Abstract
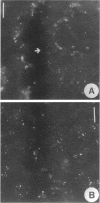
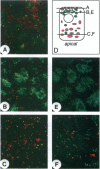
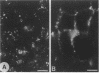
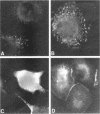
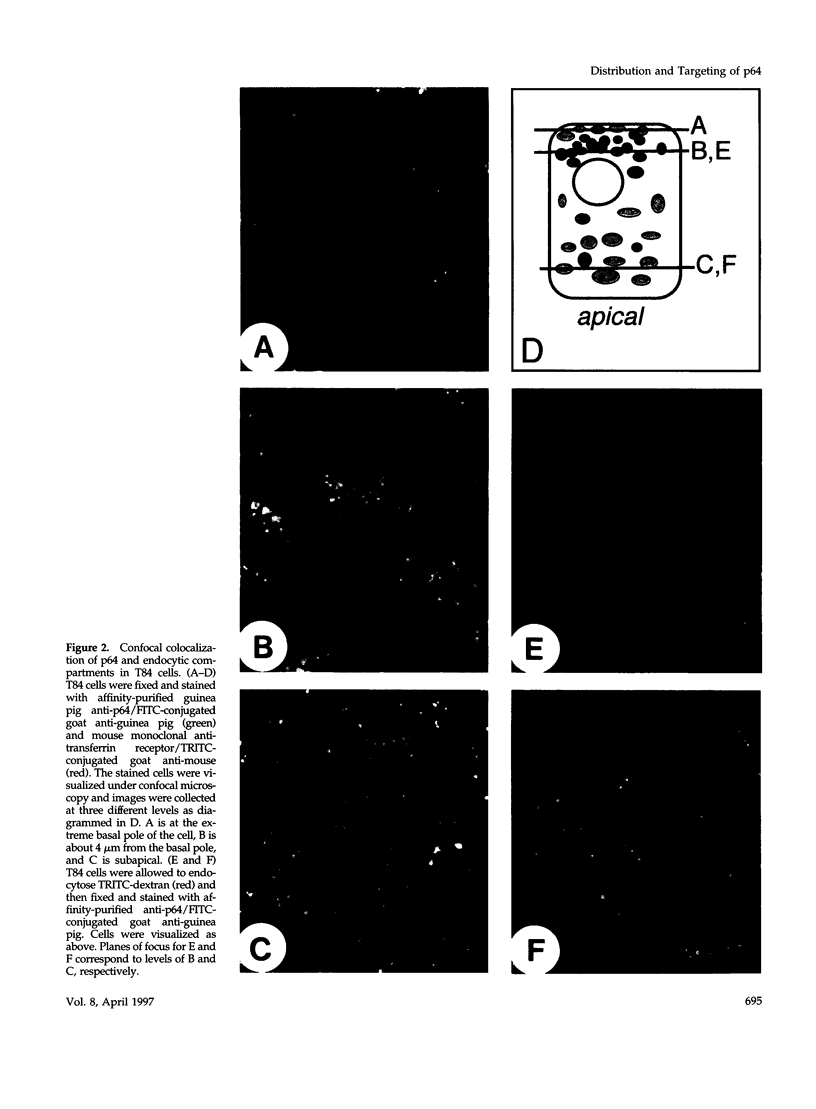
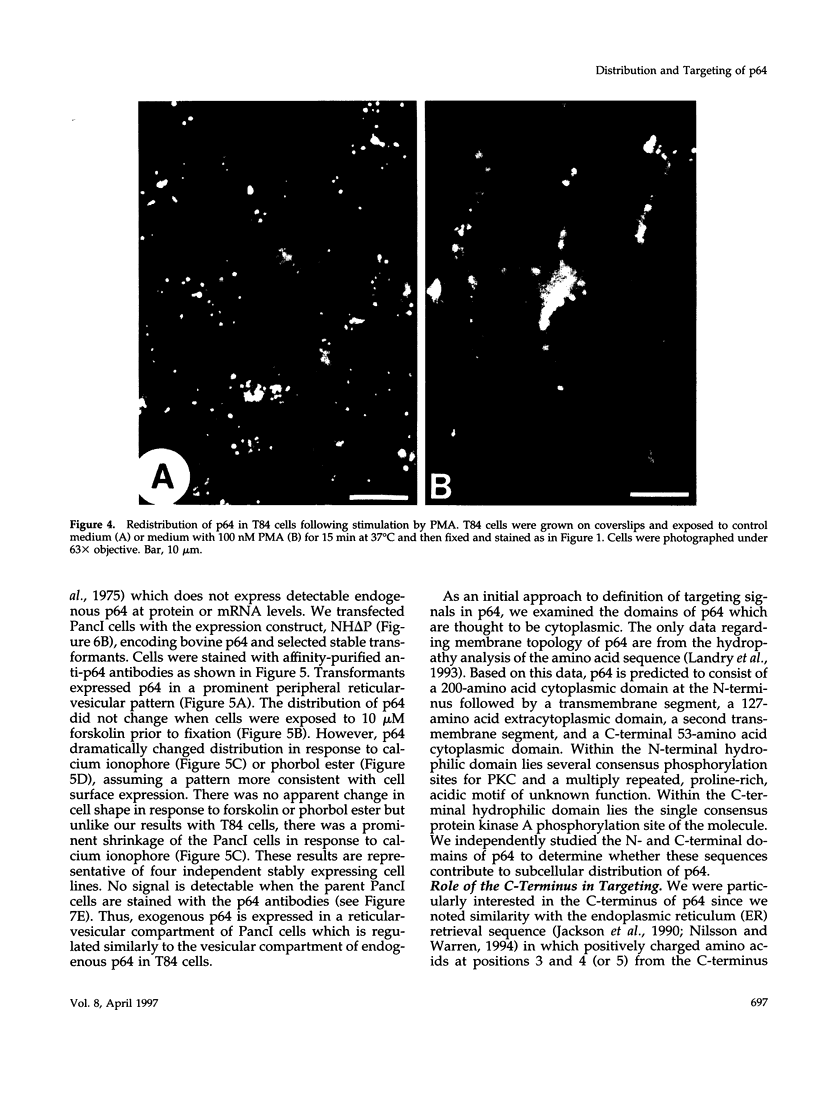
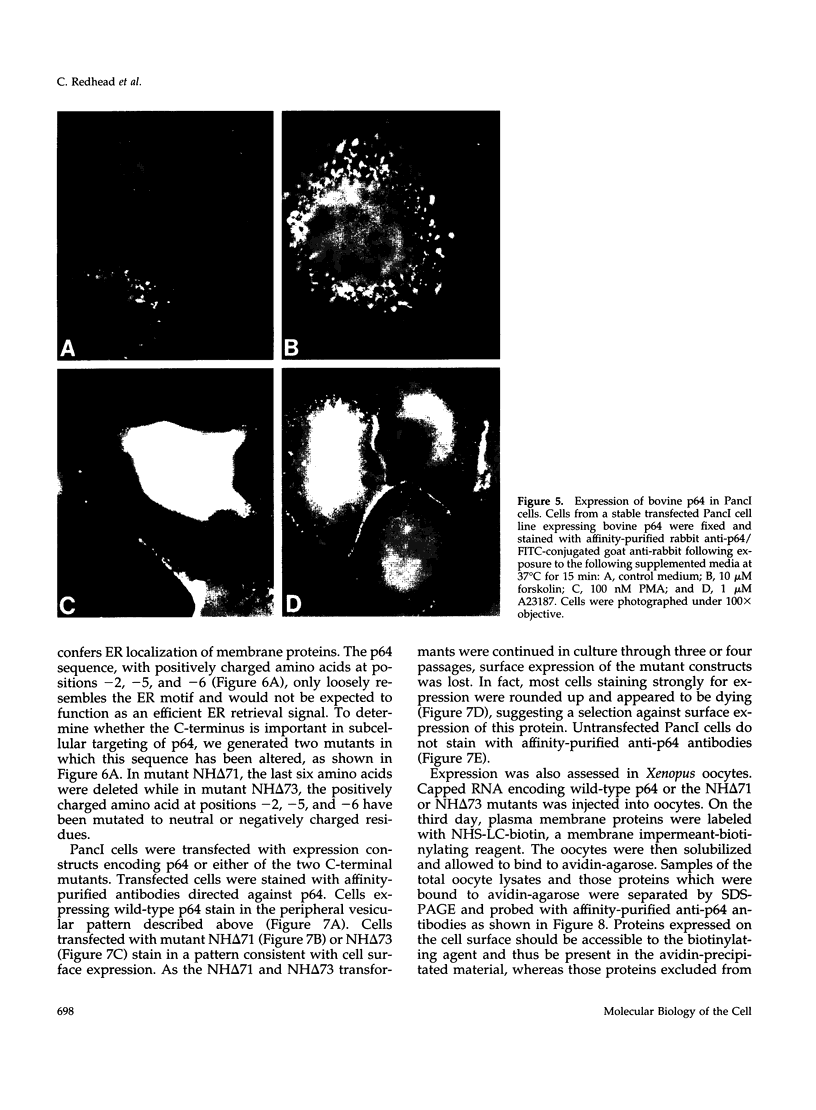
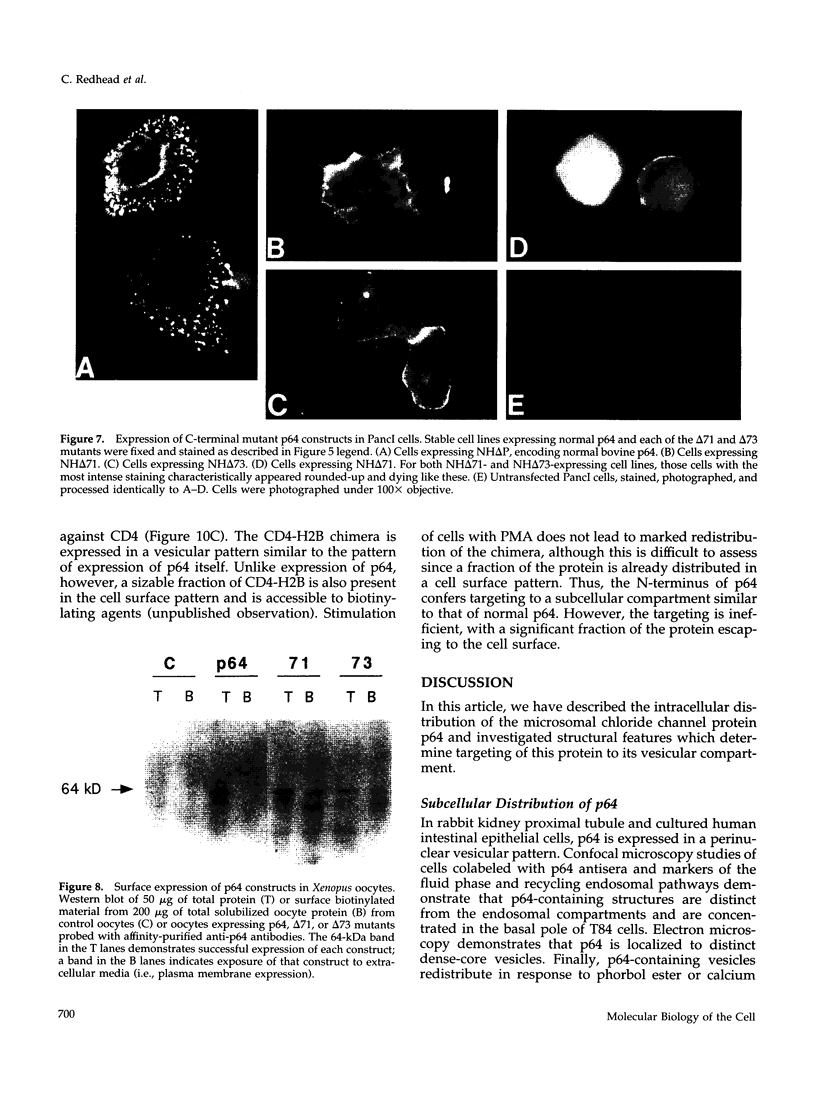
p64 is an intracellular chloride channel originally identified in bovine kidney microsomes. Using a combination of immunofluorescent and electron microscopic technique, we demonstrate that p64 resides in the limiting membranes of perinuclear dense core vesicles which appear to be regulated secretory vesicles. Heterologous expression of p64 in PancI cells, a cell type which does not normally express p64, results in targeting to a similar compartment. Mutagenesis experiments demonstrate that both the N- and C-terminal domains of the protein independently contribute to subcellular distribution of the protein. The C-terminal domain functions to prevent expression of p64 on the plasma membrane and the N-terminal domain is necessary to deliver p64 to the appropriate membrane compartment.
Full text
PDF




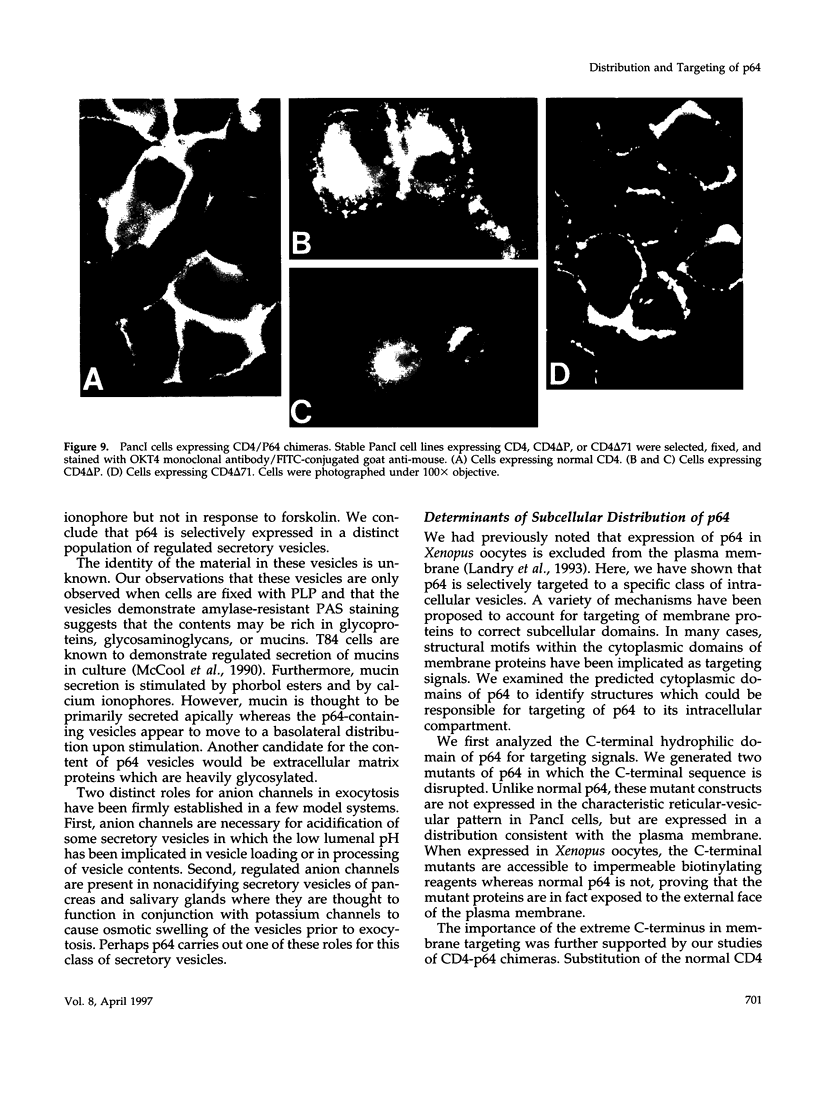
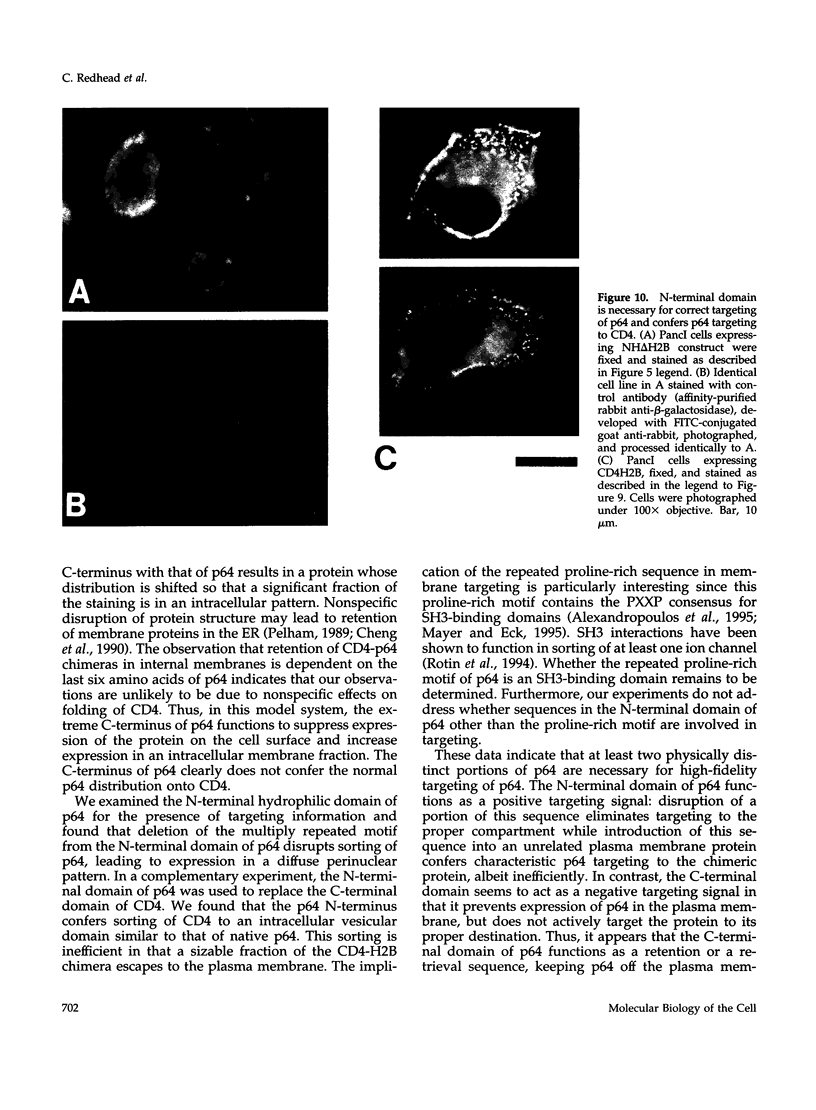


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. Proton-translocating ATPases. Annu Rev Cell Biol. 1986;2:179–199. doi: 10.1146/annurev.cb.02.110186.001143. [DOI] [PubMed] [Google Scholar]
- Alexandropoulos K., Cheng G., Baltimore D. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3110–3114. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P., Rudnick G., Castle J. D. Osmotic properties and internal pH of isolated rat parotid secretory granules. J Biol Chem. 1984 Nov 10;259(21):13567–13572. [PubMed] [Google Scholar]
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990 Dec 13;348(6302):637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Barasch J., Gershon M. D., Nunez E. A., Tamir H., al-Awqati Q. Thyrotropin induces the acidification of the secretory granules of parafollicular cells by increasing the chloride conductance of the granular membrane. J Cell Biol. 1988 Dec;107(6 Pt 1):2137–2147. doi: 10.1083/jcb.107.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Bauerfeind R., Huttner W. B. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993 Aug;5(4):628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986 Mar;68(3):375–381. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Nordmann J. J. Chloride and magnesium dependence of vasopressin release from rat permeabilized neurohypophysial nerve endings. Neurosci Lett. 1989 Dec 4;106(3):305–309. doi: 10.1016/0304-3940(89)90181-x. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Deetjen H. H., Piiper A., Schulz I. Secretagogue and second messenger-activated Cl- permeabilities in isolated pancreatic zymogen granules. Pflugers Arch. 1989 Oct;415(1):29–36. doi: 10.1007/BF00373138. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Eckhardt L., Schulz I. Ionic and osmotic dependence of secretion from permeabilised acini of the rat pancreas. Pflugers Arch. 1989 Feb;413(4):385–394. doi: 10.1007/BF00584488. [DOI] [PubMed] [Google Scholar]
- Gasser K. W., DiDomenico J., Hopfer U. Secretagogues activate chloride transport pathways in pancreatic zymogen granules. Am J Physiol. 1988 Jan;254(1 Pt 1):G93–G99. doi: 10.1152/ajpgi.1988.254.1.G93. [DOI] [PubMed] [Google Scholar]
- Huflejt M. E., Blum R. A., Miller S. G., Moore H. P., Machen T. E. Regulated Cl transport, K and Cl permeability, and exocytosis in T84 cells. J Clin Invest. 1994 May;93(5):1900–1910. doi: 10.1172/JCI117181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J., Hartl F. U. Post-translational protein import and folding. Curr Opin Cell Biol. 1994 Aug;6(4):499–509. doi: 10.1016/0955-0674(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry D. W., Akabas M. H., Redhead C., Edelman A., Cragoe E. J., Jr, Al-Awqati Q. Purification and reconstitution of chloride channels from kidney and trachea. Science. 1989 Jun 23;244(4911):1469–1472. doi: 10.1126/science.2472007. [DOI] [PubMed] [Google Scholar]
- Landry D., Sullivan S., Nicolaides M., Redhead C., Edelman A., Field M., al-Awqati Q., Edwards J. Molecular cloning and characterization of p64, a chloride channel protein from kidney microsomes. J Biol Chem. 1993 Jul 15;268(20):14948–14955. [PubMed] [Google Scholar]
- Lieber M., Mazzetta J., Nelson-Rees W., Kaplan M., Todaro G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975 May 15;15(5):741–747. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Matter K., Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994 Aug;6(4):545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Eck M. J. SH3 domains. Minding your p's and q's. Curr Biol. 1995 Apr 1;5(4):364–367. doi: 10.1016/s0960-9822(95)00073-x. [DOI] [PubMed] [Google Scholar]
- McCool D. J., Marcon M. A., Forstner J. F., Forstner G. G. The T84 human colonic adenocarcinoma cell line produces mucin in culture and releases it in response to various secretagogues. Biochem J. 1990 Apr 15;267(2):491–500. doi: 10.1042/bj2670491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Milgram S. L., Mains R. E., Eipper B. A. Identification of routing determinants in the cytosolic domain of a secretory granule-associated integral membrane protein. J Biol Chem. 1996 Jul 19;271(29):17526–17535. doi: 10.1074/jbc.271.29.17526. [DOI] [PubMed] [Google Scholar]
- Mulberg A. E., Tulk B. M., Forgac M. Modulation of coated vesicle chloride channel activity and acidification by reversible protein kinase A-dependent phosphorylation. J Biol Chem. 1991 Nov 5;266(31):20590–20593. [PubMed] [Google Scholar]
- Naim H. Y., Roth M. G. Characteristics of the internalization signal in the Y543 influenza virus hemagglutinin suggest a model for recognition of internalization signals containing tyrosine. J Biol Chem. 1994 Feb 11;269(6):3928–3933. [PubMed] [Google Scholar]
- Nilsson T., Warren G. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr Opin Cell Biol. 1994 Aug;6(4):517–521. doi: 10.1016/0955-0674(94)90070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Pazoles C. J., Creutz C. E., Ramu A., Pollard H. B. Permeant anion activation of MgATPase activity in chromaffin granules. Evidence for direct coupling of proton and anion transport. J Biol Chem. 1980 Aug 25;255(16):7863–7869. [PubMed] [Google Scholar]
- Pazoles C. J., Pollard H. B. Evidence for stimulation of anion transport in ATP-evoked transmitter release from isolated secretory vesicles. J Biol Chem. 1978 Jun 10;253(11):3962–3969. [PubMed] [Google Scholar]
- Redhead C. R., Edelman A. E., Brown D., Landry D. W., al-Awqati Q. A ubiquitous 64-kDa protein is a component of a chloride channel of plasma and intracellular membranes. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3716–3720. doi: 10.1073/pnas.89.9.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Bar-Sagi D., O'Brodovich H., Merilainen J., Lehto V. P., Canessa C. M., Rossier B. C., Downey G. P. An SH3 binding region in the epithelial Na+ channel (alpha rENaC) mediates its localization at the apical membrane. EMBO J. 1994 Oct 3;13(19):4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma T., Ichida T. Roles of potassium and chloride ions in cAMP-mediated amylase exocytosis from rat parotid acini. Cell Struct Funct. 1991 Oct;16(5):405–409. doi: 10.1247/csf.16.405. [DOI] [PubMed] [Google Scholar]
- Tamir H., Piscopo I., Liu K. P., Hsiung S. C., Adlersberg M., Nicolaides M., al-Awqati Q., Nunez E. A., Gershon M. D. Secretogogue-induced gating of chloride channels in the secretory vesicles of parafollicular cells. Endocrinology. 1994 Nov;135(5):2045–2057. doi: 10.1210/endo.135.5.7525261. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. Proton pump-generated electrochemical gradients in rat liver multivesicular bodies. Quantitation and effects of chloride. J Biol Chem. 1988 Feb 25;263(6):2603–2611. [PubMed] [Google Scholar]
- Wandinger-Ness A., Bennett M. K., Antony C., Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990 Sep;111(3):987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H., de Souza D. O., de Meis L. Regulation of glutamate transport into synaptic vesicles by chloride and proton gradient. J Biol Chem. 1996 May 17;271(20):11726–11731. doi: 10.1074/jbc.271.20.11726. [DOI] [PubMed] [Google Scholar]