Abstract
Nonviral gene delivery is now considered a promising alternative to viral vectors. Among nonviral gene delivery agents, polyethylenimine (PEI) has emerged as a potent candidate for gene delivery to the lung. PEI has some advantages over other polycations in that it combines strong DNA compaction capacity with an intrinsic endosomolytic activity. However, intracellular (mainly the nuclear membrane) and extracellular obstacles still hamper its efficiency in vitro and in vivo, depending on the route of administration and the type of PEI. Nuclear delivery has been increased by adding nuclear localization signals. To overcome nonspecific interactions with biological fluids, extracellular matrix components and nontarget cells, strategies have been developed to protect polyplexes from these interactions and to increase target specificity and gene expression. When gene delivery into airway epithelial cells of the conducting airways is necessary, aerosolization of complexes seems to be better suited to guarantee higher transgene expression in the airway epithelial cells with lower toxicity than observed with either intratracheal or intravenous administration. Aerosolization, indeed, is useful to target the alveolar epithelium and pulmonary endothelium. Proof-of-principle that PEI-mediated gene delivery has therapeutic application to some genetic and acquired lung disease is presented, using as genetic material either plasmidic DNA or small-interfering RNA, although optimization of formulation and delivery protocols and limitation of toxicity need further studies.
Keywords: gene transfer, gene therapy, polyethylenimine, airway epithelial cells, lung, RNA interference
Introduction
Gene therapy has become a promising strategy for the treatment of inheritable or acquired lung diseases that are currently considered incurable. The lung is an attractive target for gene therapy since it is easily accessible and represents the organ where lethal mendelian (eg, cystic fibrosis) and acquired (eg, cancer) diseases occur.
Viral vectors have been applied to deliver therapeutic genes into living cells, but their broad use is limited by the inability of repeated administration and the severe safety risks (Lehrman 1999; Liu and Muruve 2003), based upon their immunogenicity and their oncogenic potential (Thomas et al 2003; Cavazzana-Calvo and Fischer 2007).
In light of these concerns, nonviral gene delivery has emerged as a promising alternative. The use of synthetic nonviral vectors confers several advantages, due to their ease of preparation, purification and chemical modification. Moreover, they display high flexibility regarding the size of the transgene delivered (Lollo et al 2000). However, nonviral carriers linked electrostatically with plasmid DNA encounter various barriers at the tissue and cellular level. First, positively charged complexes have to interact with the molecules of the extracellular matrix (included proteoglycans) and of the basal membranes. The following step is interaction with the plasma membrane via electrostatic interactions with anionic cell surface groups, such as proteoglycans. Endocytosis is then followed by routing though the endo-lysosomal compartments, release into the cytoplasm, migration into the nucleus, and finally deconsendation of the DNA into a transcribable form.
Among the first nonviral vectors used, cationic liposomes have displayed limited efficiency and high toxicity, due to high levels needed to overcome tissue and cellular constraints, although recent research has highlighted the possibility to optimize their formulation to promote gene expression in vivo without increasing cytotoxicity (Ding et al 2007). Nevertheless, the liposomal class of drug delivery agents has a dominant position in clinical practice today (Zhang et al 2008). Liposomal products include anti-fungals (AmBisome, Abelcet), antineoplastics (DepoCyt, Doxil, DaunoXome, Myocet), and antivirals (Epaxal, Inflexal V).
For these reasons, research has focussed mainly on alternative nonviral vectors, including cationic polymers that have intrinsic properties that help to override the above mentioned barriers. In the last decade, the cationic polymer polyethylenimine (PEI) has received much attention for its interesting properties as a gene transfer agent. Given the vast application of PEI as a gene delivery agent, in this review we will describe the biophysical features of PEI, its biological properties in reference above all to the lung, and its eventual use in therapeutic applications to lung diseases.
Physico-chemical properties of PEI complexes and gene transfer efficiency in vitro
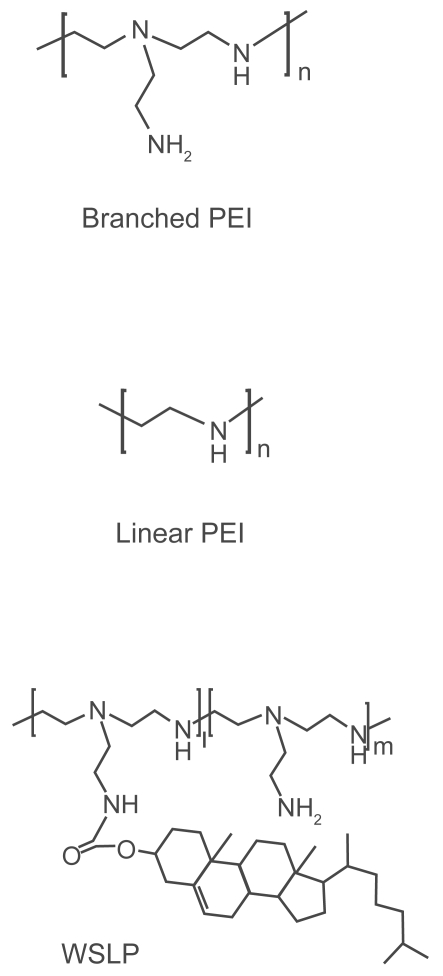
The use of cationic polymers became popular after introduction of PEI by Behr and colleagues (Boussif et al 1995) to mediate delivery of DNA. This polymer is available mainly in either linear or branched forms (Figure 1), and different commercially available PEIs in the range of 25,000 to 800,000 Daltons have successfully been employed for in vitro and in vivo gene delivery of DNA either alone or as complex with additional components such as viral proteins and targeting moieties (Boussif et al 1995; Baker and Cotten 1997; Baker et al 1997; Meunier-Durmort et al 1997). PEI is a versatile vector, since it has been used successfully for in vitro delivery of nucleic acids including small interfering RNA (siRNA) (Read et al 2005; Urban-Klein et al 2005; Grayson et al 2006), rybozymes (Aigner et al 2002; Merdan et al 2002), and oligonucleotides (Boussif et al 1995; Dheur et al 1999) in addition to plasmidic DNA. PEI differs from cationic lipids at several levels, including behavior inside the cell (Rejman et al 2006). For example, PEI polyplexes enter the cells via both clathrin- and caveole-dependent routes, whereas DOTAP lipoplexes enter only via the clathrin-dependent pathway (Rejman et al 2005). It is known that caveolae – mediated endocytosis does not result in trafficking of gene transfer agents to the lysosomal compartment, where degadation of DNA by nuclease may occur. This could be one of the reasons why PEI polyplexes have been shown to be more efficient than cationic lipids in vitro (Huh et al 2007), as well as in vivo (Bragonzi et al 1999).
Figure 1.
Chemical structure of linear PEI, branched PEI and water-soluble lipopolymer (WSLP). Copyright © 2006. Reprinted with permission from Park TG, Jeong JH, Kim SW. 2006. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev, 58:467–86.
Indeed, some characteristics featured by PEIs make them attractive and proficient vehicles for gene delivery. First of all, PEI has a high cationic charge density, since every third atom is a potentially protonable amino nitrogen. Approximately 20% of the nitrogens of PEI are protonated under physiological conditions (Suh et al 1994; Tang and Szoka 1997). As a result, the polymer can change its ionization state over a broad pH range and, as a consequence, PEI has a high intrinsic endosomolytic activity as compared to other polycations, such as polylysine (Boussif et al 1995). Second, PEI is very efficient in condensing plasmid DNA and siRNA, thus protecting polynucleotides from nuclease degradation (Ferrari et al 1999; Urban-Klein et al 2005).
Structural characteristics such as the molecular weight and the degree of branching influence the efficacy of PEI-derived nonviral vectors in mediating delivery of plasmid DNA and siRNAs. The molecular weight of PEI most suitable for gene transfer ranges between 5 and 25 kDa, as assessed on L929 murine fibroblasts. Higher molecular weights correlate with increased cytotoxicity, presumably due to aggregation of huge clusters of the cationic polymer on the outer cell membrane, which thereby induces necrosis (Fischer et al 1999). PEI with a molecular weight less than 1.8 kDa shows almost no transfection but is less toxic to human endothelial cell-derived cell line EA.hy 926 (Godbey et al 1999a). A series of five poly[(ethylene imine)-co-N-(2-hydroxyethyl-ethylene imine)] copolymers with similar molecular weights and different degrees of branching was established to study structure-function relationship with regard to physicochemical and biological properties as gene delivery systems. Higher branched polymers showed stronger complexation and condensation of DNA, formed smaller polymer/DNA complexes, and induced the expression of plasmids in 3T3 mouse fibroblasts to a higher extent than less branched polymers (Fischer et al 2002). However, other studies have shown that the application of small polyplexes reduces the transfection efficiency in Neuro 2A, K562 and B16F10 cells, compared to the larger complexes, although this effect seems to be dependent on the cell line used (Ogris et al 1998, 2001). The presence of serum did not affect, or indeed slightly increased, the efficiency of PEI 25 kDa-mediated plasmid DNA delivery in COS-1 and Calu-3 cells (Florea et al 2002). Similarly, the transfection efficiency of a GFP-expressing plasmid was almost the same or even slightly increased when PEI 800 kDa-cholesterol was used in HeLa cells in the presence of serum at different N/P ratios (Chen et al 2007). No significant difference in the expression of luciferase could be observed between 3T3 fibroblasts transfected with a low molecular weight PEI in the presence or in the absence of serum (Fischer et al 1999).
Efficient delivery systems for therapeutic siRNAs have been demanded for clinical applications. The delivery systems should be designed to solve intrinsic problems of naked siRNA: the lack of stability in the blood stream and a low intracellular transfection efficiency, even though the target compartment is the cytoplasm and not the nucleus. PEI/siRNA complexes have been shown to give robust reduction in target gene expression in several different cellular models (either in co-transfection experiments or in cells stably expressing a marker gene) (Read et al 2005; Urban-Klein et al 2005; Kim et al 2006a; Werth et al 2006) (Table 1). For instance, one study showed that noncovalent complexation of synthetic siRNAs with a commercially available linear low molecular weight PEI (JetPEI) determined a 60% decrease of luciferase activity in luciferase-expressing SKOV-3 ovarian carcinoma cells even in the presence of serum in the culture medium, demonstrating that upon PEI-complexation, siRNA is stabilized and internalized by the cells where it exerts its full bioactivity. VEGF siRNA-PEG complexed with branched PEI 25 kDa showed greater stability and higher silencing than naked siRNA in the presence of serum in human prostate carcinoma cells (PC-3) (Kim et al 2006a). Direct comparison among different PEIs showed that branched PEI 25 kDa at N/P ratio of 6 and 8 displayed a very strong affinity for siRNAs and mediated efficient down-regulation of luciferase in luciferase-expressing HR5-CL11 cell line (a HeLa derivative), whereas nor branched PEI 800 kDa neither linear PEI 22 kDa were able to exert this effect (Grayson et al 2006). Only polyplexes formed by PEI 25 kDa and displaying small size (59–80 nm) and positive zeta potentials (almost 40 mV) were able to deliver siRNA, while linear PEI 22 KDa gave inconsistent results. These results suggest that the predictability of siRNA transfection is not straightforward and that variable transfection results might also occur with other PEI architectures.
Table 1.
Selected examples of in vitro and in vivo efficacy of PEI-mediated siRNAs
| Target | Formulation | Cell lines/in vivo model | Reference |
|---|---|---|---|
| Tumours | |||
| VEGF | siRNAs-s-PEG/PEI | Human prostate carcinoma cells (PC-3) | (Kim et al 2006a) |
| VEGF | siRNA/low molecular weight PEI | Human prostate carcinoma cells (PC-3) | (Werth et al 2006) |
| VEGF-R2 | siRNA/RGD-PEG-PEI | Subcutaneous tumor xenograft formed by N2A neuroblastoma cells in nude mice (i.v.) | (Schiffelers et al 2004) |
| HER-2 | siRNA/linear low molecular weight PEI (JetPEI) | SKOV-3 ovarian carcinoma cells Subcutaneous tumour xenograft formed by SKOV-3 cells in athymic nude mice (i.p.) | (Urban-Klein et al 2005) |
| Pleiotrophin | siRNA/linear low molecular weight PEI (JetPEI) | U87 glioblastoma cells Subcutaneous tumour xenograft formed by U87 cells in athymic nude mice (i.p, s.c.) Orthotopic mouse glioblastoma model (i.c.) | (Grzelinski et al 2006) |
| Viral infection | |||
| Influenza A nucleocapsid protein | JetPEI | Influenza virus infection in C57Bl/6 mice (i.v.) | (Ge et al 2004) |
| Influenza A nucleocapsid protein | Nonacylated PEI | Influenza virus infection in C57Bl/6 mice (i.v.) | (Thomas et al 2005) |
Notes: Routes of administration are indicated: i.c.: intracranial; i.p.: intraperitoneal; i.t.: intratechal; s.c.: subcutaneous.
Abbreviation: VEGF, vascular endothelial growth factor.
Size and charge of PEI complexes and their relation to in vitro and in vivo activity
PEI/DNA complexes have been found to form toroid structures of 40–80 nm (Tang and Szoka 1997), as estimated by electron microscopy. The higher the N/P ratio the lower the size of PEI complexes. Atomic force microscopy (AFM) studies have revealed that the morphology of PEI complexes may have different structures depending on the level of saturation of DNA by PEI (Dunlap et al 1997). Complete condensates were rounded globular shaped (with the size of 20–40 nm), while unsaturated complexes ranged from bundled, folded loops of DNA sorrounding central cores to loose coils with isolated nodes of condensation. Complexes formed between a 22 nt double-stranded siRNA and PEI were spherical with a mean diameter of ~41.5 nm as determined by AFM analysis. The absence of free siRNA molecule ends, as well as the absence of free siRNA molecules, indicated the complete complexation of the siRNAs (Grzelinski et al 2006). In the same study, PEI complexes with DNA plasmids were somewhat larger (95 nm), indicating no direct correlation between the size of nucleic acids and the size of their complexes (while siRNA is 22 bp the plasmid is 5000 bp). The small size (59–80 nm) of bioactive PEI/siRNA complexes was confirmed by dynamic light scattering (Grayson et al 2006).
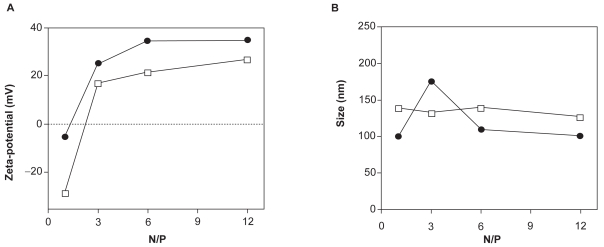
The size and zeta-potential of PEI/DNA polyplexes were found not to be dependent on the molecular weight (2, 25, or 750 kDa) or geometry (linear versus branched) of PEI, but mainly on the nitrogen to phosphate (N/P) ratio used (Choosakoonkriang et al 2003). Florea and colleagues (Florea et al 2002) showed that the zeta potential of polyplexes (formed with branched 600–1000, 60, and 25 kDa) in a low-salt envirnonment (10 mM HEPES) increased proportionally with higher PEI concentration, reaching a plateau at high N/P ratio. While the surface charge show this behavior, the size of linear PEI 22 kDa complexes formed in 5% glucose does not change with increasing N/P ratio, as shown in Figure 2 (Kichler et al 2002).
Figure 2.
Zeta potential (A) and size (B) of non-PEGylated (•) and PEGylated (•) linear PEI 22 kDa complexes prepared in 5% glucose at different N/P ratios were measured using a Malvern Zetasizer. Note that the grafting with PEG residues allows to partially shield the surface charge of the polyplexes. The size of the particles generated with the PEI-PEG conjugate at N/P above 3 was slightly bigger than that observed with PEI (125 vs 100 nm). Copyright © 2002. Reprinted with permission from Kichler A, Chillon M, Leborgne C, et al 2002. Intranasal gene delivery with a polyethylenimine-PEG conjugate. J Control Release, 81:379–88.
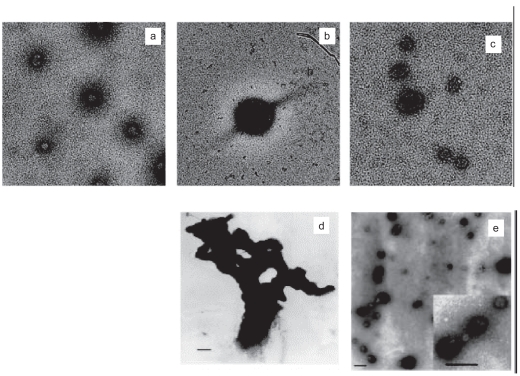
On the other hand, the size of the PEI/DNA particles is clearly dependent on the conditions used for the preparation of the formulations and the type of PEI. Whereas branched PEI 25 kDa gene vectors formulated in distilled water and 5% glucose were kinetically stable, having diameters of ~98 and ~89 nm respectively, PEI complexes formulated in HBS (150 mM NaCl) were larger (~153 nm) and slowly aggregating (Rudolph et al 2005b). Conversely, the surface charge, as assessed by zeta potential measurement, was less positive in the highest ion concentration medium (water: ~56 mV; 5% glucose: ~45 mV; HBS: ~12 mV). Electron microscopy studies revealed homogeneous populations of nearly globuar particles in all the solvents used (Figure 3). Using linear PEI 22 kDa to complex DNA in high salt solution gives quite large particles (≥1 μm) that work exceedingly well in vitro (Boussif et al 1995). However, formulation with the same linear PEI 22 kDa in an equi-osmotic but salt-free solution of 5% glucose produces particles with mean size ranging between 30 and 60 nm (Goula et al 1998b) (Figure 3).
Figure 3.
Electron microscopy of PEI/DNA complexes. PEI 25 kDNA complexes prepared in water (a) HBS (b) and 5% glucose (c) Copyright © 2005. Reprinted with permission from Rudolph C, Schillinger U, Ortiz A, et al 2005b. Aerosolized nanogram quantities of plasmid DNA mediate highly efficient gene delivery to mouse airway epithelium. Mol Ther, 12:493–501. Linear PEI 22 kDa complexes prepared in 150 mM NaCl (d) or 5% glucose (e) Copyright © 1998. Reprinted with permission from Goula D, Remy JS, Erbacher P, et al. 1998b. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther, 5:712–7.
A correlation between complexes size and transfection efficiency in vitro has been shown (Wightman et al 2001). Linear PEI 22 kDa transfected murine neuroblastoma Neuro2a and C-26 colon carcinoma cells more efficiently, as compared to branched PEI 800 kDa and PEI 25 kDa complexes, when formulated in high salt medium. More over, both branched and linear PEIs generated in salt-free 5% glucose had reduced activity as compared to complexes generated in high-salt HBS. Only complexes generated with linear PEI 22 kDa in high salt condition grew in size to reach ~746 nm over 3 hours, indicating that high transfection efficiency of PEI 22 kDa/DNA may be derived from its pronounced ability to aggregate in salt containing conditions.
To correlate the activity of PEIs in vivo with the activity observed in vitro, intravenous administration of polyplexes generated in different formulations (ie, high vs low salt) was carried out in BALB/C mice (Wightman et al 2001). Interestingly, PEI 22 kDa/DNA complexes generated in high salt were less active than the complexes formed in low salt (100-fold less). This apparent disagreement between in vitro and in vivo data was solved by demonstrating that when the salt concentration of linear PEI 22 kDa, previously formulated in low salt, was increased to 90% physiological concentration these complexes rapidly aggregated. Overall, these data indicate that when complexes initially formulated in salt-free environment are introduced into the tail vein of mice the salt environment around the applied complexes would change rapidly determining an increase in their size. This speculation has been corroborated by other studies on intravenous administration in mice (see below).
A similar discrepancy between in vitro and in vivo data has been noted with a topical administration of PEI polyplexes to the lung. Intratracheal injection of TAT-PEG-PEI polyplexes in mice demonstrated differences of a ca. 600% higher transfection efficiency compared to PEI which was not reflected in the in vitro experiments in A549 cells (Kleemann et al 2005).
In conclusion, the multidimensional tissue structure of the lung and extracellular protein/enzyme network reveal difficulties in extrapolation of in vitro results to successful gene delivery into the complex lung systems.
Cytotoxicity in vitro
Unmodified PEIs can induce cytotoxicity, often defined by assessing either the release of lactate dehydrogenase (LDH) or a decrease in the in vitro metabolic activity of cells (eg, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay) (Fischer et al 1999; Godbey et al 2001; Putnam et al 2001; Florea et al 2002). The higher the N/P ratio and the DNA amount used for transfection, the higher the cytotoxicity of linear PEI 25 kDa polyplxes, as assessed in various cell lines (HEK293T, COS-7, HeLa, NCCIT) (Huh et al 2007). Godbey and colleagues (2001) have shown that when complexes formed between branched PEIs (600 Da, 10 kDa, 25 kDa) and a GFP-expressing plasmid were used to transfect the endothelial cell line EA.hy 926, the secreted levels of tissue-type plasminogen activator, plasminogen activator inhibitor type 1 and von Willeband factor increased above the levels recorded in untreated cells. Moreover, there appeared to be two distinct types of cell death, resulting from the use of either free PEI (which acts within 2 h) or PEI/DNA complexes (which cause death 7–9 h after transfection). The immediate cell death has been linked to the membrane destabilizing activity of free PEI, which contributes to as much as 85% of the total PEI in the PEI/DNA complexes used for transfection (Clamme et al 2003a).
In contrast to high molecular weight PEI (1600 kDa), a low molecular weight PEI (10 kDa) with a low degree of branching was not cytotoxic in L929 mouse fibroblasts and ECV 304 endothelial cells at the concentrations useful for efficient cell transfection (Fischer et al 1999), indicating that high cationic charge densities, a compact and highly branched structure as well as high molecular weights all negatively affect the biocompatibility of PEI. On the other hand, branched PEIs with different MWs (25, 60, and 600–1000 kDa) were shown to be comparable in their cytotoxic effect in COS-1 (green monkey fibroblasts) and in well-differentiated human submucosal airway epithelial cells (Calu-3), with a slightly more pronounced effect with PEI 25 kDa (Florea et al 2002).
The molecular mechanisms underlying immediate and delayed cytotoxicity following PEI gene transfer have been recently elucidated. Branched PEI 25 kDa and linear high molecular weight PEI (750 kDa) can both induce membrane damage and initiate apoptosis in three clinically relevant human cell lines (Jurkat T cells, umbilical vein endothelial cells, and THLE3 hepatocyte-like cells) (Moghimi et al 2005). Phase I toxicity was defined as early necrotic-like changes (30 min) resulting from compromized membrane integrity, assessed by LDH release and phosphatidylserine translocation from the inner plasma membrane to the outer cell surface. Phase II toxicity (24 h) was due to activation of a mitochondrially mediated apoptotic program, resulting from PEI-induced channel formation in the outer mitochindrial membrane. These results confirm a previous study which suggested 25 kDa PEI could be a pro-apoptotic agent, as evaluated by Hoechst 33258 staining in COS-1 and Calu-3 cells (Florea et al 2002).
The concentration of branched PEI 25 kDa that was found to mediate RNA interference in HR5-CL11 cells was not cytotoxic and the relative cytotoxicities of the three PEI structures was 800 kDa branched PEI > 22 kDa linear PEI > 25 kDa branched PEI (Grayson et al 2006). These results are comparable to those reported by others with plasmid DNA, where low molecular weight branched PEI was shown to be less cytotoxic than high molecular weight branched PEI (Fischer et al 1999).
In the attempt to decrease PEI cytotoxicity, different strategies have been pursued (Park et al 2006) including PEI-grafted PEGs, water-soluble lipopolymers, and biodegradable PEIs. PEI-grafted PEGs (PEI-g-PEG) with different PEG grafying ratios were synthesized (Petersen et al 2002). In 3T3 fibroblasts, cell cytotoxicity (LDH assay) of PEI-g-PEG was greatly reduced and was independent of molecular weight of PEG but affected by the degree of PEG substitution. Despite PEGylation, all copolymers showed transfection efficiency (luciferase levels) at least as high as homopolymer PEI. Furthermore, at higher N/P ratios, the copolymers turned out to be even more efficient than PEI.
The water-soluble lipopolymer (WLSP) (Figure 1) was synthesized by conjugating cholesteryl chloroformate to a low molecular weight PEI (1.8 kDa) (Han et al 2001). The hydrophobic cholesterol moiety of WLSP enabled the polymer to form micelles in acqueous solution. WSLP/pDNA complexes were nontoxic to CT-26 colon adenocarcinoma and 293T human embryonic kidney transformed cells when formulated at the N/P ratio of 10 and below as determined by MTT assay. In contrast, PEI 25 KDa/DNA complexes were toxic to these cells. Erythrocytes aggregated when incubated with PEI 25 kDa/pCMV-Luc complexes at high DNA concentrations, but there was little aggregation with WSLP/DNA complexes. WSLP/pCMV-Luc complexes demonstrated higher transfection efficiency in both CT-26 and 293T cells compared to PEI 25 kDa- or PEI 1.8 kDa-based formulations.
Biodegradable PEIs have been synthetized by cross-linking low molecular weight PEI (0.8 kDa) with either PEG-bis-succinimidyl succinate (Ahn et al 2002) or disulfide-containing crosslinkers, such as dithiobis(succinimidy lproprionate) and dimethyl 3–3′-dithiobisproprionimidate (Gosselin et al 2001) and exhibited decreased cytotoxicity and improved transfection efficiencies. For example, viability (MTT assay) of 293T cells was over 80% for all the synthesized cross-linked PEG/PEI copolymers, whereas cell viability was around 40% with PEI 25 kDa (Ahn et al 2002). The transfection efficiency (β-gal assay) of these copolymers was three times that of the initial PEI 0.8 kDa. Novel biodegradable poly(ester amine)s (PEAs) based on polycaprolactone and different low molecular weight PEIs (0.6, 1.2, and 1.8 kDa) was prepared and characterized (Arote et al 2007). Upon incubation at 37 °C and at physiological pH, all PEAs showed typical degradation pattern where the susceptible ester linkages got hydrolyzed leading to formation of low molecular weight degradation products which are nontoxic in nature. PEAs were nontoxic to 293T, HepG2 and HeLa cells and increased luciferase levels in these cells up to 25-fold as compared with PEI 25 kDa.
Intracellular trafficking of PEI complexes in vitro
Binding and uptake
It is generally accepted that interaction of polyplexes with cells is nonspecific and involves mainly electrostatic interactions between the positively charged complexes and the negatively charged cell surface, as showed in various cell types (3T3 mouse fibroblasts, HepG2 human hepatocytes, COS-7 simian kidney cells, HeLa human carcinoma cells, and MRC-5 human lung embryonic cells) (Boussif et al 1995). The positively charged DNA polyplexes can interact with negatively charged molecules such as glycosaminoglycans (GAGs). Heparan sulfate (HS) is an anionic GAG that is found in the extracellular space and on the surface of most cells, often in the structure of proteoglycans (PGs) (Kramer and Yost 2003). Unmodified cationic complexes interact nonspecifically with negatively charged glycoproteins, HS and PGs (Behr 1994; Labat-Moleur et al 1996; Mislick and Baldeschwieler 1996; Mounkes et al 1998) located on the surface of cell membranes. A report suggests that the uptake of PEI polyplexes by HeLa cells occurs through actin-mediated phagocytosis as a result of the adhesion of polyplexes to syndecan PG molecules followed by their clustering in lipid rafts (Kopatz et al 2004). However, the interactions between the positively charged DNA complexes and negatively charged cellular GAGs may hamper specific ligand-receptor interaction and extracellular GAGs or PGs may reduce the DNA delivery to the target cells.
Endocytosis (the vescicular uptake of extracellular macromolecules) has been established as the main mechanism for the internalization of nonviral vectors into cells, with a size range of 100–200 nm (Khalil et al 2006). Kinetically, three modes of endocytosis can be defined: fluid-phase, adsorptive, and receptor mediated endocytosis. While fluid-phase endocytosis refers to bulk uptake of solutes according to their concentration in the extracellular fluid, in adsorptive and receptor-mediated endocytosis, macromolecules are bound to the cell surface and concentrated before internalization. Endocytosis can also be classified into two broad categories: phagocytosis (for the uptake of large particles), which is restricted to specialized mammalian cells, and pynosytosis (for the uptake of luid and solutes), which occurs in all cells. Although four morphologically distinct pynocytic pathways have been characterized (clathrin-mediated endocytosis, caveolae, macropynocytosis, and clathrin/caveolae-independent endocytosis), most pynocytic pathways involve receptor-ligand interactions.
Following their interaction with anionic surface molecules on the cell surface, polyplexes enter the cell predominantly via adsorptive endocytosis in EA.hy.926 endothelial cells (Godbey et al 1999b) or fluid-phase endocytosis in L929 mouse fibroblasts (Remy-Kristensen et al 2001). Both microscopy analysis and endocytosis-interfering drugs were used for demonstrating these pathways of internalization (Ogris et al 2001; Remy-Kristensen et al 2001; Bieber et al 2002). Using colocalization with endocytic markers, Remy-Kristensen and colleagues have demonstrated that polyplexes formed between branched PEI 25 KDa and DNA enter the cells and accumulate within 10 min in endosomes that were about 200 nm in diameter following a typical pinocytosis pathway (Remy-Kristensen et al 2001). A rapid accumulation of polyplexes in the lysosomal compartment, by endocytosis, has been showed using both subcellular fractionation and confocal/electron microscopy techniques in PaTu 8902 cells, derived from a human pancreatic adenocarcinoma (Bieber et al 2002).
Using endocytosis-interfering drugs, Rejman and colleagues (2005) has provided strong evidence that polyplexes are internalized both by clathrin-mediated and by caveolae-mediated endocytosis in A549 and HeLa cells. Moreover, they showed that only the caveolae-dependent route leads to effective transfection while the DNA contained in the polyplexes internalized by clathrin-mediated endocytosis could not be released and was taken with the complex into the lysosomal compartment to be degraded. It has also been shown that the internalization pathway mediating successful transfection depended on both cell type and polyplex type applied (von Gersdorff et al 2006). The use of specific uptake inhibitors (chlorpromazine and filipin III) revelaed that in COS-7 cells the clathrin-dependent pathway was dominant. In HUH-7 hepatocarcinoma cells, gene transfer by linear PEI 22 kDa was mainly dependent on the clathrin-dependent route, whereas transfection by branched PEI 25 kDa was mediated by both clathrin- and caveole/lipid raft-dependent pathways. Finally, in HeLa cells, both pathway were involved, although the lipid-raft-dependent route was more relevant.
The intracellular fate of PEI polyplexes was studied in detail by using lactosylated PEI 25 kDa (Lac-PEI) in the human tracheal epithelial ∑CFTEo- cell line (Grosse et al 2005). Electron microscopy studies showed that the cellular processing of complexes varied with their size and the polycation derivative used: large complexes (>200 nm) made with all polycationic vectors studied were internalized by macropinocytosis. In contrast, intermediate (100–200 nm) glycosylated polylysine and PEI complexes primarily entered through clathrin-coated pits. Complexes were than found in endosomal vesicles, accumulated in lysosomes or in vesicles near the nucleus. The population of small complexes (<100 nm) obtained with PEI derivatives were internalized through caveolae and pursued a traffic pattern of potocytosis to the endoplasmic reticulum.
Although not studied in as much detail as PEI/DNA complexes, the nonspecific endocytosis via clathrin-coated pits is the likely mechanism of internalization of PEI/siRNA complexes. The complexes formed with branched PEI 25 kDa were smaller than 150 nm (Grayson et al 2006; Grzelinski et al 2006), which is considered the size limit for this route of internalization.
Endosomal escape
PEI, as a nonviral vector, combines a high membrane-destabilizing potential with a high DNA condensing activity, thus protecting DNA from degradation and therefore increasing the probability that intact plasmid DNA will reach the nucleus (Klemm et al 1998; Zhang and Smith 2000). However, polyplexes should overcome the endosomal-lysosomal barrier. The “proton-sponge” hypothesis, which has been postulated by Behr (1994), relates the intrinsic endosomolytic activity of PEI to its capacity to buffer the endosomal compartment, causing the osmotic swelling of the vescicle and its rupture with the releasing of the polyplexes into the cytoplasm. On the basis of this phenomenon, the fusion with lysosomes may thereby be prevented, circumventing DNA degradation by lysosomal DNAse activity. Membrane holes have been observed by electron microscopy and they were attributed to the direct interaction of branched PEI with the internal side of the endosomal membrane (Bieber et al 2002). While acidification seems to facilitate vesicle rupture (Kichler et al 2001; Forrest and Pack 2002), the membrane damage occurred in a dose-dependent manner (Clamme et al 2003b) and was present even in the absence of acidification when high molecular weight-branched PEI derivatives are used (Bieber et al 2002). A report directly supporting the “proton sponge hypothesis” demonstrates that increased PEI buffer capacity results in endosomal Cl− entry because of H+/Cl− charge coupling, promoting osmotic swelling and endosome leak/lysis, as shown in Chinese hamster ovary (CHO)-K1 cells (Sonawane et al 2003).
Interestingly, some experiments, which were carried out using 35S-DNA/branched PEI, have revealed that the endosomolitic activity may depend on the particle size, suggesting that larger polyplexes entrapped in endosomes may facilitate endosomolysis (Boussif et al 1995; Ogris et al 1998; Wattiaux et al 2000; Blessing et al 2001), while the efficient gene delivery mediated by small particles relies on the addition of endosomolytic compounds (Blessing et al 2001).
In the attempt to further improve gene delivery, several virus-derived endosomolytic proteins or peptides were incorporated onto branched PEI/DNA polyplexes (Table 2). The membrane-active peptide JTS-1 (Gottschalk et al 1996; Sparrow et al 1998), the Haemophilus influenzae hemagglutinin-derived peptides (INF) (Lear and DeGrado 1987; Plank et al 1994; Wolfert and Seymour 1998; Wagner 1999) and GALA peptide (Subbarao et al 1987; Parente et al 1990, 1990b) all assume a random coil structure at pH 7. Upon acidification, a conformational transition occurs, which enables the subsequent interaction with the phospholipids membranes, resulting in pore formation or the induction of membrane fusion and/or lysis (Parente et al 1988). The membrane-active dimeric influenza peptide INF5 (Plank et al 1994) has been attached to both transferrin-branched PEI/DNA and branched PEI/DNA polyplexes, increasing transgene expression up to 10-fold (Kircheis et al 1997). However, the optimum expression levels observed were not significantly higher than the maximum expression at high N/P ratios without INF5.
Table 2.
Peptide-guided PEI-mediated gene delivery
| Function | Peptide | Sequence | Target organ/Cell lines | Reference |
|---|---|---|---|---|
| Binding | RGD-peptide | ACDCRGDCFC | CT-26 subcutaneous tumor in mice | (Kim et al 2006b) |
| RGD-peptide | ACRGDMFGCA | HUVECs | (Schiffelers et al 2004) | |
| N2A neuroblastoma cells | ||||
| SVR-bag- 4 endothelial cells | ||||
| N2A subcutaneous tumor in mice | ||||
| Tet1 | HLNILSTLWKYRC | PC-12 neuron-like cells | (Park et al 2007) | |
| 3T3 murine fibroblasts | ||||
| Primary rat astrocytes | ||||
| Murine DRG neurons | ||||
| CD13 ligand | CNGRC | HUVECs | (Moffatt et al 2005) | |
| HT-1080 human fibrosarcoma cells | ||||
| H1299 non-small-cell lung cancer cells | ||||
| H1299 subcutaneous tumor in athymic nude mice | ||||
| CD13 ligand | CNGRC | H1299 non-small-cell lung cancer cells | (Moffatt et al 2006) | |
| H1299 subcutaneous tumor in athymic nude mice | ||||
| Internalization | IB1 | CDSAFVTVDWGRSMSLC | Calu – 3 | (Florea et al 2003) |
| TAT | GRKKRRQRRRPPQ | COS-7 | (Rudolph et al 2003) | |
| 16HBE human bronchial cells | ||||
| Primary nasal respiratory epithelium | ||||
| TAT | GRKKKRRQRC | A549 lung epithelial cells | (Kleemann et al 2005) | |
| Intratracheal instillation to the mouse lung | ||||
| KALA | WEAKLAKALAKALAKHLAKALAKALKACEA | C2C12 mouse myoblasts | (Min et al 2006) | |
| Human Chang Liver cells | ||||
| 3T3 mouse fibroblasts | ||||
| HeLa | ||||
| C3-subcutaneous mouse model | ||||
| Endosome-destabilizing | IFN5 | GLFGAIAGFIENGWEGMIDGWYG | H225 human melanoma cells | (Kircheis et al 1997) |
| KALA | WEAKLAKALAKALAKHLAKALAKALKACEA | 293T cells | (Lee et al 2001) | |
| Nuclear import | SV40-large T antigen | PKKKRKVEDPYC | 3T3 murine fibroblasts | (Zanta et al 1999) |
| HeLa carcinoma cells | ||||
| BNL CL.2 murine hepatocytes | ||||
| Murine DRG neurons | ||||
| Human macrophages |
Abbreviations: DRG, dorsal root ganglion; HUVECs, human umbilical vein endhotelial cells.
KALA peptide (Wyman et al 1997; Lee et al 2001) resembles the membranolytic activity of GALA with DNA condensing properties derived from the lysine residues (Wyman et al 1997). After site-specific mono-PEGylation, PEG-KALA was attached to the surface of branched PEI/DNA polyplexes (Lee et al 2001). The PEGylation sterically prevented particle aggregation and increasing concentrations of either branched PEI or PEG-KALA led to an enhanced transfection efficiency.
The tranfection potential of branched PEI 25 kDa/KALA complexes was evaluated in various cells in culture. In most cell lines (HeLa, C2C12 mouse myoblasts, 3T3 mouse fibroblasts, human Chang Liver cells) PEI/KALA exhibited tranfection efficiencies higher then PEI 25 kDa (Min et al 2006). The performance of PEI/KALA was tested in the C3 subcutanetenous mouse tumor model. Luciferase plasmid DNA complexed with either PEI/KALA or PEI was directly injected in the tumor. The tumors injected with PEI/KALA/DNA complexes showed 2.5–3.5-fold higher luciferase activity than those injected with PEI/DNA.
However, concerns have arisen about the high costs of peptide synthesis and the risk of immunogenic reactions. At the moment, since the DNA release from PEI polyplexes into the cytoplasm is sufficiently supported by its own endosomolytic activity, the usefulness of these endosome-destabilizing peptides is controversial.
Another approach consists of using HIV–1 virus trans-activator protein (TAT) or its derivatives, which are know to be cell penetrating peptides (Jones 2007) with fusogenic properties (Martin and Rice 2007). Rudolph and colleagues (2003), using complexes formed between PEI and oligomers of the arginine-rich motif of the HIV-1 TAT protein, have demonstrated how they are capable of improving the transfection efficiency of PEI/DNA complexes in vitro in A549 cells, although not in vivo after an intratracheal administration. Conversely, TAT-PEG-PEI 25 kDa polyplexes mediated much lower levels of luciferase in A549 cells than PEI, while higher transfection efficiencies for TAT-PEG-PEI were detected in mice upon intratracheal instillation (Kleemann et al 2005).
Interaction with cytosolic components and nuclear import
Once PEI/DNA complexes are released from the endosomal vesicles, they must reach the perinuclear region and enter the nucleus. Generally, plasmid DNA larger than 2000 bp, released from endosomes, remains nearly immobile within the viscous cytoplasm (Lukacs et al 2000) and is degraded by cytosolic nucleases (Lechardeur et al 1999; Pollard et al 2001). Further, when isolated DNA is micro-injected into the cytoplasm of COS-7 cells, less than 0.1% of this DNA reaches the nucleus (Pollard et al 1998). PEI is able to prolong the survival of plasmid DNA in a dose dependent manner, facilitated by the complexation of DNA, without affecting nuclease activity itself (Pollard et al 1998, 2001). While Okuda and colleagues have suggested that cytosolic soluble proteins (protease sensitive) can induce DNA dissociation from PEI/DNA complexes (Okuda et al 2004), others have demonstrated that RNA can dissociate DNA from complexes in a dose- and polymer-dependent fashion (Bertschinger et al 2006; Huth et al 2006). RNA (in particular tRNA) is present in the cytoplasm at high concentrations and show high structural similarity to DNA. Data suggest that the release of DNA from polyplexes is primarly due to charge interaction but not sequence-dependent (Huth et al 2006).
Many evidences show that entire gene vector complexes are largely excluded from entry into nonmitotic nuclei (Itaka et al 2004; Grosse et al 2006; Huth et al 2006). Others have found PEI/plasmid DNA complexes inside the nucleus, albeit at low levels (Godbey et al 1999b; Grosse et al 2004). For example, for both lactosylated and unsubstituted PEI 25 kDa PEIs, nuclear localization was observed for less than 5% of intracellular complexes in an immortalized human tracheal epithelial cell line (∑CFTE29o-) (Grosse et al 2004). Electron microscopy studies combined with in situ PCR and FISH performed in our laboratory in pneumocyte type II A549 cells show that PEI/DNA complexes are excluded from nuclei in intact cells while the plasmid is found inside isolated nuclei (Biffiet al unpublished results).
The mechanism by which plasmid DNA reaches the nuclear compartment is still poorly understood. The nuclear envelope is perforated by nuclear pore complexes (NPC), each of which assembles 8 smaller diffusion channels to allow macromolecules with an upper size limit of 50 kDa to diffuse freely (Talcott and Moore 1999) and a large channel for signal-mediated transport of macromolecules larger than 50 kDa (Böttger et al 1998), which can expand to an upper diameter of 26 nm (8.0 × 106 Da), depending on the species and the metabolic state of the cell (Lungwitz et al 2005). Therefore, the transfection efficiency is largely influenced by both the polyplexes size (Kreiss et al 1999) and plasmid copy number (Walker et al 2004). PEI/DNA complexes, which form particles within the 100–200 nm range, are thus excluded from nuclear entry through the NPC.
For active transport through the pore, a nuclear localization signal (NLS) is required (Tachibana et al 2001). A NLS is typically composed of clusters of four or more basic aminoacids flanked by α-helix breakers proline or glycine. Each NLS can interact with importin α which therefore becomes able to interact with importinα. This ternary complex can be uptaken through the nuclear pore by an energy-dependent mechanism (Pouton 1998).
Grosse and colleagues, using the human tracheal epithelial cell line ©CFTE29o-, have shown that the nuclear import either of pDNA/PEI or of pDNA/lactose-PEI increases when the cells are undergoing mitosis (Grosse et al 2004). These results are consistent with those reported by Brunner and colleagues using transferrin-PEI (Brunner et al 2000; Brunner et al 2002) and by other groups with unconjugated PEIs (Remy-Kristensen et al 2001; Mannisto et al 2005, 2007). Branched PEI 25 KDa-mediated transfection seems to be more dependent on the cell mitosis than that mediated by linear PEI 22 kDa (Brunner et al 2002).
On the contrary, it has been shown that many cells are still able to be transfected when growth-arrested (Pollard et al 1998; Godbey et al 1999b; Brunner et al 2002; Oh et al 2002). While in the former case, this is due to a passive nuclear importation during mitosis, mechanisms of transfection of mitotically quiescent cells are still indeciphered. This property could be another interesting feature of PEIs in view of gene transfer to the airway epithelium, whose half life has been shown to be more than 100 days (Blenkinsopp 1967).
In order to increase the nuclear import of DNA complexed by PEI, Zanta and colleagues (1999) developed a CMV-luciferase construct containing a single, covalently linked SV40 large T-antigen NLS (Table 2). In this system, the luciferase reporter gene was capped on both ends with an ODN hairpin structure, increasing resistance to exonucleases up to 25-fold and complexed to branched PEI 25 kDa or linear PEI 22 kDa. The transfection efficiency of these vectors in 3T3 and HeLa cells was 100–1,000-fold higher (even at low DNA concentration) than that observed when no NLS or a mutated NLS was used.
All together, these data support the notion that plasmid DNA, in its free form or still complexed with PEI, enters the nucleus either in the mitotic phase or in post-mitotic nuclei. This brings concerns about the toxicity of PEI at the level of whole gene expression (see above).
Interestingly, it has been demonstated in acellular systems that transcription is not impaired when PEI is closely attached to the template DNA (Bieber et al 2002) and that transcription inhibition was observed when plasmid DNA was either loosely (N/P < 5) or tightly condensed (N/P > 15) (Honoré et al 2005). It is then of interest that the ratios allowing the most efficient transcription (5–15) are also those allowing the most efficient transfection rates in vitro (Boussif et al 1995; Ogris et al 1998; Pollard et al 1998) and in vivo (Boussif et al 1995; Bragonzi et al 2000).
In vivo transfection of lung by PEIs
In recent years it has been shown that PEI is a good vehicle for plasmidic DNA and siRNAs delivery in vivo after lung instillation (Ferrari et al 1997; Bragonzi et al 2000), kidney perfusion (Boletta et al 1997), intracerebral injection (Abdallah et al 1996), or systemic delivery to the lung (Goula et al 1998a; Bragonzi et al 1999; Thomas et al 2005) or to the liver (Chemin et al 1998). In this section we will discuss those parameters which influence PEI-mediated gene transfer in vivo, with particular emphasis on the lung.
Impact of routes of administration and biophysical parameters
The lung is an attractive target for gene therapy interventions and a variety of approaches such as intravenous, intranasal, intratracheal instillation or aerosol delivery have been utilized to target genes to the airways. PEI has been shown to be a suitable vehicle for gene transfer into lung epithelial cells in vivo (mouse and rabbit) with higher efficiency than cationic lipids (Lemkine and Demeneix 2001; Bragonzi and Conese 2002). Recently, aerosolization of PEI 25 kDa was shown to be of equal efficiency as compared to the cationic lipid GL67, a gold standard for cystic fibrosis gene therapy, in the sheep (McLachlan et al 2007). High transfection efficiency of PEI as compared with cationic lipids might be ascribed to its high intrinsic endosomolytic activity because of a strong buffer capacity at virtual any pH (see above). However, whether this mechanism (based on the “proton sponge” hypothesis) is applicable following in vivo gene transfer is less clear in view of likely dilutional effects, due to airway surface liquid if topical administration is to be considered, as well as potential toxicities of the polymer that restrict local concentrations.
Intratracheal instillation of vectors into mouse lungs is still extensively used as a preclinical model to study vector efficacy, toxicity, and the action of delivered transgenes. Unfortunately, intratracheal instillation can hardly be envisaged to be a practical administration route for human applications and therefore, the aerosolization seems to offer an optimal noninvasive means of targeting the lung (Densmore 2006). The mode of gene vector application to the lung, ie, aerosol delivery vs direct instillation, exerts a pronounced effect on the gene transfer efficiency. Rudolph and colleagues (2005b) demonstrated that aerosolized nanogram quantities of DNA complexed to PEI yielded transfection levels 1 order of magnitude higher than microgram quantities of DNA applied directly to the lungs of mice via intratracheal intubation.
It must be said that the effects of the physicochemical properties of polyplexes on biological phenomena and transport processes are not yet fully understood and many efforts have done to elucidate the role of factors such as the polyplexes formulation, the solvent used and the polymer structure and pourity. The formulation of polyplexes, which is generally expressed in terms of N/P ratio, plays an important role in the transfection efficiency. PEI 25 kDa/DNA complexes of N/P ratios at 1,2, and 4 did not show luciferase activity in the lung following an intratracheal administration, whereas the transfection efficiency was enhanced by increasing the N/P ratio at values higher than 6 (Rudolph et al 2000). Formulation of complexes at N/P ratios <6 results in particles which bear negative or just slightly positive charges that are not able to arrange distinct cell adhesion and subsequent endocytosis. These findings are supported by observations made by Ferrari et al who did not detect luciferase activity in the lungs of newborn rabbits after intratracheal instillation of linear PEI 22 kDa at N/P = 1.
Moreover, the kind of the solvent used for vector formulation and delivery have an impact on gene transfer efficiency and PEI/DNA complexes formulated in various solvents and applied to mouse lungs, seem to have a completely different transfecting properties. After intratracheal instillation, in the mouse lung, complexes prepared in water express higher levels of transfection as compared to solvent like HBS (Rudolph et al 2000). One of the typical explanation for this phenomena is the fact that the colloidal stability of PEI/DNA complexes is very sensitive to the ionic strength of the surrounding solution and high salt concentrations, leading to salt-induced aggregation of complexes, make the transfection more difficult. Interestingly, the solvent used to prepare the complexes could be important not only for the effect on the complexes structure itself but even for being directly effective on the airway epithelium. Rudolph and colleagues (2005b), using PEI/DNA complexes formulated in water and administered via aerosol, have shown that water is able to induce a permeabilization of the apical membrane as well as airway swelling and both these factors seem to be correlated with a better transfection efficiency.
Since PEI exists as a branched polymer as well as in linear form, many in vivo experiments have been carried out to compare these two different structures. Efficiency depends on the route of administration and on PEI molecular structure. In mice, both branched PEI 25 kDa and linear PEI 22 kDa complexes formulated to have a N/P ratio of 5–10 and administered through the trachea gave similar levels of lung luciferase expression although targeting different cell populations (Bragonzi et al 2000). On the other hand, Stammberger and colleagues showed that linear PEI 22 kDa determined higher luciferase activity than branched PEI 25 kDa in ventilated lungs upon an intratracheal instillation in rats (Stammberger et al 2002).
Intranasal application of linear PEI 22 kDa polyplexes in mice has been shown to be superior to branched PEI 25 kDa vectors, yielding higher transgene expression (Wiseman et al 2003). Branched PEI 25 kDa is more efficient, when administered by aerosolization, as compared with both the linear PEI 22 kDa and the linear PEI 25 kDa (Rudolph et al 2005a). Linear PEI 25 kDa can produce large complexes aggregated whereas the linear PEI 22 kDa produce complexes which are very similar to those produced by branched PEI 25 kDa. These data indicate that particle aggregation seems not to influence gene delivery by PEI through aerosolization.
When the polyplexes are administered intravenously, linear PEI 22 kDa appears to be more efficient than the branched PEI 25 kDa (Bragonzi et al 2000). Lung transfection using nonviral vectors via the intravenous route is mainly attributed to their physical properties that give rise to prolonged retention in the pulmonary circulation and efficient interactions with the pulmonary vasculature (Song et al 1998; Li et al 1999a). Linear PEI 22 kDa/DNA complexes share a similar mechanism in lung transfection. PEI/DNA complexes formulated in 5% glucose and delivered into adult mice through the tail vein gave very high luciferase levels in the lung, at the level of endothelial cells of small vessels and penumocytes (Goula et al 1998a). At 2 h after injection large aggregates containing DNA were occasionally seen adhering to the wall of blood vessels (Goula et al 2000).
Organ DNA distribution and expression of plasmid DNA in vivo can be modulated significantly via the selection of PEIs with different physicochemical properties, including molecular weight, structure, and PEI/DNA complexation ratios. Although different PEIs (branched 25 kDa, branched 2 kDa, and linear 25 kDa) did not differ in prolonging the mean retention time of DNA in the blood as compared to naked DNA (Oh et al 2001; Jeong et al 2007), they deeply differed in organ distribution of the plasmid and its expression (Jeong et al 2007). Of the branched PEIs complexed with DNA at the same N/P ratio of 10, PEI 25 kDa showed a prolonged retention of plasmid DNA in the liver, as compared to PEI 2 kDa. At the same N/P ratio, linear PEI 25 kDa exhibited lung plasmid DNA levels orders of magnitude higher than was seen with the branched 25 kDa until 3 days post-dose. These results were paralleled by expression kinetics of plasmid DNA, with linear PEI 25 kDa providing mRNA expression levels order of magnitude higher in the lung than branched PEI 25 kDa over an 8-day period.
Finally, it has to be mentioned that efficiency and lung specificity of PEI-mediated delivery greatly depend upon the purity of the polymer (Thomas et al 2005). The removal of 11% residual acyl groups from the commercial linear 25 kDa by acid hydrolysis at elevated temperature enhanced its plasmid DNA delivery effciency 21 times in vitro in A549 cells and as much as 10,000 times in mice with a concomitant 1500-fold enhancement in lung specificity of gene delivery upon retroorbitally injection. To confirm that the DNA transfection efficiency of linear PEI is markedly affected by its extent of deacylation, three additional PEIs of 22-,87, and 217 kDa molecular weights were synthesized ab initio by acyl-catalyzed hydrolysis of poly(2-ethyl-2-oxazoline), yielding the pure polycations. PEI87 and PEI217 exhibited the highest DNA transfection efficiency in vitro; moreover, PEI87 delivered DNA to the mouse lung as efficiently as the pure PEI25 but at lower concentration and, importantly, with a 200-fold lung specificity. Upon systemic delivery in mice of the complexes of a siRNA against a model gene, firefly luciferase, PEI25 and PEI87 afforded a 77% and 93%, respectively, suppression of the gene expression in the lungs.
Persistence of gene expression
The persistence of transgene expression over the time is another important parameter to take in consideration as an ideal vector system should be able to confer a long duration of expression. Unfortunately, PEI/DNA complexes as well as the other non viral vectors are characterized by a poor persistence of gene expression. After intratracheal instillation of PEI/DNA complexes, the highest level of gene expression has been found after 24 h and a gradual decrease is observed over a time period of 7 days (Rudolph et al 2000). On the other hand, Gautam and colleagues (2001a) have shown that the transgene can be mantained for about 1 month following aerosolization of polyplexes. This short length of gene expression holds true also in other organs. For example, PEI-mediated gene delivery to rat hypoglossal nuclei via intramuscular tongue injection lasted for only two weeks (Wang et al 2001).
The loss of transgene expression may be explained in one of several ways: loss of the vector, transcriptional silencing of the transgene promoter, loss of transfected cells through cell turnover, or the generation of immune response to transgene product or the transfected cell itself. The majority of pre-clinical and clinical airway gene transfer studies have used strong viral promoters in order to achieve high-level gene expression in the lung. The human immediate-early cytomegalovirus (CMV) ehancer/promoter which is one of the most widely used, is known to undergo transcriptional inactivation in several tissues, likely for the production of cytokines including TNFα and IFNγ (Kawakami et al 2006). Although not specifically used with PEIs, it is worth mentioning that several investigators have evaluated alternative promoters in the context of nonviral gene delivery to the lung, including the human ubiquitin (Gill et al 2001; Yew et al 2001) and the elongation factor 1α (Gill et al 2001), obtaining sustained (up to 6 months) gene expression in mice airways.
Given this short duration of expression, repeat dosing with no reduction in the level of PEI-mediated gene expression is an objective to be achieved. Wiseman and colleagues showed by means of intranasal administration of PEI 22 kDa complexes in mice that a second delivery seven days after the initial treatment only gives around 20% of the initial gene expression level (Wiseman et al 2003). This decrease was unlikely to be caused by antibody production against reporter gene product as it was still observed when the first plasmid injected encodes a different protein, and in nude mice which cannot elicit T-cell dependent antibody response. Dames and colleagues using PEI 25 kDa, obtained fairly constant gene expression levels in mice lungs even 9 days after the first PEI/DNA aerosol treatment although the third dose of PEI-DNA, which was administered 6 days after the first dose, could not completely restore gene expression levels to the initial values (Dames et al 2006).
In an attempt to further increase the level of transfection in the lung after an intravenous administration, Bragonzi et al observed that the administration of PEI 22 kDa/DNA complexes 16 h after the first injection did not give any increase in transfection activity (Bragonzi et al 1999). On the contrary, when two injections of PEI 22 kDa complexes were administered at a 15-min interval, one log increase was observed. Orson et al using ternary complexes made of PEI-DNA and murine serum albumin (MSA) observed that repeated administration of the same dose of luciferase-encoding plasmid did not increase the maximal detected activity, but did result in a relatively constant gene expression level, demonstrating that the response to repeated injection was not inhibited (Orson et al 2002). Similarly, we have observed that double intratracheal administration of complexes made of PEI 25 kDa/DNA and MSA with a 3-day interval did not reduced luciferase expression in the lung but even increased its level (Di Gioia et al 2008).
An in vivo study with PEI-g-PEG demonstrated a prolonged gene expression in the spinal cord without experiencing any attenuation of gene expression after repeated intratechal injection of PEI-g-PEG/DNA complexes (Shi et al 2003). In contrast, 70% attenuation of the gene expression was observed after repeated administration of PEI/DNA complexes to the spinal cord at a 2-week interval. The attenuation was associated with apoptotic death, suggesting that PEGylation of PEI could have reduced intrinsic cytotoxicity of PEI.
With intravenous administration, reduced gene expression after a second dose was found to be related to induction of inflammatory cytokines in response to unmethylated CpG motifs in the plasmid DNA (Li et al 1999b). Decreased gene expression following a second dose may also be due the toxic side effects associated with PEI, which have been reported following systemic injection of PEI/DNA complexes (Chollet et al 2002).
To overcome the limited transgene expression in the lung obtained with nonviral vectors, a new gene delivery vector based on the Sleeping Beauty (SB) transposase was used. The SB transposase is an enzyme that recognizes the ends of a transposon (indirect repeats, IRs), excises the transposon from its location in the genome, and reinserts it elsewhere into chromosomal DNA. By introducing a therapeutic gene between transposon IRs and supplying the transposase function, it is possible to use this transposon as a vector for gene therapy. Belur and colleagues demonstrated in the lungs of all animals transgene expression after 24 h after intravenous injection with the luciferase transposon complexed to linear PEI 22 kDa, but expression up to 3 months required co-delivery of a plasmid encoding the SB transposase (Belur et al 2003). Transgene expression was localized to the alveolar region of the lung, with transfection of mainly type II pneumocytes.
The SB transposase/transposon system, however, mediates integration at random sites in the genome and therefore could result in insertional mutagenesis similar to integrating viral vectors. Phage φC31 integrase is a site-specific recombinase that mediates efficient integration of plasmid DNA into host cell genomes. It was demonstrated that integrase φC31 mediates site-specific integration in vitro and in vivo by recombining the attB recognition site in an episomal plasmid and one or more pseudo-attP sites in the host chromosome. This system has been tested in gene therapy approaches for different diseases, such ase hemophilia, epidermolysis bullosa, hereditay tyrosinemia type 1, and muscular dystrophy. Recently, Aneja and colleagues (2007) have shown expression of luciferase after intravenous injection of linear PEI 22 kDa/DNA complexes could be observed after 2 weeks only in the lungs of mice which were co-injected with the integrase-encoding plasmid. Only seven out of the fifteen treated mice showed integration in the mouse lung at the mpsL1 site at 8–9 months post-application, emphasizing the need for optimization of this sytem to maintain a sustained gene expression in the lung environment.
Localization of transfected cells in the lung
Many factors can influence the localization of PEI/DNA complexes in the lung tissue, such as the route of administration or the type of PEI used. Diseases such as cystic fibrosis and asthma would need gene delivery to the airway epithelial cells of the conducting airways, whereas gene transfer into alveolar epithelial cells would be necessary to treat surfactant protein B deficiency and α1-antitrypsin deficiency. After injection of plasmid DNA formulated with PEI directly in the blood system, high levels of transgene expression can be found mostly in the lung alveolar tissue in mice (Goula et al 1998a, 2000; Bragonzi et al 1999). These data have been confirmed by means of electron microscopy which demonstrated how both type I and type II pneumocytes, as well as endothelial and myoepithelial cells can be transfected (Dif et al 2006). Levels and sites of expression are similar in normal and in CFTR-mutant mice. After intravenous administration, linear PEI 22 kDa complexes can transfect up to 5% of pulmonary cells (Zou et al 2000). Beta-galactosidase histochemistry confirmed alveolar cells, including pneumocytes, to be the main target.
After nebulization, gene expression is mainly localized in the bronchial epithelium of the large airways (Gautam et al 2001a; Rudolph et al 2005a), and Rudolph and colleagues (2005a) argued that this kind of distribution could be due to a different rate of clearance of polyplexes by macrophages whose number is much higher in the alveoli than in the rest of respiratory tree. Recently, the same pattern of gene expression has been confirmed using cell-specific antibodies and GFP by intranasal instillation in mice (Davies et al 2007). Bragonzi et al observed that, upon intratracheal instillation, both PEI 22 kDa and PEI 25 kDa had similar levels of transgene expression but that the pattern of tissue distribution was different with PEI 25 kDa complexes being distributed mainly in the large bronchi and PEI 22 kDa localized in the distal tract of the broncho-alveolar tree (Bragonzi et al 2000).
In vivo toxicity
Though PEI is one of the most efficient nonviral vectors, both its pronounced cellular toxicity and its severe systemic side effects as well as its pro-inflammatory properties limit the use of PEI polyplexes for gene transfer in vivo. Several authors reported that PEI is able to induce multiple responses such as FasL-mediated antigen-induced apotosis (Regnstrom et al 2003), liver necrosis, activation of lung endothelium, adhesion of aggregated platelets and shock after systemic injection of elevated doses (Chollet et al 2002). Kawakami and colleagues (2006) observed that the intravenous administration of PEI/DNA complexes induced the production of serum proinflammatory cytokines (TNF-α, interferon-γ, and interleukin-12), which are then reduced upon the administration of the linear PEI polyplexes as compared with that of branched PEI complexes.
In a recent study carried out by intratracheal instillation, the toxic effects of PEI-DNA and chitosan complexes have been analysed using a microarray platform (Regnstrom et al 2006). Interestingly, PEI-DNA complexes seem to upregulate genes involved in inflammatory processes, such as the cyclooxygenase 1 and 2, indicating possible involvement in the development of adverse reactions. Furthermore, genes involved in reaction to stress, such as chaperone genes and members of the p38 mitogen-activated protein kinase pathway, were upregulated only in the PEI group, suggesting a possible explanation for the better performance of PEI than chitosan in gene delivery to the lung.
PEI polyplexes stimulate the immune system (Regnstrom et al 2003) and can cause from moderate to serious inflammation of lungs, which is evidenced by neutrophils infiltration and reduced arterial oxygen saturation (Rudolph et al 2000). In contrast to these observations, transient inflammation restricted to the lungs was observed by measurement of cytokine levels in the lungs, bronchoalveolar lavage fluid, and serum when PEI gene vectors were applied via aerosol administration (Gautam et al 2001b).
Barriers to efficient gene transfer to the lung
Direct instillation of PEI polyplexes would be needed in congenital and acquired lung diseases. In the lung, the mucus layer secreted by globet cells, apical membrane gly-conjugates, and the airway epithelium with tight junctions inhibiting the intercellular transport remain the major barriers for efficient topical nonviral-based gene delivery (Pilewski 2002; Kolb et al 2006). Compared to PEI, the stability of TAT-PEG-PEI polyplexes was significantly higher in the presence of high concentration of heparin, Alevofact® (a natural bovine surfactant preparation) as well as broncho-alveolar lavage fluid, indicating that PEG-PEI complexes with DNA are endowed with increased complexation and condensation properties (Kleemann et al 2005).
In cystic fibrosis (CF), airways inspissated mucus and mucus plaques will enhance the barrier to airway gene transfer even further. The CF mucus, extremely thick and viscoelastic due to the presence of high amounts of DNA, proteins (albumin, actin and mucins), phospholipids, and inflammatory products, is the primary barrier which induces a failure of viral and nonviral vectors in transducing airway epithelial cells (reviewed in (Ferrari et al 2002) and (Conese et al 2007)). While the exposure to surfactant proteins does not have any effect or even enhances PEI-mediated gene transfer (Ernst et al 1999), normal mucus has been found to inhibit gene delivery to native sheep tracheal epithelium maintained at an air-liquid interface (Ferrari et al 2001). Similarly, sputum and bronchoalveolar lavage fluid (BALF) recovered from CF patients were demonstrated to inhibit PEI-mediated gene transfer efficiency (Rosenecker et al 2003). Recently, we have shown that addition of human serum albumin (HSA) to preformed PEI/DNA complexes increased gene transfer efficiency of PEI by 500–1000-fold in immortalized airway epithelial cells (Carrabino et al 2005). CF sputum inihibited PEI-mediated gene transfer, whereas in the presence of HSA, PEI showed increased levels of gene transfer. The presence of HSA in the complexes may have impeded interaction between positively charged PEI polyplexes and negatively charged albumin present in the CF sputum following increased vessel permeability due to chronic inflammation.
The role of bacteria infection in the gene transfer, specifically the massive colonization of CF airways by Pseudomonas aeruginosa and other bacteria, causes a profound modification in the airway “microenvironment” and this process must be taken into account when a nonviral vector is studied. Rejman and colleagues (2007), using mice infected by either P. aeruginosa or E. coli observed that the infection with P. aeruginosa caused a 5-fold increase in gene transfer whereas infection with E. coli had no effect. Moreover, by studying biodistribution of fluorescently labelled PEI-DNA complexes, the same group showed that prior treatment with P. aeruginosa increased penetration of the complexes deeper into the epithelium than in untreated animals. In P. aeruginosa-treated animals fluorescence was detected not only in the airway epithelium itself but also in the parenchyma. They concluded that infection with P. aeruginosa causes disruption of the tight junctions between the cells and thus of the barrier function of the epithelium. As a consequence, PEI-DNA complexes injected intratracheally into infected animals gain access to the basolateral side of the cells and to spaces across the epithelial lining, giving rise to substantially increased transfection efficiency. These results strongly suggest that putative “receptors” for PEI/DNA complexes are not apically concentrated and indicate that, in the absence of bacterial infection, a targeting moiety should be added for efficient transfection of bronchial/bronchiolar cells in vivo.
Pegylation and targeting of PEI polyplexes
The major limitations of positively charged PEI polyplexes include: limited efficiency of gene delivery, toxicity at higher concentration, and potentially adverse interaction with negatively charged macromolecules in serum and cell surfaces (Plank et al 1996). Moreover, they can interact with the extracelullar matrix and nontarget cells. PEI polyplexes can be shielded by coating them with different compounds, among which the most used is polyethylene glycol (PEG) (Kircheis et al 2001; Lungwitz et al 2005; Park et al 2006) (Figure 4). PEI-PEG/DNA complexes, generated in 5% glucose, have slightly reduced surface charge (~20 vs ~40 mV) and bigger size (125 vs 100 nm) as compared with PEI/DNA polyplexes (Kichler et al 2002) (Figure 2). A beneficial effect of PEGylation that was observed is that the PEG-PEI conjugates are less cytotoxic than the nonmodified polymers. This, however, may be related to the fact that less PEG-PEI/DNA complexes are uptaken by the cells (Kichler et al 2002). It was also found that PEG chains dramatically increase the solubility of the DNA complexes. At DNA concentration of 350 μg/ml, small precipitates were found when linear PEI 22 kDa was used at a N/P ratio of 12. In contrast, PEG-PEI/DNA complexes with up to 1,500 μg/ml of DNA could be generated (Kichler et al 2002).
Figure 4.
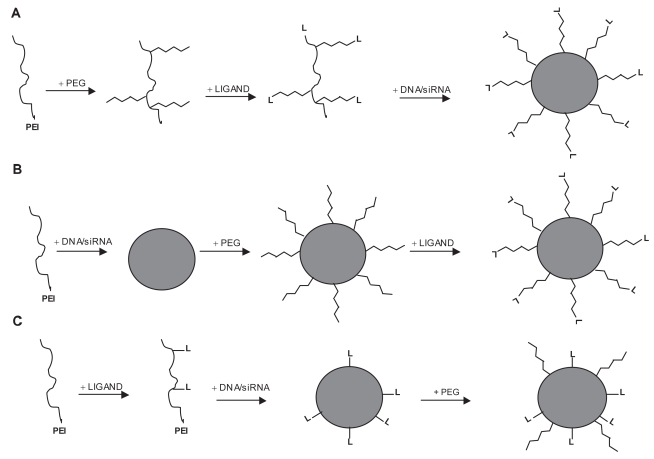
Schematic representation of three strategies used for the formation of PEGylated ligand-containing PEI/DNA and PEI/siRNA complexes. (A) After PEGylation of PEI, the ligand reacts with functionalized distal end of the hydrophilic arm. The last step then consists of condensing the DNA/siRNA with the ligand-PEG-PEI conjugate. (B) PEI/DNA or PEI/siRNA complexes are first generated. The resulted polyplexes are modified by a heterobifunctional PEG which reacts with aminogroups of PEI. Ligands are finally incorporated into the complexes by conjugation with the distal end of the PEG. (C) The first step consists of covalently coupling the ligand to PEI. Addition of plasmid DNA or siRNA leads to the formation of ligand-PEI/DNA(siRNA) complexes which are subsequently modified with PEG chains. Modified with permission from Kichler A. 2004. Gene transfer with modified polyethylenimines. J Gene Med, 6:S3–S10.
A hydrophilic shielding component, such as PEG, is introduced into the complexes also to reduce aggregation (Sung et al 2003). For instance, while PEI polyplexes prepared in high ionic strenght medium (150 mM NaCl) showed a drastic increase in size due to aggregation, the TAT-PEG-PEI polyplexes remained stable over a period of 20 min (Kleemann et al 2005). However, polyplex aggregation under physiological conditions is still an area of controversy and must be characterized thoroughly (Neu et al 2005). While aggregation of polyplexes in the lung vessels seems to be favorable for efficient lung cell transfection, especially by linear 22 kDa PEI, this behavior may not be proficient for transfection in distant sites (eg, cancer).
Targeting PEI polyplexes using peptides
Valid alternative approaches to PEGylation include the development of polyplexes displaying ligands for cellular receptors on their surface, the ligand having the additional effect of shielding positive charges. Different types of ligands such as sugar residues, growth factors, integrins, peptides, proteins and antibodies have been used for the targeting of PEI/DNA complexes in vivo (Kircheis et al 2001; Lungwitz et al 2005; Park et al 2006). In order to favorably modify the behavior of PEI/DNA complexes for systemic administration, different strategies have been developed to generate PEGylated ligand-PEI/DNA complexes (Figure 4).
Although several studies have ascertained that the conjugation of negatively charged ligands to PEI is sufficient to reduce the surface charge of PEI polyplexes, rendering not necessary the addition of PEG chains for this purpose (Kichler 2004), recent studies have shown that PEG-PEI-ligand complexes were used with success in targeting tumors and neurons using peptides (Table 2).
One group demonstrated that conjugates of branched PEI 25 KDa PEGlyated with an RGD peptide vehicling siRNA inhibiting vascular endothelial growth factor receptor-2 (VEGF-R2, also named KDR/flk-1) was able to accumulate specifically in subcutaneously grown neuroblastoma N2A tumor, when administered intravenously, and mediate inhibition of both tumor angiogenesis and growth rate (Schiffelers et al 2004). The same results were obtained when a conjugate of PEI grafted with PEG-RGD and a plasmid encoding the soluble form of VGF-R1 (flt-1) was tested in the subcutaneous CT-26 adenocarcinoma model (Kim et al 2006b).
Targeting CD13 is an alternative strategy for specific targeting of cancers. It has been amply demonstrated that the CD13/aminopeptidase N protein is expressed exclusively on tumor cells, as well as endothelial cells of angiogenic tumor vasculature but not normal vasculature (Pasqualini et al 2000). Intravenous injection of a PEG/PEI/βgal vector harboring the CNGRC peptide, which binds specifically CD13, to nude mice bearing subcutaneous H1299 lung tumors resulted in as much as a 12-fold increase in β-gal expression in tumors as compared with expression in lungs. The CNGRC/PEG/PEI/YFP vector targeted delivery to both tumor cells and tumor endothelial cells (Moffatt et al 2005). As a further refinement, a multifunctional vector for p53-mediated gene therapy was created (Moffatt et al 2006). The SV40 large T peptide-NLS and oligonucleotide-based DNA nuclear targeting signal (DNTS) were coupled to the CNRGRC/PEG/PEI vector carrying the p53 gene in a EBV-based episomal system. This targeting vector was the most effective in reducing tumur volumes in the subctaneous H1299 tumor model as compared to the vector without NLS and DNTS coupling and increased animal survival up to 90% as opposed to only 20% with the untargeted PEI/DNA/β-gal vector.
PEI-mediated gene delivery to the central nervous system (CNS) of mice and rats using PEI/DNA complexes has been reported (Abdallah et al 1996; Goula et al 1998b; Wang et al 2001; Li et al 2004). However, a certain toxicity (apoptosis) has been demonstrated after intracisternal administration of PEI/DNA complexes (Li et al 2004). To specifically target neurons, branched PEI 25 kDa was conjugated to two peptides: the Tet1 peptide, which competes effectively with tetanus toxin for receptor binding to the neurons and neurotensin (NT), a 13-aminoacid neuropeptide. Targeted PEI complexes showed specific binding to differentiated PC-12 neuron-like cells but not to nonneuronal 3T3 fibroblasts (Park et al 2007). Significant binding to and uptake by primary neurons, derived from dorsal root ganglion isolated from embryonic mice, was only observed with Tet1-PEI formulations. However, since terminally differentiated neurons are not-dividing, gene expression was not observed under these conditions, indicating that Tet1-PEI formulation should be modified with peptides that improve intracellular trafficking. Since efficient gene transfer to neurons was observed in vivo by application of untargeted PEI complexes to CNS via different delivery methods, it is also possible that Tet1 peptide affects PEI capability of transfecting differentiated neurons.
Table 2 summarizes the peptides used for guided PEI-mediated gene delivery in vitro and in vivo, including those harboring binding, internalizing, endosome-destabilizing and nuclear import activities.
Targeting PEI complexes to the lung
As discussed above, PEI polyplexes transfect preferentially epithelial cells of the conducting airways if administered by intratracheal or aerosolization routes. Conversely, alveolar cells are preferably transfected with an intravenous admnistration. The airway epithelium of bronchi and bronchioli is the target for a successful gene therapy approach to cystic fibrosis (CF) and asthma, whereas the pulmonary endothelium and type I and II pneumocytes are the cell types to be transfected for treating pulmonary metastases, pulmonary hypertension, α1-antitrypsin deficiency and surfactant deficiency (Driskell and Engelhardt 2003). Moreover, serous cells of airway submucosal glands express extremely high levels of the gene mutated in CF, ie, CFTR (Engelhardt et al 1992), suggesting that these regions may also be important gene therapy targets in CF.
Lac-PEI was investigated as a model for interaction with surface lectins (Fajac et al 2003; Grosse et al 2004; Grosse et al 2005). While in 70% confluent undifferentiated human tracheal gland serous CF-KM4 cells, Lac-PEI is more efficient than unsubstituted PEI and lactosylated polylysine in the presence of chloroquine, in differentiated CF-KM4 and human bronchial epithelial cells grown as an explant-outgrowth, a low gene transfer efficiency was observed (Fajac et al 2003).
Recently, the function of lactoferrin as a novel targeting ligand for gene delivery to human airway epithelial cells has been investigated. Lactoferrin has been demonstrated to have many receptors on BEAS-2B bronchial epithelial cells (Ghio et al 1999) and not on A549 cells (type II pneumocytes) (Elfinger et al 2007). Interestingly, even with low degrees of lactoferrin coupling to branched PEI 25 kDa, the gene expression was about 5-fold higher than PEI alone in BEAS-2B cells. On A549 cells, no increase in gene expression was observed with lactoferrin/PEI complexes (Elfinger et al 2007).
To discover novel ligands, phage display libraries has been screened for peptide binding with high affinity to airway epithelial cells. Romanczuk and colleagues (1999) found that the peptide SDQLASPYSHPR, coupled to an adenoviral vector using bifunctional PEG, increased gene transfer to well-differentiated human airway epithelial cells as compared to wild-type vector and to that bearing a scrambled peptide. Jost and colleagues (2001) used the peptide THALWHT, to deliver a luciferase reproter gene to confluent 16HBE14o- bronchial cells, but obtaining only 10% of the levels reached by a liposomal gene transfer agent. Writer and colleagues (2004) found five peptides which were incorporated into gene delivery formulations with the cationic lipid Lipofectin and shown to confer a high degree of transfection efficiency and specificity in 1HAEo- cells. The most efficient peptide, LPHKSMP, consistently produced transfection efficiencies around 10 times that obtained with the scrambled version of the peptide. Two peptides, RFDSLKV and GRGDGVD, were identified by Vaysse and colleagues (2000) by biopanning on CFT-2, a human foetal tracheal cell line derived from a CF patient. The latter peptide contains an integrin-targeting RGD motif. When investigated in vivo upon systemic administration of peptides formulated with a cationic lipid, the peptide RFD-SLKV determined an increase of lipid-mediated transfection of the lung (Vaysse et al 2002). Finally, Florea and colleagues (2003) identified an internalizing peptide (CDSAFVTVDWGRSMSLC) which increased up to 6-fold PEI-mediated transfection of differentiated human submucosal gland Calu-3 cells. Interestingly, confocal laser-scanning microscopy revealed basolateral localization in membrane-associated and internalizing vesicles. This pattern and the presence of a DGWR sequence in the peptide “receptor” that closely resembles the RGD motif, known to be recognized and constitutively internalized by α5β3 and α5β1 integrins (Parkes and Hart 2000), suggest that peptide endocytosis might be facilitated by an integrin receptor. Indeed, integrins have been considered as possible targets for receptor mediated gene delivery to airway epithelial cells (Meng et al 2004). However, caution should be used in interpreting this last study, since in vivo transfection of murine lung by intratracheal administration of Lipofectin/integrin targeting peptide/DNA complexes showed a sixfold enhancement of luciferase expression when lungs were pre-treated with EGTA, a Ca2+-chelator able to disrupt tight juntions, indicating that nonviral vectors are able to transfect well diffeentiated polarized airway epithelial cells via integrin only when the basolateral membrane is exposed.
An alternative strategy is to target known receptors and it has been successfully used in human CF airway epithelial cells and CF mice to correct primary and secondary defects due to CFTR mutations. Drapkin and colleagues (2000) found that human primary airway epithelial cells express the urokinase receptor (uPAR) on the apical surface and that a peptide derived from the amino-terminal portion of uPA (CLNGGTC) is able to stimulate fluid-phase endocytosis. Gene transfer of wild-type CFTR gene to CF airway epithelia by an adenoviral vector and correction of the cAMP-stimulated current was further enhanced in the presence of uPA Finally, coupling of the uPA peptide to the PEG-modified adenoviral vector enhanced gene transfer to airway epithelia 10-fold. Ziady and colleagues (2002) administered molecular conjugates that contain a peptide (CSIPPEVKFNKPFVYLI) targeting the serpin-enzyme complex receptor and these beared the CFTR gene to the nasal epithelium of CF mutant mice. They observed partial correction of the chloride transport defect as assessed by in vivo nasal potential difference measurements and restoration of nitric oxide synthase-2, which is downregulated in the epithelium of mice and humans with CF.
From a gene therapy standpoint, submucosal glands will most definitely be difficult to target from the airway lumen. Several strategies have been proposed to circumvent this potential problem. One such method has utilized the polymeric IgA receptor to target DNA/protein complexes through the blood to glandular cells by transcytosis (Ferkol et al 1995, 2003).
Since the RGD peptide-containing polyplexes transfect preferentially angiogenic vasculature in tumors when administered intravenously (see above), alternative approaches must be found to target the pulmonary endotehelium and hence penumocytes in nonangiogenic conditions. One group has shown that a conjugate of linear PEI 22 kDa with antiplatelet endothelial cell adhesione molecule (PECAM) was 20-fold more efficient than unconjugated PEI in tranfecting the mouse lung after an intravenous administration with lower toxicity with respect to circulating TNF-α levels (Li et al 2000).
PEI as potential nonviral vector for the treatment of lung diseases
Since PEI can achieve high levels of transgene expression in the lung, it could be potentially useful as therapeutic approach for many lung diseases such as cancer and asthma.
Cancer
The inhibition of tumor metastasis to the lung, which poses a huge challenge in most clinical settings, was one of the first attempts carried out to study the function of PEI as nonviral vector. Many researchers reasoned that local delivery of agents via aerosol or intranasal instillation may offer a unique therapeutic opportunity. Intranasal or aerosol therapy delivers these agents directly to the lung, increasing the concentration in the tumor area and potentially decreasing systemic toxic effects.
Interleukin (IL)-12 is a cytokine that has antiangiogenic as well as immunostimolatory activity (Del Vecchio et al 2007). IL-12 activates NK activity, facilitates the induction of cytotoxic and Th1 T cells, and stimulates the production of interferon-gamma. Many of these activities have been implicated in the antitumor effect of IL-12 (Brunda et al 1993; Nastala et al 1994). Jia et al have demonstrated that PEI could be used to deliver the IL-12 gene to the mouse lung by both aerosol and intranasal administration, resulting in selective IL-12 expression with no spillover to the blood stream (Jia et al 2002, 2003). These investigators observed that mice that received PEI/IL-12, both intanasally and by aerosol twice weekly for 4–6 weeks, presented a decrease in the osteosarcoma lung metastases compared to the untreated mice. Interestingly, the same group observed that when IL-12, which is able to up-regulate Fas expression, was combined with one of the most used alkylating agents (ifosfamide), the therapeutic effect of this drug was enhanced (Duan et al 2006).
Another experimental approach to the anti-cancer therapy depends on the inhibition of growth and/or promotion of apoptosis of cancer cells. p53 codes for a nuclear phosphoprotein which is capable of modulating the expression of certain genes implicated in the regulation of cell division and apoptosis. The mutated p53 protein looses its anti-proliferative properties favoring a de-regulation of cellular multiplication with the accumulation of genetic aberrations. The delivery of p53 tumor suppressor gene complexed to PEI by aerosol can inhibit lung metastasis induced by a melanoma (Gautam et al 2000) or by osteosarcoma cell line (Densmore et al 2001). Recently, it has been demonstrated that aerosol delivery of the PDCD4 gene (encoding for a protein that is able to decrease antiapoptotic protein BCL-XL, but increases the proapoptotic proteins such as BAX, BAD, BID as well as inhibiting the synthesis or activation of AP-1 protein) might be a useful system in anti-cancer therapy of lungs. This study suggests that combined actions such as facilitating apoptosis, controlling cell cycle and suppression of AP-1 activity by PDCD4 may provide useful tool for designing lung tumor prevention and treatment by which PDCD4 functions as a transformation suppressor in the future (Hwang et al 2007).
Lung transplantation
The treatment of acute and chronic airway injury caused by immune response in particular conditions, such as the lung transplantation, is another important field of application of PEI-mediated gene therapy. Using PEI as nonviral vector and a SB transposase/transposon system encoding the human gene indoleamine-2, 3-dioxygenase (hIDO), which is an enzyme that possesses both T cell-suppressive and anti-oxidant properties, Liu and colleagues have demonstrated that a single dose of intratracheal-administered hIDO-expressing transposon complexed with PEI is effective in preventing the development of lung allograft fibrotic injury as well as the ischemia-reperfusion injury, in rats (Liu et al 2006, 2007). It is noteworthy that the high level of lung IDO activity cannot be achieved by giving animals this enzyme directly, as stable and purified protein is not available at the present. Moreover, this novel pharmacological intervention may be usful in most lung diseases characterized by fibrosis with impaired pulmonary function such as asthma, cystic fibrosis, idiopathic pulmonary fibrosis and pulmonary hypertension.
Asthma
Another application of PEI polyplexes, where local delivery can be successfully made, is asthma. Seong and colleagues generated antisense oligodeoxynucleotides (AS-ODNs) against murine IL-4 mRNA as IL-4 plays a crucial role as an inflammatory mediator in allergic asthma via inducing Th2 inflammation and IgE synthesis. When administered intranasally, the IL-4 AS-ODNs/PEI complexes were capable of inhibiting IL-4 secretion in a sensitized murine model of airway inflammation (Seong et al 2006).
RNA interference
RNA interference (RNAi), the mechanism by which the expression of a specific protein can be reduced or eliminated, has emerged as a potential therapeutic agent for treating numerous diseases, including cancer and other diseases from genetic disorders to viral infection (de Fougerolles et al 2007). RNAi pathways are guided by small RNAs that include small-interfering RNAs (siRNAs) and microRNAs, the latter being called the ‘endogenous’ pathway. In gene delivery, it is possible to harness the endogenous pathway in one of two ways: either by using a viral vector to express short hairpin RNA (shRNA) that resembles microRNA precursors, or by introducing siRNAs that mimic the cleavage product into the cytoplasm.
PEI-siRNA complexes have been reported to be efficient in vitro and to show therapeutic benefit in vivo in a number of disease models. In Table 1, we report selected examples referred to cancer and infectious diseases. Since the application of PEI/siRNA to tumors has already been described in other sections of this review, we focus here on respiratory infectious diseases, which are one of the main targets of nonviral delivery of siRNAs by polycationic polymers to the lung (Thomas et al 2007). To this end, several groups have been optimizing the physical structure of PEI to improve in vivo siRNA delivery (Thomas et al 2005; Grayson et al 2006; Werth et al 2006). Ge et al have shown that short siRNAs specific for conserved regions of influenza virus genes, especially those encoding nucleocapsid protein (NP), can prevent and treat influenza virus infection in mice (Ge et al 2004). In this study, virus production in lungs of infected mice was reduced by siRNAs given either before or after virus infection, by using slow iv administration of small volumes containing siRNAs in complexes with PEI. Interestingly, these results have been confirmed by another study which showed that retroorbitally administered PEI-NP siRNA resulted in 94% drop of virus titers in the lungs of influenza-infected animals (Thomas et al 2005). It is noteworthy to mention that previous vaccine development studies have shown that even a 90% reduction in the virus titer leads to the survival from a lethal challenge (Epstein et al 2002).
Clinical application of PEI-mediated gene delivery
The versatility and efficiency of PEI-based gene transfer to the lung in different in vivo settings warrants further investigation in terms of possible therapeutic applications. The following steps have to be considered for moving PEI into the clinic:
If gene delivery agents are to be considered a drug, a stable formulation is an essential requisite. In acqueous solution, nonviral delivery systems suffer from poor long-term physical stability including the loss of transfection efficacy and the tendency to aggregate. It has been shown that lyophilization of PEI complexes may under certain conditions prevent these effects leading to the preservation of physical stability and biological activity for DNA (Talsma et al 1997; Brus et al 2004) and siRNA (Werth et al 2006). Importantly, a controlled degradation to intracellualar nontoxic products has to be included to tackle cytotoxicity. Hence, the use of biodegradable PEIs should be pursued.
The type of lung disease and the cell type(s) to be targeted defines which route of administration and the type of polyplexes should be used. Intravenous injection “target” primarly the lung and achieve high transfection levels in alveolar cells. Chemical modifications with hydrophilic residues to avoid interactions with ionic macromolecules (such as PEG) and grafting with cell-specific ligands or antibodies are the current strategies to increase lung specificity. Indeed, transgene expression in nontarget cells could lead to side-effects. In particular, the uptake of pDNA by immune cells is a major obstacle to nonviral gene therapy approaches, because the uptake sometimes triggers a severe immune reaction which reduces the level and duration of transgene expression in target cells. Aerosolization is the preferred method for topical delivery of PEI/DNA complexes that results in the higher efficiency/toxicity ratio. However, cilia beating and mucociliary clearance can be difficult hurdles to be overcome. Thus, the local confinement of nucleic acid delivery is an important requirement for gene delivery to the airway epithelium. Magnetofection is a novel technique which could guide aerosols to specific regions of the lung using an external magnetic field (Plank 2008).
Although toxic and immunological reactions are lower than those elicited by cationic lipids and viral vectors, they are not fully absent. This is due to the bacterial nature of plasmid DNA, rich in CpG motifs, a constraint which can be overcome by using plasmid with no such motifs (Yew et al 2002). The potential of PEI for stimulating immune response when complexed with siRNA has not yet been studied. However, it is known that RNA molecules less than 20 bp in length are generally believed to avoid induction of interferon pathways (Fish and Kruithof 2004).
Gene therapy approaches for genetic or acquired disorders (such as cystic fibrosis or asthma) requires long-term and sustained transgene expression levels in vivo. This is not available with PEIs and the currently used genetic material, although some studies are currently evaluating the use and optimization of site-spcific integrase (Aneja et al 2007).
In terms of lung disease which could potentially be cured by PEI-mediated gene delivery, those targeted by intravenous route of administration are likely to be the first, including This category includes cancer (primary or metastatic) and alveolar-borne diseases, such as acute respiratory distress syndrome.
Conclusion
In the present review, we have illustrated some of the interesting features of PEI-mediated gene delivery and barriers which impede its clinical application at the levels of organ, tissues and cells. Proof-of-principle that PEI can have therapeutic effect in both genetic and acquired lung diseases has been given. However, specific studies aimed to overcome some of the extracellular barriers (eg, mucus) have not been performed, reflecting the absence of appropriate animal models. Only when these caveats will be addressed, can therapeutic use of PEIs become a reality.
Acknowledgments
We would like to thank Dr Stephan J. Reshkin for critically reading the manuscript. This work was supported by the Italian Ministero della Sanità with the law 548/1993.
References
- Abdallah B, Hassan A, Benoist C, et al. A powerful nonviral vector for In Vivo gene transfer into the adult mammalian brain: Polyethylenimine. Hum Gene Ther. 1996;7:1947–54. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- Ahn CH, Chae SY, Bae YH, et al. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J Control Release. 2002;80:273–82. doi: 10.1016/s0168-3659(01)00547-8. [DOI] [PubMed] [Google Scholar]
- Aigner A, Fischer D, Merdan T, et al. Delivery of unmodified bioactive ribozymes by an RNA-stabilizing polyethylenimine (LMW-PEI) efficiently down-regulates gene expression. Gene Ther. 2002;9:1700–7. doi: 10.1038/sj.gt.3301839. [DOI] [PubMed] [Google Scholar]
- Aneja MK, Imker R, Rudolph C. Phage phiC31 integrase-mediated genomic integration and long-term gene expression in the lung after nonviral gene delivery. J Gene Med. 2007;9:967–75. doi: 10.1002/jgm.1090. [DOI] [PubMed] [Google Scholar]
- Arote R, Kim TH, Kim YK, et al. A biodegradable poly(ester amine) based on polycaprolactone and polyethylenimine as a gene carrier. Biomaterials. 2007;28:735–44. doi: 10.1016/j.biomaterials.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Baker A, Cotten M. Delivery of bacterial artificial chromosomes into mammalian cells with psoralen-inactivated adenovirus carrier. Nucleic Acids Res. 1997;25:1950–6. doi: 10.1093/nar/25.10.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Saltik M, Lehrmann H, et al. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997;4:773–82. doi: 10.1038/sj.gt.3300471. [DOI] [PubMed] [Google Scholar]
- Behr J-P. Gene transfer with synthetic cationic amphiphiles: Propsects for gene therapy. Bioconjugate Chem. 1994;5:382–9. doi: 10.1021/bc00029a002. [DOI] [PubMed] [Google Scholar]
- Belur LR, Frandsen JL, Dupuy AJ, et al. Gene insertion and long-term expression in lung mediated by the Sleeping Beauty transposon system. Mol Ther. 2003;8:501–7. doi: 10.1016/s1525-0016(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Bertschinger M, Backliwal G, Schertenleib A, et al. Disassembly of polyethylenimine-DNA particles in vitro: Implications for polyethylenimine-mediated DNA delivery. J Control Release. 2006;116:96–104. doi: 10.1016/j.jconrel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Bieber T, Meissner W, Kostin S, et al. Intracellular route and transcriptional competence of polyethylenimine-DNA complexes. J Control Release. 2002;82:441–54. doi: 10.1016/s0168-3659(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res. 1967;46:144–54. doi: 10.1016/0014-4827(67)90416-8. [DOI] [PubMed] [Google Scholar]
- Blessing T, Kursa M, Holzhauser R, et al. Different strategies for formation of pegylated EGF-conjugated PEI/DNA complexes for targeted gene delivery. Bioconjug Chem. 2001;12:529–37. doi: 10.1021/bc0001488. [DOI] [PubMed] [Google Scholar]
- Boletta A, Benigni A, Lutz J, et al. Nonviral gene delivery to the rat kidney with polyethylenimine. Hum Gene Ther. 1997;8:1243–51. doi: 10.1089/hum.1997.8.10-1243. [DOI] [PubMed] [Google Scholar]
- Böttger M, Zaitsev SV, Otto A, et al. Acid nuclear extracts as mediators of gene transfer and expression. Biochim Biophys Acta. 1998;1395:78–87. doi: 10.1016/s0167-4781(97)00128-0. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragonzi A, Boletta A, Biffi A, et al. Comparison between cationic polymers and lipids in mediating systemic gene delivery to the lungs. Gene Therapy. 1999;6:1995–2004. doi: 10.1038/sj.gt.3301039. [DOI] [PubMed] [Google Scholar]
- Bragonzi A, Conese M. Non-viral approach toward gene therapy of cystic fibrosis lung disease. Curr Gene Ther. 2002;2:295–305. doi: 10.2174/1566523023347832. [DOI] [PubMed] [Google Scholar]
- Bragonzi A, Dina G, Villa A, et al. Biodistribution and transgene expression with nonviral cationic vector/DNA complexes in the lungs. Gene Ther. 2000;7:1753–60. doi: 10.1038/sj.gt.3301282. [DOI] [PubMed] [Google Scholar]
- Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimeta-static activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S, Furtbauer E, Sauer T, et al. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol Ther. 2002;5:80–6. doi: 10.1006/mthe.2001.0509. [DOI] [PubMed] [Google Scholar]
- Brunner S, Sauer T, Carotta S, et al. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–7. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- Brus C, Kleemann E, Aigner A, et al. Stabilization of oligonucleotide-polyethylenimine complexes by freeze-drying: physicochemical and biological characterization. J Control Release. 2004;95:119–31. doi: 10.1016/j.jconrel.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Carrabino S, Di Gioia S, Copreni E, et al. Serum albumin enhances polyethylenimine-mediated gene delivery to human respiratory epithelial cells. J Gene Med. 2005;7:1555–64. doi: 10.1002/jgm.799. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Fischer A. Gene therapy for severe combined immunodeficiency: are we there yet? J Clin Invest. 2007;117:1456–65. doi: 10.1172/JCI30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin I, Moradpour D, Wieland S, et al. Liver-directed gene transfer: a linear polyethlenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo. J Viral Hepat. 1998;5:369–75. doi: 10.1046/j.1365-2893.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- Chen JL, Wang H, Gao JQ, et al. Liposomes modified with polycation used for gene delivery: preparation, characterization and transfection in vitro. Int J Pharm. 2007;343:255–61. doi: 10.1016/j.ijpharm.2007.05.045. [DOI] [PubMed] [Google Scholar]
- Chollet P, Favrot MC, Hurbin A, et al. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J Gene Med. 2002;4:84–91. doi: 10.1002/jgm.237. [DOI] [PubMed] [Google Scholar]
- Choosakoonkriang S, Lobo B, Koe G, et al. Biophysical characterization of PEI/DNA complexes. J Pharm Sci. 2003;92:1710–22. doi: 10.1002/jps.10437. [DOI] [PubMed] [Google Scholar]
- Clamme JP, Azoulay J, Mely Y. Monitoring of the formation and dissociation of polyethylenimine/DNA complexes by two photon fluorescence correlation spectroscopy. Biophys J. 2003a;84:1960–8. doi: 10.1016/S0006-3495(03)75004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamme JP, Krishnamoorthy G, Mély Y. Intracellular dynamics of the gene delivery vehicle polyethylenimine during transfection: investigation by two-photon fluorescence correlation spectroscopy. Biochim Biophys Acta. 2003b;1617:52–61. doi: 10.1016/j.bbamem.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Conese M, Copreni E, Piro D, et al. Gene and Cell Therapy for the Treatment of Cystic Fibrosis. Adv Gene Mol Cell Ther. 2007;1:99–119. [Google Scholar]
- Dames P, Ortiz A, Schillinger U, et al. Aerosol gene delivery to the murine lung is mouse strain dependent. J Mol Med. 2006 doi: 10.1007/s00109-006-0130-9. [DOI] [PubMed] [Google Scholar]
- Davies LA, Seguela C, Varathalingam A, et al. Identification of transfected cell types following non-viral gene transfer to the murine lung. J Gene Med. 2007;9:184–96. doi: 10.1002/jgm.1007. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A, Vornlocher HP, Maraganore J, et al. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–53. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- Densmore CL. Advances in noninvasive pulmonary gene therapy. Curr Drug Deliv. 2006;3:55–63. doi: 10.2174/156720106775197547. [DOI] [PubMed] [Google Scholar]
- Densmore CL, Kleinerman ES, Gautam A, et al. Growth suppression of established human osteosarcoma lung metastases in mice by aerosol gene therapy with PEI-p53 complexes. Cancer Gene Ther. 2001;8:619–27. doi: 10.1038/sj.cgt.7700343. [DOI] [PubMed] [Google Scholar]
- Dheur S, Dias N, van Aerschot A, et al. Polyethylenimine but not cationic lipid improves antisense activity of 3′-capped phosphodiester oligonucleotides. Antisense Nucleic Acid Drug Dev. 1999;9:515–25. doi: 10.1089/oli.1.1999.9.515. [DOI] [PubMed] [Google Scholar]
- Di Gioia S, Rejman J, Carrabino S, et al. Role of biophysical parameters on ex vivo and in vivo gene transfer to the airway epithelium by polyethylenimine/albumin complexes. Biomacromolecules. 2008 doi: 10.1021/bm701190p. [DOI] [PubMed] [Google Scholar]
- Dif F, Djediat C, Alegria O, et al. Transfection of multiple pulmonary cell types following intravenous injection of PEI-DNA in normal and CFTR mutant mice. J Gene Med. 2006;8:82–9. doi: 10.1002/jgm.831. [DOI] [PubMed] [Google Scholar]
- Ding W, Hattori Y, Higashiyama K, et al. Hydroxyethylated cationic cholesterol derivatives in liposome vectors promote gene expression in the lung. Int J Pharm. 2007 doi: 10.1016/j.ijpharm.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Drapkin PT, O’Riordan CR, Yi SM, et al. Targeting the urokinase plasminogen activator receptor enhances gene transfer to human airway epithelia. J Clin Invest. 2000;105:589–96. doi: 10.1172/JCI8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RA, Engelhardt JF. Current status of gene therapy for inherited lung diseases. Annu Rev Physiol. 2003;65:585–612. doi: 10.1146/annurev.physiol.65.092101.142426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Jia SF, Koshkina N, et al. Intranasal interleukin-12 gene therapy enhanced the activity of ifosfamide against osteosarcoma lung metastases. Cancer. 2006;106:1382–8. doi: 10.1002/cncr.21744. [DOI] [PubMed] [Google Scholar]
- Dunlap DD, Maggi A, Soria MR, et al. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997;25:3095–101. doi: 10.1093/nar/25.15.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfinger M, Maucksch C, Rudolph C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials. 2007;28:3448–55. doi: 10.1016/j.biomaterials.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–47. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Epstein SL, Tumpey TM, Misplon JA, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst N, Ulrichskotter S, Scmalix WA, et al. Interaction of liposomal and polycationic transfection complexes with pulmonary surfactant. J Gene Med. 1999;1:331–40. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<331::AID-JGM60>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Fajac I, Thevenot G, Bedouet L, et al. Uptake of plasmid/glycosylated polymer complexes and gene transfer efficiency in differentiated airway epithelial cells. J Gene Med. 2003;5:38–48. doi: 10.1002/jgm.318. [DOI] [PubMed] [Google Scholar]
- Ferkol T, Cohn LA, Phillips TE, et al. Targeted delivery of antiprotease to the epithelial surface of human tracheal xenografts. Am J Respir Crit Care Med. 2003;167:1374–9. doi: 10.1164/rccm.200209-1119OC. [DOI] [PubMed] [Google Scholar]
- Ferkol T, Perales JC, Eckman E, et al. Gene transfer into airway epithelim of animals by targeting the polymeric immunoglobulin receptor. J Clin Invest. 1995;95:493–502. doi: 10.1172/JCI117690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Geddes DM, Alton EW. Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv Drug Deliv Rev. 2002;54:1373–93. doi: 10.1016/S0169-409X(02)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Kitson C, Farley R, et al. Mucus altering agents as adjunct for nonviral gene transfer to airway epithelium. Gene Ther. 2001;8:1380–86. doi: 10.1038/sj.gt.3301525. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Moro E, Pettenazzo A, et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Ther. 1997;4:1100–6. doi: 10.1038/sj.gt.3300503. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Pettenazzo A, Garbati N, et al. Polyethylenimine shows properties of interest for cystic fibrosis gene therapy. Biochim Biophys Acta. 1999;1447:219–25. doi: 10.1016/s0167-4781(99)00153-0. [DOI] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li Y, et al. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–9. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- Fischer D, von Harpe A, Kunath K, et al. Copolymers of ethylene imine and N-(2-hydroxyethyl)-ethylene imine as tools to study effects of polymer structure on physicochemical and biological properties of DNA complexes. Bioconjug Chem. 2002;13:1124–33. doi: 10.1021/bc025550w. [DOI] [PubMed] [Google Scholar]
- Fish RJ, Kruithof EK. Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors. BMC Mol Biol. 2004;5:9. doi: 10.1186/1471-2199-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea B, Meaney C, Junginger H, et al. Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. AAPS Pharm Sci. 2002;4:E12. doi: 10.1208/ps040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea BI, Molenaar TJ, Bot I, et al. Identification of an internalising peptide in differentiated Calu-3 cells by phage display technology; application to gene delivery to the airways. J Drug Target. 2003;11:383–90. doi: 10.1080/10611860310001642389. [DOI] [PubMed] [Google Scholar]
- Forrest ML, Pack DW. On the kinetics of polyplex endocytic trafficking: implications for gene delivery vector design. Mol Ther. 2002;6:57–66. doi: 10.1006/mthe.2002.0631. [DOI] [PubMed] [Google Scholar]
- Gautam A, Densmore CL, Golunski E, et al. Transgene expression in mouse airway epithelium by aerosol gene therapy with PEI-DNA complexes. Mol Ther. 2001a;3:551–6. doi: 10.1006/mthe.2001.0300. [DOI] [PubMed] [Google Scholar]
- Gautam A, Densmore CL, Waldrep JC. Inhibition of experimental lung metastasis by aerosol delivery of PEI-p53 complexes. Mol Ther. 2000;2:318–23. doi: 10.1006/mthe.2000.0138. [DOI] [PubMed] [Google Scholar]
- Gautam A, Densmore CL, Waldrep JC. Pulmonary cytokine response associated with PEI-DNA aerosol gene therapy. Gene Ther. 2001b;8:254–57. doi: 10.1038/sj.gt.3301369. [DOI] [PubMed] [Google Scholar]
- Ge Q, Filip L, Bai A, et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci U S A. 2004;101:8676–81. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Carter JD, Dailey LA, et al. Respiratory epithelial cells demonstrate lactoferrin receptors that increase after metal exposure. Am J Physiol. 1999;276:L933–40. doi: 10.1152/ajplung.1999.276.6.L933. [DOI] [PubMed] [Google Scholar]
- Gill DR, Smyth SE, Goddard CA, et al. Increased persistence of lung gene expression using plasmids containing the ubiquitin elongation factor 1alpha promoter. Gene Ther. 2001;8:1539–46. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999a;45:268–75. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci U S A. 1999b;96:5177–81. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–80. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- Gosselin MA, Guo W, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug Chem. 2001;12:989–94. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- Gottschalk S, Sparrow JT, Hauer J, et al. A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996;3:448–57. [PubMed] [Google Scholar]
- Goula D, Becker N, Lemkine G, et al. Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Ther. 2000;7:499–504. doi: 10.1038/sj.gt.3301113. [DOI] [PubMed] [Google Scholar]
- Goula D, Benoist C, Mantero S, et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther. 1998a;5:1291–5. doi: 10.1038/sj.gt.3300717. [DOI] [PubMed] [Google Scholar]
- Goula D, Remy JS, Erbacher P, et al. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998b;5:712–7. doi: 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23:1868–76. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- Grosse S, Aron Y, Honore I, et al. Lactosylated polyethylenimine for gene transfer into airway epithelial cells: role of the sugar moiety in cell delivery and intracellular trafficking of the complexes. J Gene Med. 2004;6:345–56. doi: 10.1002/jgm.515. [DOI] [PubMed] [Google Scholar]
- Grosse S, Aron Y, Thevenot G, et al. Potocytosis and cellular exit of complexes as cellular pathways for gene delivery by polycations. J Gene Med. 2005;7:1275–86. doi: 10.1002/jgm.772. [DOI] [PubMed] [Google Scholar]
- Grosse S, Thevenot G, Monsigny M, et al. Which mechanism for nuclear import of plasmid DNA complexed with polyethylenimine derivatives? J Gene Med. 2006;8:845–51. doi: 10.1002/jgm.915. [DOI] [PubMed] [Google Scholar]
- Grzelinski M, Urban-Klein B, Martens T, et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–66. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- Han S, Mahato RI, Kim SW. Water-soluble lipopolymer for gene delivery. Bioconjug Chem. 2001;12:337–45. doi: 10.1021/bc000120w. [DOI] [PubMed] [Google Scholar]
- Honoré I, Grosse S, Frison N, et al. Transcription of plasmid DNA: Influence of plasmid DNA/polyethylenimine complex formation. J Control Release. 2005;107:537–46. doi: 10.1016/j.jconrel.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Huh SH, Do HJ, Lim HY, et al. Optimization of 25 kDa linear polyethylenimine for efficient gene delivery. Biologicals. 2007;35:165–71. doi: 10.1016/j.biologicals.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Huth S, Hoffman F, von Gersdorff K, et al. Interaction of polyamine gene vectors with RNA leads to the dissociation of plasmid DNA-carrier complexes. J Gene Med. 2006;8:1416–24. doi: 10.1002/jgm.975. [DOI] [PubMed] [Google Scholar]
- Hwang SK, Jin H, Kwon JT, et al. Aerosol-delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP-1 activity in the lungs of AP-1 luciferase reporter mice. Gene Ther. 2007;14:1353–61. doi: 10.1038/sj.gt.3302983. [DOI] [PubMed] [Google Scholar]
- Itaka K, Harada A, Yamasaki Y, et al. In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J Gene Med. 2004;6:76–84. doi: 10.1002/jgm.470. [DOI] [PubMed] [Google Scholar]
- Jeong GJ, Byun HM, Kim JM, et al. Biodistribution and tissue expression kinetics of plasmid DNA complexed with polyethylenimines of different molecular weight and structure. J Control Release. 2007;118:118–25. doi: 10.1016/j.jconrel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Jia SF, Worth LL, Densmore CL, et al. Aerosol gene therapy with PEI: IL-12 eradicates osteosarcoma lung metastases. Clin Cancer Res. 2003;9:3462–8. [PubMed] [Google Scholar]
- Jia SF, Worth LL, Densmore CL, et al. Eradication of osteosarcoma lung metastases following intranasal interleukin-12 gene therapy using a nonviral polyethylenimine vector. Cancer Gene Ther. 2002;9:260–6. doi: 10.1038/sj.cgt.7700432. [DOI] [PubMed] [Google Scholar]
- Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11:670–84. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost PJ, Harbottle RP, Knight A, et al. A novel peptide, THALWHT, for the targeting of human airway epithelia. FEBS Lett. 2001;489:263–9. doi: 10.1016/s0014-5793(00)02236-5. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Ito Y, Charoensit P, et al. Evaluation of proinflammatory cytokine production induced by linear and branched polyethylenimine/plasmid DNA complexes in mice. J Pharmacol Exp Ther. 2006;317:1382–90. doi: 10.1124/jpet.105.100669. [DOI] [PubMed] [Google Scholar]
- Khalil IA, Kogure K, Akita H, et al. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- Kichler A. Gene transfer with modified polyethylenimines. J Gene Med. 2004;6:S3–S10. doi: 10.1002/jgm.507. [DOI] [PubMed] [Google Scholar]
- Kichler A, Chillon M, Leborgne C, et al. Intranasal gene delivery with a polyethylenimine-PEG conjugate. J Control Release. 2002;81:379–88. doi: 10.1016/s0168-3659(02)00080-9. [DOI] [PubMed] [Google Scholar]
- Kichler A, Leborgne C, Coetynaux E, et al. Polyethylenimine-mediated gene delivery: a mechanistic study. J Gene Med. 2001;3:135–44. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]
- Kim SH, Jeong JH, Lee SH, et al. PEG conjugated VEGF siRNA for anti-angiogenic gene therapy. J Control Release. 2006a;116:123–9. doi: 10.1016/j.jconrel.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Yockman JW, Jeong JH, et al. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J Control Release. 2006b;114:381–8. doi: 10.1016/j.jconrel.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Kircheis R, Kichler A, Wallner M, et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997;4:409–18. doi: 10.1038/sj.gt.3300418. [DOI] [PubMed] [Google Scholar]
- Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev. 2001;53:341–58. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- Kleemann E, Neu M, Jekel N, et al. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG-PEI. J Control Release. 2005;109:299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Klemm AR, Young D, Lloyd JB. Effects of polyethyleneimine on endocytosis and lysosome stability. Biochem Pharmacol. 1998;56:41–6. doi: 10.1016/s0006-2952(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kolb M, Martin G, Medina M, et al. Gene therapy for pulmonary diseases. Chest. 2006;130:879–84. doi: 10.1378/chest.130.3.879. [DOI] [PubMed] [Google Scholar]
- Kopatz I, Remy JS, Behr JP. A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J Gene Med. 2004;6:769–76. doi: 10.1002/jgm.558. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. Heparan sulfate core proteins in cell-cell signaling. Annu Rev Genet. 2003;37:461–84. doi: 10.1146/annurev.genet.37.061103.090226. [DOI] [PubMed] [Google Scholar]
- Kreiss P, Cameron B, Rangara R, et al. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999;27:3792–8. doi: 10.1093/nar/27.19.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat-Moleur F, Steffan A-M, Brisson C, et al. An electron microscopy study into the mechanism of gene transfer with lipopolyamines. Gene Ther. 1996;3:1010–7. [PubMed] [Google Scholar]
- Lear JD, DeGrado WF. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987;262:6500–5. [PubMed] [Google Scholar]
- Lechardeur D, Sohn KJ, Haardt M, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–97. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- Lee H, Jeong JH, Park TG. A new gene delivery formulation of polyethylenimine/DNA complexes coated with PEG conjugated fusogenic peptide. J Control Release. 2001;76:183–92. doi: 10.1016/s0168-3659(01)00426-6. [DOI] [PubMed] [Google Scholar]
- Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–8. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- Lemkine GF, Demeneix BA. Polyethylenimines for in vivo gene delivery. Curr Opin Mol Ther. 2001;3:178–82. [PubMed] [Google Scholar]
- Li S, Tan Y, Viroonchatapan E, et al. Targeted gene delivery to pulmonary endothelium by anti-PECAM antibody. Am J Physiol Lung Cell Mol Physiol. 2000;278:L504–11. doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- Li S, Tseng WC, Stolz DB, et al. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999a;6:585–94. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- Li S, Wu S-P, Whitmore M, et al. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am J Physiol (Lung Cell Mol Physiol) 1999b;276:L796–L804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang J, Lee CG, et al. CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes. Gene Ther. 2004;11:109–14. doi: 10.1038/sj.gt.3302135. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu L, Fletcher BS, et al. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. Faseb J. 2006;20:2384–6. doi: 10.1096/fj.06-6228fje. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu L, Visner GA. Nonviral gene delivery with indoleamine 2,3-dioxygenase targeting pulmonary endothelium protects against ischemia-reperfusion injury. Am J Transplant. 2007;7:2291–300. doi: 10.1111/j.1600-6143.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–40. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- Lollo CP, Banaszczyk MG, Chiou HC. Obstacles and advances in non-viral gene delivery. Curr Opin Mol Ther. 2000;2:136–42. [PubMed] [Google Scholar]
- Lukacs GL, Haggie P, Seksek O, et al. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275:1625–9. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T, et al. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–66. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Mannisto M, Reinisalo M, Ruponen M, et al. Polyplex-mediated gene transfer and cell cycle: effect of carrier on cellular uptake and intracellular kinetics, and significance of glycosaminoglycans. J Gene Med. 2007;9:479–87. doi: 10.1002/jgm.1035. [DOI] [PubMed] [Google Scholar]
- Mannisto M, Ronkko S, Matto M, et al. The role of cell cycle on polyplex-mediated gene transfer into a retinal pigment epithelial cell line. J Gene Med. 2005;7:466–76. doi: 10.1002/jgm.693. [DOI] [PubMed] [Google Scholar]
- Martin ME, Rice KG. Peptide-guided gene delivery. Aaps J. 2007;9:E18–29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan G, Baker A, Tennant P, et al. Optimizing aerosol gene delivery and expression in the ovine lung. Mol Ther. 2007;15:348–54. doi: 10.1038/sj.mt.6300058. [DOI] [PubMed] [Google Scholar]
- Meng Q-H, Robinson D, Jenkins GR, et al. Efficient transfection of non-proliferating human airway epithelial cells with a sytnthetic vector system. J Gene Med. 2004;6:210–21. doi: 10.1002/jgm.483. [DOI] [PubMed] [Google Scholar]
- Merdan T, Kunath K, Fischer D, et al. Intracellular processing of poly(ethylene imine)/ribozyme complexes can be observed in living cells by using confocal laser scanning microscopy and inhibitor experiment. Pharm Res. 2002;19:140–6. doi: 10.1023/a:1014212630566. [DOI] [PubMed] [Google Scholar]
- Meunier-Durmort C, Grimal H, Sachs LM, et al. Adenovirus enhancement of polyethylenimine-mediated transfer of regulated genes in differentiated cells. Gene Ther. 1997;4:808–14. doi: 10.1038/sj.gt.3300450. [DOI] [PubMed] [Google Scholar]
- Min SH, Lee DC, Lim MJ, et al. A composite gene delivery system consisting of polyethylenimine and an amphipathic peptide KALA. J Gene Med. 2006;8:1425–34. doi: 10.1002/jgm.973. [DOI] [PubMed] [Google Scholar]
- Mislick KA, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc Natl Acad Sci U S A. 1996;93:12349–54. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt S, Wiehle S, Cristiano RJ. Tumor-specific gene delivery mediated by a novel peptide-polyethylenimine-DNA polyplex targeting aminopeptidase N/CD13. Hum Gene Ther. 2005;16:57–67. doi: 10.1089/hum.2005.16.57. [DOI] [PubMed] [Google Scholar]
- Moffatt S, Wiehle S, Cristiano RJ. A multifunctional PEI-based cationic polyplex for enhanced systemic p53-mediated gene therapy. Gene Ther. 2006;13:1512–23. doi: 10.1038/sj.gt.3302773. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Symonds P, Murray JC, et al. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–5. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Mounkes LC, Zhong W, Cipres-Palacin G, et al. Proteoglycans mediate cationic liposome-DNA complex-base gene delivery in vitro and in vivo. J Biol Chem. 1998;273:26164–70. doi: 10.1074/jbc.273.40.26164. [DOI] [PubMed] [Google Scholar]
- Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–706. [PubMed] [Google Scholar]
- Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- Ogris M, Steinlein P, Carotta S, et al. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS Pharm Sci. 2001;3:E21. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris M, Steinlein P, Kursa M, et al. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–33. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- Oh YK, Kim JP, Yoon H, et al. Prolonged organ retention and safety of plasmid DNA administered in polyethylenimine complexes. Gene Ther. 2001;8:1587–92. doi: 10.1038/sj.gt.3301516. [DOI] [PubMed] [Google Scholar]
- Oh YK, Suh D, Kim JM, et al. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Ther. 2002;9:1627–32. doi: 10.1038/sj.gt.3301735. [DOI] [PubMed] [Google Scholar]
- Okuda T, Niidome T, Aoyagi H. Cytosolic soluble proteins induce DNA release from DNA – gene carrier complexes. J Control Release. 2004;98:325–32. doi: 10.1016/j.jconrel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Orson FM, Song L, Gautam A, et al. Gene delivery to the lung using protein/polyethylenimine/plasmid complexes. Gene Ther. 2002;9:463–71. doi: 10.1038/sj.gt.3301666. [DOI] [PubMed] [Google Scholar]
- Parente RA, Nadasdi L, Subbarao NK, et al. Association of a pH-sensitive peptide with membrane vesicles: role of amino acid sequence. Biochemistry. 1990a;29:8713–9. doi: 10.1021/bi00489a030. [DOI] [PubMed] [Google Scholar]
- Parente RA, Nir S, Szoka FC., Jr Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry. 1990b;29:8720–8. doi: 10.1021/bi00489a031. [DOI] [PubMed] [Google Scholar]
- Parente RA, Nir S, Szoka FCJ. pH-dependent fusion of phosphatidylcholine small vesicles. Induction by a synthetic amphipathic peptide. J Biol Chem. 1988;263:4724–30. [PubMed] [Google Scholar]
- Park IK, Lasiene J, Chou SH, et al. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine) J Gene Med. 2007;9:691–702. doi: 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Parkes RJ, Hart SL. Adhesion molecules and gene transfer. Adv Drug Deliv Rev. 2000;44:135–52. doi: 10.1016/s0169-409x(00)00091-0. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Kain R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–7. [PMC free article] [PubMed] [Google Scholar]
- Petersen H, Fechner PM, Martin AL, et al. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem. 2002;13:845–54. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- Pilewski JM. Gene therapy for airway diseases: continued progress toward identifying and overcoming barriers to efficiency. Am J Respir Cell Mol Biol. 2002;27:117–21. doi: 10.1165/ajrcmb.27.2.f244. [DOI] [PubMed] [Google Scholar]
- Plank C. Nanomagnetosols: magnetism opens up new perspectives for targeted aerosol delivery to the lung. Trends Biotechnol. 2008;26:59–63. doi: 10.1016/j.tibtech.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Plank C, Mechtler K, Szoka FC, et al. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Human Gene Therapy. 1996;7:1437–46. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- Plank C, Oberhauser B, Mechtler K, et al. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 1994;269:12918–24. [PubMed] [Google Scholar]
- Pollard H, Remy J-S, Loussouarn G, et al. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J Biol Chem. 1998;273:7507–11. doi: 10.1074/jbc.273.13.7507. [DOI] [PubMed] [Google Scholar]
- Pollard H, Toumaniantz G, Amos JL, et al. Ca2+– sensitive cytosolic nucleases prevent efficient delivery to the nucleus of injected plasmids. J Gene Med. 2001;3:153–64. doi: 10.1002/jgm.160. [DOI] [PubMed] [Google Scholar]
- Pouton CW. Nuclear import of polypeptides, polynucleotides and supramolecular complexes. Adv Drug Deliv Rev. 1998;34:51–64. doi: 10.1016/s0169-409x(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Putnam D, Gentry CA, Pack DW, et al. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc Natl Acad Sci U S A. 2001;98:1200–5. doi: 10.1073/pnas.031577698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read ML, Singh S, Ahmed Z, et al. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnstrom K, Ragnarsson EG, Fryknas M, et al. Gene expression profiles in mouse lung tissue after administration of two cationic polymers used for nonviral gene delivery. Pharm Res. 2006;23:475–82. doi: 10.1007/s11095-006-9563-7. [DOI] [PubMed] [Google Scholar]
- Regnstrom K, Ragnarsson EG, Koping-Hoggard M, et al. PEI – a potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003;10:1575–83. doi: 10.1038/sj.gt.3302054. [DOI] [PubMed] [Google Scholar]
- Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–74. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo- and polyplexes: role of clathrin and caveolae-mediated endocytosis. J Liposome Res. 2006;16:237–47. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- Rejman J, Di Gioia S, Bragonzi A, et al. Pseudomonas aeruginosa Infection Destroys the Barrier Function of Lung Epithelium and Enhances Polyplex-Mediated Transfection. Hum Gene Ther. 2007;18:642–52. doi: 10.1089/hum.2006.192. [DOI] [PubMed] [Google Scholar]
- Remy-Kristensen A, Clamme JP, Vuilleumier C, et al. Role of endocytosis in the transfection of L929 fibroblasts by polyethylenimine/DNA complexes. Biochim Biophys Acta. 2001;1514:21–32. doi: 10.1016/s0005-2736(01)00359-5. [DOI] [PubMed] [Google Scholar]
- Romanczuk H, Galer CE, Zabner J, et al. Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice. Hum Gene Ther. 1999;10:2615–26. doi: 10.1089/10430349950016654. [DOI] [PubMed] [Google Scholar]
- Rosenecker J, Naundorf S, Gersting SW, et al. Interaction of bronchoalveolar lavage fluid with polyplexes and lipoplexes: analysing the role of proteins and glycoproteins. J Gene Med. 2003;5:49–60. doi: 10.1002/jgm.291. [DOI] [PubMed] [Google Scholar]
- Rudolph C, Lausier J, Naundorf S, et al. In vivo gene delivery to the lung using polyethylenimine and fracturated polyamidoamine dendrimers. J Gene Med. 2000;2:269–78. doi: 10.1002/1521-2254(200007/08)2:4<269::AID-JGM112>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Rudolph C, Ortiz A, Schillinger U, et al. Methodological optimization of polyethylenimine (PEI)-based gene delivery to the lungs of mice via aerosol application. J Gene Med. 2005a;7:59–66. doi: 10.1002/jgm.646. [DOI] [PubMed] [Google Scholar]
- Rudolph C, Plank C, Lausier J, et al. Oligomers of the arginine-rich motif of the HIV-1 TAT peptide are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278:11411–8. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- Rudolph C, Schillinger U, Ortiz A, et al. Aerosolized nanogram quantities of plasmid DNA mediate highly efficient gene delivery to mouse airway epithelium. Mol Ther. 2005b;12:493–501. doi: 10.1016/j.ymthe.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Schiffelers RM, Ansari A, Xu J, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong JH, Lee KM, Kim ST, et al. Polyethylenimine-based antisense oligodeoxynucleotides of IL-4 suppress the production of IL-4 in a murine model of airway inflammation. J Gene Med. 2006;8:314–23. doi: 10.1002/jgm.848. [DOI] [PubMed] [Google Scholar]
- Shi L, Tang GP, Gao SJ, et al. Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord. Gene Ther. 2003;10:1179–88. doi: 10.1038/sj.gt.3301970. [DOI] [PubMed] [Google Scholar]
- Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- Song YK, Liu F, Liu D. Enhanced gene expression in mouse lung by prolonging the retention time of intravenously injected plasmid DNA. Gene Ther. 1998;5:1531–7. doi: 10.1038/sj.gt.3300770. [DOI] [PubMed] [Google Scholar]
- Sparrow JT, Edwards VV, Tung C, et al. Synthetic peptide-based DNA complexes for nonviral gene delivery. Adv Drug Deliv Rev. 1998;30:115–31. doi: 10.1016/s0169-409x(97)00111-7. [DOI] [PubMed] [Google Scholar]
- Stammberger U, Uduehi AN, Kubisa B, et al. Non-viral gene delivery to atelectatic and ventilated lungs. Ann Thorac Surg. 2002;73:432–6. doi: 10.1016/s0003-4975(01)03388-4. discussion 436–7. [DOI] [PubMed] [Google Scholar]
- Subbarao NK, Parente RA, Szoka FCJ, et al. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26:2964–72. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- Suh J, Paik H-J, Hwang BK. Ionization of poly(ethylenimine) and poly(allylamine) at various pH’s. Bioorganic Chemistry. 1994;22:318–27. [Google Scholar]
- Sung SJ, Min SH, Cho KY, et al. Effect of polyethylene glycol on gene delivery of polyethylenimine. Biol Pharm Bull. 2003;26:492–500. doi: 10.1248/bpb.26.492. [DOI] [PubMed] [Google Scholar]
- Tachibana R, Harashima H, Shinohara Y, et al. Quantitative studies on the nuclear transport of plasmid DNA and gene expression employing nonviral vectors. Adv Drug Deliv Rev. 2001;52:219–26. doi: 10.1016/s0169-409x(01)00211-3. [DOI] [PubMed] [Google Scholar]
- Talcott B, Moore MS. Getting across the nuclear pore complex. Trends Cell Biol. 1999;9:312–8. doi: 10.1016/s0962-8924(99)01608-6. [DOI] [PubMed] [Google Scholar]
- Talsma H, Cherng J, Lehrmann H, et al. Stabilization of gene delivery systems by freeze-drying. Int J Pharm. 1997;157:233–8. doi: 10.1016/s0378-5173(97)00244-5. [DOI] [PubMed] [Google Scholar]
- Tang MX, Szoka F. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–32. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Chen J, et al. Non-viral siRNA delivery to the lung. Adv Drug Deliv Rev. 2007;59:124–33. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Ge Q, et al. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102:5679–84. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Klein B, Werth S, Abuharbeid S, et al. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–6. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- Vaysse L, Burgelin I, Merlio JP, et al. Improved transfection using epithelial cell line-selected ligands and fusogenic peptides. Biochim Biophys Acta. 2000;1475:369–76. doi: 10.1016/s0304-4165(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Vaysse L, Guillaume C, Burgelin I, et al. Proteolipidic vectors for gene transfer to the lung. Biochem Biophys Res Commun. 2002;290:1489–98. doi: 10.1006/bbrc.2002.6343. [DOI] [PubMed] [Google Scholar]
- von Gersdorff K, Sanders NN, Vandenbroucke R, et al. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol Ther. 2006;14:745–53. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Wagner E. Application of membrane-active peptides for nonviral gene delivery. Adv Drug Deliv Rev. 1999;38:279–89. doi: 10.1016/s0169-409x(99)00033-2. [DOI] [PubMed] [Google Scholar]
- Walker WE, Porteous DJ, Boyd AC. The effects of plasmid copy number and sequence contect upon transfection efficiency. J Control Release. 2004;94:245–52. doi: 10.1016/j.jconrel.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Ma N, Gao SJ, et al. Transgene expression in the brain stem effected by intramuscular injection of polyethylenimine/DNA complexes. Mol Ther. 2001;3:658–64. doi: 10.1006/mthe.2001.0324. [DOI] [PubMed] [Google Scholar]
- Wattiaux R, Laurent N, Wattiaux-De Coninck S, et al. Endosomes, lysosomes: their implication in gene transfer. Adv Drug Deliv Rev. 2000;41:201–8. doi: 10.1016/s0169-409x(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Werth S, Urban-Klein B, Dai L, et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;112:257–70. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Wightman L, Kircheis R, Rossler V, et al. Different behaviour of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med. 2001;3:362–72. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- Wiseman JW, Goddard CA, McLelland D, et al. A comparison of linear and branched polyethylenimine (PEI) with DCChol/DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene Ther. 2003;10:1654–62. doi: 10.1038/sj.gt.3302050. [DOI] [PubMed] [Google Scholar]
- Wolfert MA, Seymour LW. Chloroquine and amphipathic peptide helices show synergistic transfection in vitro. Gene Ther. 1998;5:409–14. doi: 10.1038/sj.gt.3300606. [DOI] [PubMed] [Google Scholar]
- Writer MJ, Marshall B, Pilkington-Miksa MA, et al. Targeted gene delivery to human airway epithelial cells with synthetic vectors incorporating novel targeting peptides selected by phage display. J Drug Target. 2004;12:185–93. doi: 10.1080/10611860410001724459. [DOI] [PubMed] [Google Scholar]
- Wyman TB, Nicol F, Zelphati O, et al. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36:3008–17. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- Yew NS, Przybylska M, Ziegler RJ, et al. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Ther. 2001;4:75–82. doi: 10.1006/mthe.2001.0415. [DOI] [PubMed] [Google Scholar]
- Yew NS, Zhao H, Przybylska M, et al. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol Ther. 2002;5:731–8. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- Zanta MA, Belguise-Valladier P, Behr J-P. Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci U S A. 1999;96:91–6. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gu FX, Chan JM, et al. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Smith BD. High-generation polycationic dendrimers are unusually effective at disrupting anionic vesicles: membrane bending model. Bioconjug Chem. 2000;11:805–14. doi: 10.1021/bc000018z. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Kelley TJ, Milliken E, et al. Functional evidence of CFTR gene transfer in nasal epithelium of cystic fibrosis mice in vivo following luminal application of DNA complexes targeted to the serpin-enzyme complex receptor. Mol Ther. 2002;5:413–9. doi: 10.1006/mthe.2002.0556. [DOI] [PubMed] [Google Scholar]
- Zou SM, Erbacher P, Remy JS, et al. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J Gene Med. 2000;2:128–34. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<128::AID-JGM95>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]