson AW. Leukotriene B4 in inflammation. Crit Rev Immunol. 1990;10:1–12. [PubMed] [Google Scholar]
- Geiszt M, Kapus A, Német K, et al. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes: alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–8. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- Guichard C, Pedruzzi E, Dewas C, et al. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J Biol Chem. 2007;280:37021–32. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- Hallett MB. Holding back neutrophilaggression; the oxidase has potential. Clin Exp Immunol. 2003;132:181–4. doi: 10.1046/j.1365-2249.2003.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka E, Carvalho BTC, Condino-Neto A, et al. Hyperresponsiveness of neutrophils from gp91phox deficient patients to lipopolysaccharide and serum amyloid A. Immunol Lett. 2004;94:43–6. doi: 10.1016/j.imlet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Halaszovich CR, et al. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes, evidence for currents through long TRP channel2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–53. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultqvist M, Olofsson P, Gelderman KA, et al. A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006;3:1625–36. doi: 10.1371/journal.pmed.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone MA, Wolberg G, Zimmerman TP. Chemotactic peptide induces cAMP elevation in human neutrophils by amplification of the adenylate cyclase response to endogenously produced adenosine. J Biol Chem. 1989;264:20177–80. [PubMed] [Google Scholar]
- Ishiura Y, Fujimura M, Nobata K, et al. Phosphodiesterase 3 inhibition and cough in elderly asthmatics. Cough (London, England) 2005;1:11. doi: 10.1186/1745-9974-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T. Na+/Ca2+ exchange as a drug target – insights from molecular pharmacology and genetic engineering. Ann N Y Acad Sci. 2007;1099:516–28. doi: 10.1196/annals.1387.039. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Price Evans DA, Tariq M, et al. Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med. 2003;31:1938–46. doi: 10.1097/01.CCM.0000074724.71242.88. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Masuya M, Tanaka I, et al. Plasma concentration of itraconazole and its antifungal prophylactic efficacy in patients with neutropenia after chemotherapy for acute leukemia. J Infect Chemother. 1999;5:213–16. doi: 10.1007/s101560050038. [DOI] [PubMed] [Google Scholar]
- Komatsu J, Koyama H, Maeda N, et al. Earlier onset of neutrophil-mediated inflammation in the ultraviolet-exposed skin of mice deficient in myeloperoxidase and NADPH oxidase. Inflamm Res. 2006;55:200–6. doi: 10.1007/s00011-006-0071-3. [DOI] [PubMed] [Google Scholar]
- Leppert D, Hausser SL, Kishiyama JL, et al. Stimulation of matrix metalloproteinase-dependent migration of T-cells by eicosanoids. FASEB J. 1995;9:1473–81. doi: 10.1096/fasebj.9.14.7589989. [DOI] [PubMed] [Google Scholar]
- Li SW, Westwick J, Poll CT. Receptor-operated Ca2+ influx channels in leukocytes: a therapeutic target? Trends Pharmacol Sci. 2002;23:63–70. doi: 10.1016/s0165-6147(00)01897-6. [DOI] [PubMed] [Google Scholar]
- Ma H-T. Requirements of the inositol triphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–52. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- Marian E, Baraldo S, Visentin A, et al. Up-regulated membrane and nuclear leukotriene B4 receptors in COPD. Chest. 2006;129:1523–30. doi: 10.1378/chest.129.6.1523. [DOI] [PubMed] [Google Scholar]
- McDonald PP, McColl SR, Braquet P, et al. Autocrine enhancement of leukotriene synthesis by endogenous leukotriene B4 and platelet-activating factor in human neutrophils. Br J Pharmacol. 1994;111:852–60. doi: 10.1111/j.1476-5381.1994.tb14816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos M, Vericat ML, Garcia-Rafanell J, et al. Topical anti-inflammatory properties of flutrimazole, a new imidazole antifungalagent. Inflamm Res. 1996;45:20–5. doi: 10.1007/BF02263500. [DOI] [PubMed] [Google Scholar]
- Milla C, Yang S, Cornfield DN, et al. Myeloperoxidase deficiency enhances inflammation after allogeneic marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L706–14. doi: 10.1152/ajplung.00015.2004. [DOI] [PubMed] [Google Scholar]
- Moore AR, Willoughby DA. The role of cAMP regulation in controlling inflammation. Clin Exp Immunol. 1995;101:387–9. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in pathogenesis of lung injury. Curr Opin Hematol. 2006;13:21–7. doi: 10.1097/01.moh.0000190113.31027.d5. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Elmore M, Kellie S, et al. The relationship between cytosolic Ca2+, sn-1,2-diacylglyceroland inositol 1,4,5-triphosphate elevation in platelet-activating factor-stimulated rabbit platelets. Influence of protein kinase C on production of signal molecules. Biochem J. 1991;278:255–61. doi: 10.1042/bj2780255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M. Inflammatory cells and oxygen radicals. Curr Drug Targets Inflamm Allergy. 2005;4:503–4. doi: 10.2174/1568010054526322. [DOI] [PubMed] [Google Scholar]
- Parekh A, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–30. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Löfdahl C-G, Postma DS, et al. Effect of inhaled for-moteroland budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WR., Jr Mechanisms of disease: Leukotrienes. N Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Pettit EJ, Hallet MB. Two distinct Ca2+ storage and release sites in human neutrophils. J Leuk Biol. 1998;63:225–32. doi: 10.1002/jlb.63.2.225. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–10. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada BK, Geiszt M, Van Bruggen R, et al. Calcium signalling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clin Exp Immunol. 2003;132:53–60. doi: 10.1046/j.1365-2249.2003.02138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola-Pleszczynski M, Chavaillaz PA, Lemaire I. Stimulation of interleukin 2 and interferon gamma production by leukotriene B4 in human lymphocyte cultures. Prostaglandins Leukot Med. 1986;23:207–10. doi: 10.1016/0262-1746(86)90187-3. [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Cortijo J, Taha MA, et al. Roflumilast inhibits leukocyte-endothelial cell interactions, expression of adhesion molecules and microvascular permeability. Br J Pharmacol. 2007;152:481–92. doi: 10.1038/sj.bjp.0707428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenzel J, Serrander L, Bànfi B, et al. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–37. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- Seiler PU, Stypmann J, Breidthardt G, et al. Real-time RT-PCT for gene expression profiling in blood of heart failure patients – A pilot study. Basic Res Cardiol. 2004;99:230–8. doi: 10.1007/s00395-004-0467-6. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Powell H, Boyle MJ, et al. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177:148–55. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- Sivertson KL, Seeds MC, Long DL, et al. The differential effect of dexamethasone on granulocyte apoptosis involves stabilization of Mcl-1L in neutrophils but not in eosinophils. Cell Immunol. 2007;246:34–45. doi: 10.1016/j.cellimm.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He L-P, et al. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natlacad Sci USA. 2006;103:4040–5. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel HC, Anderson R. Dissociation of the PAF-receptor from NADPH oxidase and adenylate cyclase in human neutrophils results in accelerated influx and delayed clearance of cytosolic calcium. Br J Pharmacol. 2002;136:81–9. doi: 10.1038/sj.bjp.0704685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel HC, Tintinger GR, Theron AJ, et al. Itraconazole-mediated inhibition of calium entry into platelet-activating factor-stimulated human neutrophils is due to interference with production of leukotriene B4. Clin Exp Immunol. 2007;150:144–50. doi: 10.1111/j.1365-2249.2007.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland I, Kisich K, Hauk PJ, et al. High constitutive glucocorticoid receptor β in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med. 2001;193:585–93. doi: 10.1084/jem.193.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, et al. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyladenosine A2A receptor agonists. Br J Pharmacol. 2001;132:1017–26. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette ME, Krump E, Picard S, et al. Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A(2a) receptor agonists and crucial role of autocrine activation by leukotriene B(4) Mol Pharmacol. 1999;56:1055–62. doi: 10.1124/mol.56.5.1055. [DOI] [PubMed] [Google Scholar]
- Tauber AI. Protein kinase C and the activation of human neutrophil NADPH-oxidase. Blood. 1987;69:711–20. [PubMed] [Google Scholar]
- Theron AJ, Steel HC, Tintinger GR, et al. Endogenous adenosine regulates neutrophil pro-inflammatory activities by cyclic AMP-dependent accelerated clearance of cytosolic calcium. Inflamm Res. 2002;51:1–9. doi: 10.1007/pl00012434. [DOI] [PubMed] [Google Scholar]
- Tintinger GR, Anderson R. Counteracting effects of NADPH oxidase and the Na+/Ca2+ exchanger on membrane repolarisation and store-operated uptake of Ca2+ by chemoattractant-activated human neutrophils. Biochem Pharmacol. 2004;67:2263–71. doi: 10.1016/j.bcp.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Tintinger GR, Theron AJ, Anderson R, et al. The anti-inflammatory interactions of epinephrine with human neutrophils in vitro are achieved by cyclic AMP-mediated accelerated resequestration of cytosolic calcium. Biochem Pharmacol. 2001b;61:1319–28. doi: 10.1016/s0006-2952(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Tintinger GR, Theron AJ, Potjo M, et al. Reactive oxidants regulate membrane repolarization and store-operated uptake of calcium by formyl peptide-activated human neutrophils. Free Radic Biol Med. 2007;42:1851–7. doi: 10.1016/j.freeradbiomed.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Tintinger GR, Theron AJ, Steel HC, et al. Accelerated calcium influx and hyperactivation of neutrophils in chronic granulomatous disease. Clin Exp Immunol. 2001a;123:254–63. doi: 10.1046/j.1365-2249.2001.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy TJ. Phosphodiesterase enzymes: molecular targets for novelantiasthma agents. Am J Respir Crit Care Med. 1998;157:351–70. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- Vignola AM. PDE4 inhibitors in COPD – a more selective approach to treatment. Resp Med. 2004;98:495–503. doi: 10.1016/j.rmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu P, Ohleth KM, et al. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56:170–4. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Rieu P, Descamps-Latscha B, et al. Biology of disease. Neutrophils: Molecules, functions and pathophysiologicalaspects. Lab Invest. 2000;5:617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- Yang Y, Luo J, Kazumura K, et al. Cilostazol suppresses adhesion of human neutrophils to HUVECs stimulated by FMLP and its mechanisms. Life Sci. 2006;70:629–36. doi: 10.1016/j.lfs.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Yue C, Dodge KL, Weber G, et al. Phosphorylation of serine 1105 by protein kinase A inhibits phospholipase Cβ, stimulation by Galphaq. J Biol Chem. 1998;273:18023–7. doi: 10.1074/jbc.273.29.18023. [DOI] [PubMed] [Google Scholar]