Abstract
Aims
β-Adrenergic augmentation of Ca2+ sparks and cardiac contractility has been functionally linked to phosphorylation-dependent dissociation of FK506 binding protein 12.6 (FKBP12.6) regulatory proteins from ryanodine receptors subtype 2 (RYR2). We used FKBP12.6 null mice to test the extent to which the dissociation of FKBP12.6 affects Ca2+ sparks and mediates the inotropic action of isoproterenol (ISO), and to investigate the underlying mechanisms of cyclic ADP-ribose (cADPR) regulation of Ca2+ sparks.
Methods and results
Ca2+ sparks and contractility were measured in cardiomyocytes and papillary muscle segments from FKBP12.6 null mice, and western blot analysis was carried out on sarcoplasmic reticulum microsomes prepared from mouse heart. Exposure to ISO resulted in a three- and two-fold increase in Ca2+ spark frequency in wild-type (WT) and FKBP12.6 knockout (KO) myocytes, respectively, and Ca2+ spark kinetics were also significantly altered in both types of cells. The effects of ISO on Ca2+ spark properties in KO cells were inhibited by pre-treatment with thapsigargin or phospholamban inhibitory antibody, 2D12. Moreover, twitch force magnitude and the rate of force development were not significantly different in papillary muscles from WT and KO mice. Unlike β-adrenergic stimulation, cADPR stimulation increased Ca2+ spark frequency (2.8-fold) and altered spark kinetics only in WT but not in KO mice. The effect of cADPR on spark properties was not entirely blocked by pre-treatment with thapsigargin or 2D12. In voltage-clamped cells, cADPR increased the peak Ca2+ of the spark without altering the decay time. We also noticed that basal Ca2+ spark properties in KO mice were markedly altered compared with those in WT mice.
Conclusion
Our data demonstrate that dissociation of FKBP12.6 from the RYR2 complex does not play a significant role in β-adrenergic-stimulated Ca2+ release in heart cells, whereas this mechanism does underlie the action of cADPR.
Keywords: β-Adrenergic stimulation, cADPR, FKBP126 null cardiac myocytes, Ryanodine receptor, Ca2+ spark, Cardiac contractility
1. Introduction
In cardiomyocytes, release of Ca2+ from intracellular Ca2+ stores to the cytoplasm is controlled by ryanodine receptors subtype 2 (RYR2). Activation and inactivation of RYR2 is regulated by a number of channel modulators, including FKBP12 and FKBP12.6,1–4 calmodulin,5 protein kinase A (PKA),1,6,7 and Ca2+/calmodulin-dependent kinase (CaMKII).8–11 FKBP12.6, a 12.6 kDa protein, associates specifically with and regulates RYR2 in cardiomyocytes and smooth muscle.12,13 Adult FKBP12.6 knockout (KO) mice develop abnormal cardiac function leading to either an enlarged heart14 or exercise-induced cardiac arrhythmia and sudden death.15 We demonstrated previously that FKBP12.6 KO mice exhibit a significant alteration in Ca2+ spark properties in both sexes.13,14 These findings strongly suggest that FKBP12.6 is relevant for regulating cardiac function possibly via its association with cardiac RYR2.
It has been reported that PKA phosphorylation of RYR2 causes the dissociation of FKBP12.6 and affects the channel open probability, and hyperphosphorylation of RYR2 at a single amino acid residue, Ser-2809, leads to defective channel function in the failing heart.16 Wehrens et al.17 reported that disease-causing mutations in RYR2 reduce the binding affinity of FKBP12.6 for RYR2, and increase the channel leakage that can trigger fatal cardiac arrhythmias under physical and emotional stress. However, the theory proposed by Marks, Wehrens, and colleagues is challenged by other authors. Li et al.18 demonstrated that PKA-mediated increase in Ca2+ spark frequency is due to phosphorylation of phospholymban (PLB) but not RYR2 in mouse ventricular myocytes. Jiang et al.19 did not observe dissociation of FKBP12.6 from RYR2 in cardiac microsomal membranes treated with PKA. Stange et al.20 made site-directed substitutions of RYR2 at Ser-2809 (with Asp or Ala) and showed that FKBP12.6 binding was not abolished nor were channel functional properties significantly changed in the mutant RYR2. Xiao et al.7 found that FKBP12.6 binds to both the Ser-2809 (mouse) phosphorylated and non-phosphorylated forms of RYR2 and that a S2809D phosphomimetic mutant retains the ability to bind FKBP12.6. Furthermore, complete phosphorylation at Ser-2809 by exogenous PKA does not disrupt the FKBP12.6–RYR2 complex. An investigation of the three-dimensional location of the Ser 2030 and Ser 2809 PKA sites indicated that these PKA sites are not located close to the FKBP12.6-binding sites.21,22 Thus the theory that PKA phosphorylation causes dissociation of FKBP12.6 proteins from the RYR2 complex in cardiomyocytes is highly controversial.
Cyclic ADP-ribose (cADPR), a metabolite of β-nicotinamide adenine dinucleotide, is a novel Ca2+ mobilizing second messenger. A number of reports over the last few years have suggested that cADPR has a patho-physiological role, as reviewed by Zhlang and Li.23 It has been reported that cADPR, at micromolar concentrations, participates in the generation or modulation of intracellular Ca2+ sparks, Ca2+ waves or oscillations via RYRs,24–26 despite the fact that the role and mechanisms of cADPR regulation of Ca2+ release remain unclear and very controversial. For instance, treatment of cardiomyocytes, smooth muscle, and pancreatic β-cells with cADPR increases Ca2+ spark frequency and Ca2+ release.27–30 Growing evidence indicates that cADPR may function through its specific receptor protein FKBP12.6,30,31 i.e. cADPR binds to FKBP12.6 and causes its dissociation from RYR2, resulting in the activation of RYR channels. However, some studies are inconsistent with the above observation. Copello et al.32 reported that cADPR does not affect rabbit heart RYRs. A study on single channels indicates that cADPR does not activate single cardiac RYR channels in planar lipid bilayers.33 Guo et al.34 demonstrated that cADPR does not regulate sarcoplasmic reticulum (SR) Ca2+ release in intact cardiac myocytes at all. Although Lukyanenko et al.27 reported that cADPR altered Ca2+ spark properties in rat cardiomyocytes, the underlying mechanism of cADPR modulation of SR Ca2+ release is via enhancement of Ca2+ uptake by SERCA instead of dissociation of the FKBP12.6 from RYR2.
We have used FKBP12.6 null mice to test the extent to which the dissociation of FKBP12.6 proteins mediates the Ca2+ spark and inotropic action of isoproterenol (ISO) and to investigate the underlying mechanism. Our results indicate that alteration of Ca2+ spark properties caused by PKA phosphorylation is not through dissociation of FKBP12.6 from the RYR2 complex and that cADPR alters Ca2+ spark properties by dissociating FKBP12.6 from the RYR2 complex.
2. Methods
2.1. Isolation of cardiomyocytes and electrophysiology
Single ventricular myocytes from adult mice of either sex were prepared as described previously.14 Detailed procedures are described in Supplementary material online, Methods. All animal procedures described in this study were performed in adherence with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and with approval from the Institute of Biophysics Committee on Animal Care.
To test whether cADPR alters Ca2+ spark properties by enhancing SR Ca2+ ATPase activity and to dialyze cADPR or 2D12, cells were sealed and membranes were ruptured using a whole cell patch-clamp configuration (for detailed information see Supplementary material online).
2.2. Measurement of Ca2+ fluorescence
Single myocytes were incubated with 10 µM Fluo-4 a.m. (Molecular Probes) and Fluo-4 fluorescence was recorded using a laser scanning confocal head (BioRad Laboratories) attached to an inverted microscope (TE-300; Nikon; for detailed information see Supplementary material online).
2.3. Force measurement
The technique to measure force in isolated papillary muscles were similar to those previously described.35 To eliminate the possibility that cardiac hypertrophy may have confounding effects on the force data measurements female (C57Bl/6 and FKBP12.6 KO) mice 10–15 weeks were used.14 Muscles were slowly stretched until Lmax was obtained and stimulated at 4 Hz. The force was measured with an isometric force transducer (Radnoti, Monrovia, CA, USA) and data acquisition was carried out using Chart 3.4 (Adinstruments, Australia) installed on a microcomputer (for detailed information see Supplementary material online, Methods).
2.4. SR preparation and western blot analysis
SR microsomes from mouse heart were prepared36 and western blot analysis was performed as previously described.32 The detailed for this part, see Supplementary material online, Methods.
2.5. Data analysis
Image processing and data analyses were carried out using custom software written in MATLAB. Results were expressed as mean ± SEM. Significant differences were determined using the Student's t test. Data from three groups were compared by one-way, repeated measures ANOVA and significant differences between groups were determined by the Student–Newman–Keuls test for paired comparisons. Analysis and graphs for force measurement were completed with SigmaStat 3.0 and SigmaPlot 2000 (SPSS). Statistical significance (P ≤ 0.05) was determined within groups before and after the addition of ISO using the Wilcoxon signed rank test while between groups the Mann–Whitney rank sum test was utilized.
3. Results
3.1. β-Adrenergic stimulation regulates Ca2+ sparks in FKBP12.6-deficient cardiomyocytes
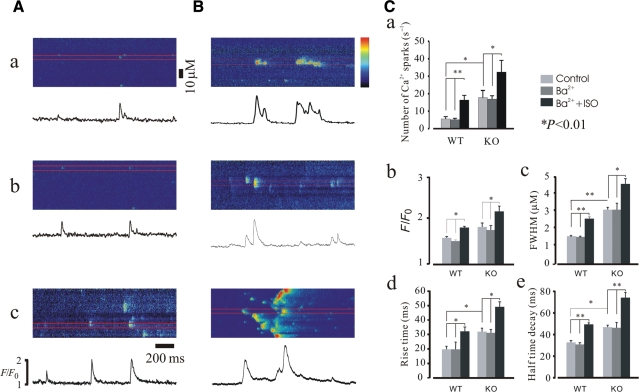
Figure 1A shows confocal microscopy line-scan images obtained from wild-type (WT) mouse cardiomyocytes. When compared with controls (Figure 1Aa and b), addition of 1 µM ISO to bath solution increased the frequency of Ca2+ sparks by about three-fold [from 5.8 ± 2.57 to 17.2 ± 3.86 per 100 µm per second, n = 286 (sparks), P < 0.05]. The kinetics of Ca2+ sparks was also significantly altered by the addition of ISO. Ca2+ fluorescence ratio (F/F0) was 1.52 ± 0.06 in controls and 1.71 ± 0.11 in the ISO group (Figure 1Cb, n = 211, P < 0.05). The full width at half maximum (FWHM) of Ca2+ sparks increased about 1.7-fold compared with controls (from 1.53 ± 0.24 µm to 2.51 ± 0.32 µm; Figure 1Cc, n = 211, P < 0.05). The rise time (RT) and half-time decay were prolonged 1.65-fold (from 19.8 ± 3.67 ms to 32.6 ± 5.12 ms) and 1.5-fold (from 33.4 ± 3.34 ms to 48.7 ± 3.1 ms), respectively (Figure 1Cd and e, n = 211, P < 0.01). Figure 1B demonstrates results obtained from KO cardiomyocytes. ISO increased Ca2+ spark frequency about two-fold [to 32.6 ± 6.14 per 100 µm per second (Figure 1Ca, n = 226, P < 0.05) from 17.5 ± 3.54 per 100 µm per second, control]. F/F0 was 1.72 ± 0.23 in controls and 2.21 ± 0.25 in the ISO group (Figure 1Cb, n = 226, P < 0.05). FWHM was increased about 1.5-fold compared with controls (from 3.1 ± 0.26 µm to 4.6 ± 0.25 µm; Figure 1Cc, n = 226, P < 0.05). RT and half-time decay of Ca2+ sparks were prolonged 1.7-fold (from 31.4 ± 3.6 ms to 49.3 ± 5.6 ms and from 46.6 ± 6.4 ms to 75.2 ± 6.8 ms, respectively; Figure 1Cd and e, n = 226, P < 0.01). These results indicate that Ca2+ spark properties were significantly altered by β-adrenergic stimulation in both WT and KO cardiomyocytes and suggest that modulation of Ca2+ release by ISO is not only via dissociation of FKBP12.6 from RYR2 but may also involve other mechanisms.
Figure 1.
Isoproterenol altered Ca2+ spark properties. Confocal line-scan images showing spontaneous Ca2+ sparks collected in WT (A) and KO (B) cells. (a) normal control experiment; (b) Ba2+ extracellular solution control experiment; (c) in the presence of 1 µM ISO. It should be noted that the Ca2+ spark characteristics were already elevated under control conditions in KO myocytes compared with those of WT cells. (C) Summary data for properties of Ca2+ sparks. Frequency (Ca), F/F0 (Cb), FWHM (Cc), RT (Cd), and half-time decay were dramatically altered by ISO in both WT and FKBP12.6 KO cardiomyocytes. The profiles (Ca2+ fluorescence ratio) shown below images were taken from the areas of the images indicated by red lines. *P < 0.05 and **P < 0.01.
It is notable that the basal Ca2+ spark properties in KO cells are similar to those of control cells treated with ISO.13,14 The effects of ISO on Ca2+ spark properties were also significantly different between WT and KO cells.
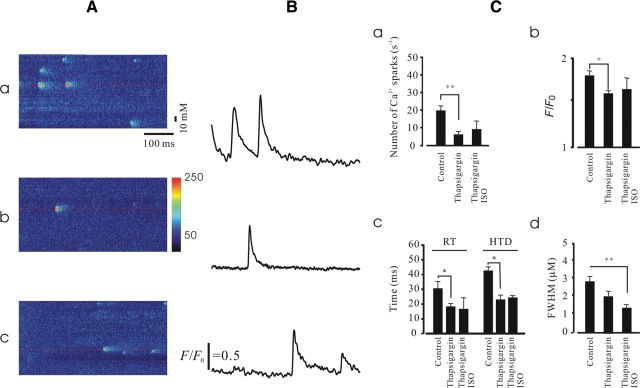
A further series of experiments was performed in KO cells to investigate the mechanism of Ca2+ spark property alteration induced by β-adrenergic stimulation in more detail. First, we sought to examine the effect of the SERCA inhibitor, thapsigargin, on the alteration of Ca2+ spark properties induced by ISO. As shown in Figure 2, as expected, thapsigargin alone exhibited an inhibitory effect on Ca2+ spark properties. In the presence of thapsigargin, the stimulatory effects of ISO on Ca2+ sparks were significantly reduced compared with the control (without thapsigargin).
Figure 2.
Blockage of the Ca2+ pump altered ISO-induced Ca2+ spark properties. The experiments were carried out in FKBP12.6 KO intact myocytes. (A) Representative line-scan images obtained from controls (a), in the presence of thapsigargin (10 µm, b), and in the presence of thapsigargin and ISO (c). (B) Profiles of relative Ca2+ fluorescence intensity taken from images (A) as indicated by red lines. (C) Summary data of Ca2+ spark properties. Note that the increased Ca2+ spark frequency (a), peak Ca2+ (b), RT/half time decay (c), and size (d) induced by ISO were significantly reduced by the application of thapsigargin (n = 126, *P < 0.05 and **P < 0.01).
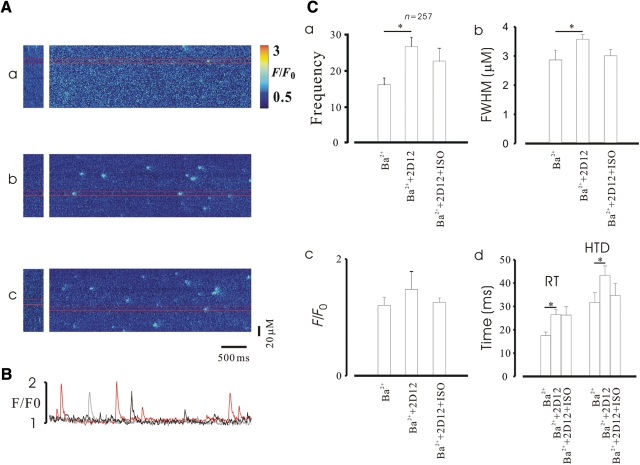
Our next series of experiments was conducted to further probe the molecular mechanism of ISO augmentation of Ca2+ spark properties in KO mice. It is known that the PLB monoclonal antibody, 2D12, reverses the inhibitory effect of PLB on SERCA in cardiomyocytes.36 So, we hypothesized that if β-adrenergic stimulation augments Ca2+ sparks via PKA phosphorylation of PLB as indicated by the above experiments, then ISO should not enhance Ca2+ sparks in KO cardiomyocytes in the presence of 2D12. After dialysis of cells with 2D12 (5 µm, through pipettes) for 15 min, Ca2+ spark frequency was increased 1.7-fold from 16.32 ± 2.6 (control) to 27.7 ± 3.2 (Figure 3A and Ca, n = 257, P < 0.01). The kinetics of Ca2+ sparks were also altered by 2D12, with the exception of peak Ca2+. However, in the presence of 2D12, ISO, as expected, could not increase Ca2+ spark frequency further compared with the control (Figure 3Ac and Ca, P > 0.05). Similarly, in the presence of 2D12 ISO also failed to change Ca2+ spark kinetics (Figure 3C).
Figure 3.
Phospholamban antibodies abrogated Ca2+ spark alteration caused by ISO in FKBP12.6 KO cardiomyocytes. (A) Representative line-scan images recorded under different conditions. (Aa) Image obtained in Ba2+ extracellular solution; (Ab) 2D12 altered Ca2+ spark properties significantly; (Ac) ISO failed to alter Ca2+ spark properties further in the presence of 2D12. (B) Profiles taken from the line-scan images (above) as indicated by gray (control), black (2D12), and red lines (2D12 + ISO). (C) Summary data for Ca2+ spark properties.
Taken together, the alteration of Ca2+ spark properties via β-adrenergic stimulation by ISO in mouse cardiomyocytes occurred via phosphorylation of PLB, and probably not through dissociation of FKBP12.6.
3.2. Normal β-adrenergic stimulation of cardiac contraction in FKBP12.6 null cardiomyocytes
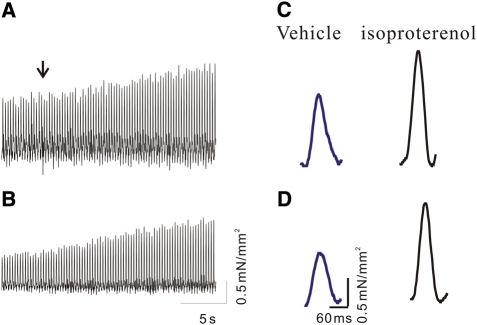
β-Adrenergic augmentation of cardiac contractility has been functionally linked to the phosphorylation-dependent dissociation of FKBP12.6 regulatory proteins from RYR2. If the disengagement of FKBP12.6 proteins from the RYR2 complex plays a significant role in inotropy, β-adrenergic stimulation would be expected to be less robust in hearts lacking FKBP12.6. Left ventricular papillary muscles from eight WT and eight FKBP12.6 KO female mice were stimulated at 4 Hz to assess cardiac contractility before and during β-adrenergic stimulation with ISO (1 µM). Baseline force was normalized to cross-sectional area, which was 0.36 ± 0.05 mm2 for WT and 0.33 ± 0.07 mm2 for FKBP12.6 KO mice (no significant difference, Figure 4A and B). Although the average magnitude of the twitch contraction (1.2 ± 0.2 mN/mm2 vs. 0.8 ± 0.1 mN/mm2) and the rate of force development (32 ± 6 mN/mm2/s vs. 24 ± 2 mN/mm2/s) was larger in WT vs. KO mice, the differences were not significant (Figure 4, also see Table 1). Importantly, ISO stimulation increased the magnitude (45% vs. 43%) and rate of stress development (47% vs. 47%) to an equivalent degree in WT and KO mice (Table 1). Similarly, twitch relaxation time was increased to an equivalent degree in both genotypes (22% vs. 23%). These data demonstrate that β-adrenergic stimulation of contractility does not require dissociation of FKBP12.6 proteins, and that other mechanisms likely predominate in the inotropic effect.
Figure 4.
β-Adrenergic stimulation of cardiac contraction. (A and B) 20 s traces demonstrating force development during β-adrenergic stimulation in (A) WT and (B) FKBP12.6 KO mice. Notice in both WT and FKBP12.6 KO mice, the amplitude of the contraction nearly doubles from baseline to peak during ISO stimulation. (C and D) Individual representative contractions from WT (C) and FKBP12.6 KO mice (D) before (blue) and during (black) β-adrenergic stimulation with ISO (1 mM).
Table 1.
Isolated left ventricular anterior papillary muscle parameters in WT and FKBP12.6 KO mice
| FKBP12.6+/+ |
FKBP12.6−/− |
|||
|---|---|---|---|---|
| Control | ISO | Control | ISO | |
| Force (mN/mm2) | 1.2 ± 0.2 | 2.1 ± 0.2* | 0.8 ± 0.1 | 1.5 ± 0.2* |
| % Change | 45 | 43 | ||
| dF/dTmax | 32 ± 6 | 63 ± 7* | 24 ± 2 | 47 ± 5* |
| % Change | 47 | 47 | ||
| Tau (ms) | 36 ± 3 | 27 ± 2* | 32 ± 2 | 24 ± 2* |
| % Change | 22 | 23 | ||
Force was normalized to cross-sectional area (mN/mm2). Baseline was determined by averaging a total of 100 ms, 75 ms before, and 25 ms after the stimulus artefact. Peak amplitude (mN/mm2) was baseline to maximal force development. dF/dTmax was the maximal rate of force development (mN/mm2/s) during contraction. The time constant of decay, tau, was determined as the time to reach 37% of initial (maximal) value. Three to five consecutive contractions were averaged to obtain values of a representative contraction. Isoproterenol was added to a final concentration of 1 mM and values calculated at peak response. A total of eight experiments were performed for each group. Values are mean ± SE.
*P ≤ 0.02 within a group. There was no statistical significance between groups.
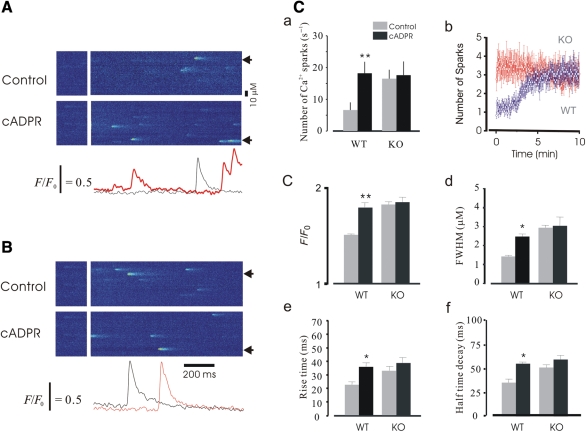
3.3. cADPR altered Ca2+ spark properties only in WT cardiomyocytes
It is known that FKBP12.6 selectively associates with cardiac RYR2,12 thus by using the FKBP12.6 KO mouse model we were able to examine the role of cADPR in both WT and FKBP12.6 KO cardiomyocytes in order to investigate the mechanism of cADPR regulation of Ca2+ release channels. Because the action of cADPR is temperature-dependent,37 we carried out the experiments at 36°C. cADPR (1 µM) was dialyzed via patch pipettes in cells from both WT and KO mice. Representative line-scan images from WT myocytes are documented in Figure 5A. cADPR caused a 2.8-fold increase in Ca2+ spark frequency from 6.6 ± 1.4 per 100 µm per second (control) to 18.2 ± 2.6 per 100 µm per second (cADPR, n = 168, P < 0.01); cADPR increased F/F0 1.2-fold from 1.51 ± 0.02 to 1.83 ± 0.08 (n = 168, P < 0.05); FWHM was 2.1 ± 0.08 µm in controls and 3.2 ± 0.14 µm in the cADPR group (n = 168, P < 0.05, Figure 5Cd); RT and half time decay of Ca2+ sparks were 22.3 ± 2.1 ms vs. 38.1 ± 2.4 ms ( n = 168, P < 0.05) and 36.4 ± 4.3 ms vs. 56.3 ± 3.8 ms (Figure 5Ce and f, n = 168, P < 0.01), respectively. As shown in Figure 5B and C, dialysis of KO cells with cADPR did not alter the Ca2+ spark properties significantly.
Figure 5.
cADPR altered the properties of Ca2+ sparks in WT cardiomyocytes. Confocal line-scan images showing spontaneous Ca2+ sparks collected from cardiomyocytes. (A) Representative images obtained from a WT cell. cADPR significantly changed the properties of Ca2+ sparks. The locations from where profiles were taken are indicated by arrows at the bottom of the images. (B) Representative line-scan images obtained from a KO cardiomyocyte. It is noteworthy that Ca2+ spark properties were not significantly altered by cADPR. (C) Summary data for Ca2+ spark properties. cADPR greatly increased Ca2+ spark frequency (a, P < 0.01, n = 168), and the increase in the frequency of Ca2+ sparks occurred after dialysis of cADPR for 3 min and reached a maximum at ∼7 min (b); peak Ca2+ (c, P < 0.01, n = 168), size (d, P < 0.05, n = 168), RT (e, P < 0.01, n = 168), and half-time decay (P < 0.01, n = 168) of Ca2+ sparks was also significantly altered by dialysis of cADPR in WT cardiomyocytes.
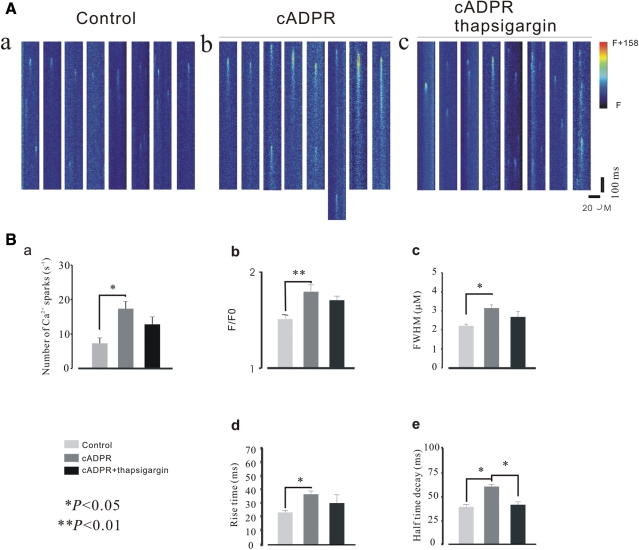
To determine whether the alteration of Ca2+ spark properties by cADPR is via enhancing the effect of SERCA as previously reported.27 We tested the effect of cADPR on Ca2+ spark properties in the presence of thapsigargin in WT cells. As shown in Figure 6, spark characteristics were not markedly changed by cADPR in the presence of thapsigargin (10 µM) except for half time decay, as was also observed in the experiments described above. However, it should be noted that these parameters were altered by cADPR though differences were not statistically significant. We further examined the effects of cADPR on Ca2+ sparks in the presence of 2D12, and a similar result as that observed in thapsigargin experiments was obtained except for half time decay which was not significantly altered (data not shown) suggesting that the alteration of Ca2+ spark properties induced by cADPR was possibly not via action on the SR Ca2+ pump.
Figure 6.
Effect of cADPR on Ca2+ spark properties in the presence of SERCA inhibitor. Spontaneous Ca2+ sparks were collected in a WT cell. (A) A representative line-scan image. (Aa) Control; (Ab) cADPR; (Ac) thapisgargin (10 µm)+ADPR. (B) Summary of Ca2+ spark properties. Except for half-time decay (e), Ca2+ spark frequency (a), peak Ca2+ (b), size (c), and RT (d) induced by dialysis of cADPR decreased, but were not abolished in the presence of thapsigargin. The number of Ca2+ sparks (n) was 186; * indicates P < 0.05, and **P < 0.01, respectively.
To test whether the increases in Ca2+ spark frequency/amplitude induced by cADPR were by enhancing SR Ca2+ ATPase activity, we analysed Ca2+ transient decay kinetics in voltage-clamped myocytes from WT mice. As shown in Figure 7A, cADPR increased peak Ca2+ from 1.71 ± 0.64 to 2.16 ± 0.16 [P < 0.05, n = 189/12 (sparks/cells)] by depolarizing membrane potential to 0 mV from a holding potential of −40 mV (100 ms duration), but RT and half-time decay were not significantly altered by cADPR in these voltage-clamped cells.
Figure 7.
Effect of cADPR on voltage clamping-induced Ca2+ sparks and caffeine-induced Ca2+ release. (A) Sample of line-scan images collected in voltage-clamped cells from WT mice in the absence (left) and presence of cADPR (right). The protocol used to trigger Ca2+ sparks is shown above the images, whereas the currents (upper) and peak Ca2+ (lower) are shown below the images. (B) Summary data of Ca2+ spark properties induced by membrane depolarization. (C) Representative tracing of caffeine-induced Ca2+ transients (top panel). When compared with the control (gray), the Ca2+ transient was significantly reduced by cADPR (green, n = 7, P < 0.05); in contrast, cADPR did not reduce Ca2+ transients in FKBP12.6 KO cells (red). However, it is notable that the Ca2+ content was lower in FKBP12.6 null cells (black) compared with that in WT cells though not statistically significant (n = 7, P = 0.06312). The bottom panel shows the comparison of average peak values (left) and the time decay (right) of caffeine-induced Ca2+ transients or SR calcium load.
We also compared the effect of cADPR on SR Ca2+ load by measuring caffeine-induced Ca2+ release. 10 mM caffeine was puffed close to the cell for 200 ms using a pipette. As shown in Figure 7C, cADPR significantly reduced Ca2+ content (by 36.2%, n = 13) in WT cardiomyocytes. In contrast, there was no significant alteration of Ca2+ content observed in KO cells (n = 16). Although statistically there was no significant difference in SR Ca2+ content between WT and KO cells, the content of SR Ca2+ in KO cells was lower to some extent compared with that in WT cells, suggesting the existence of Ca2+ leakage due to the deletion of the FKBP12.6.16 Analysis of Ca2+ transient decay kinetics showed that there was no significant difference before or after application of cADPR.
Taken together, our results show that cADPR altered Ca2+ spark properties by causing dissociation of FKBP12.6 from RYR2 binding sites.
3.4. cADPR dissociates FKBP12.6 from SR
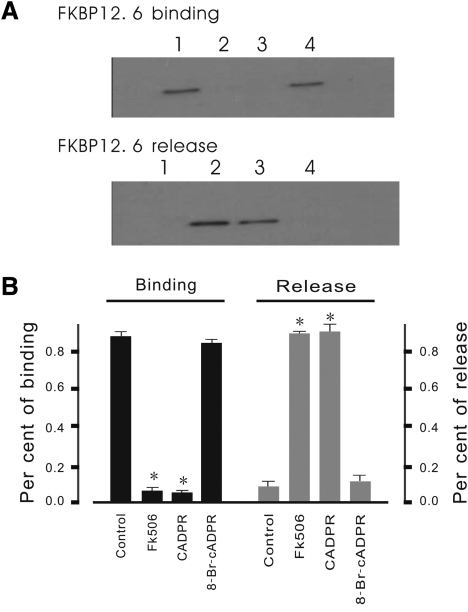
To further confirm that the effect of cADPR on Ca2+ spark properties is dependent on the dissociation of FKBP12.6 from SR, western blotting analysis was performed on SR fractions. As shown in Figure 8, endogenous FKBP12.6 was released from the SR fraction by incubation with cADPR, demonstrating that practically all of the FKBP12.6 was bound to the SR fraction in the pellet while no detectable 12.6 kDa protein band was observed in the supernatant.
Figure 8.
cADPR releases FKBP12.6 from mouse cardiac SR. (A) Western blotting analysis of FKBP12.6 binding and release. SR was incubated for 30 min at 37°C in control conditions or in the presence of cADPR, 8-Br-cADPR, or FK506. Released FKBP12.6 proteins appear in the supernatant (lower panel, lanes 1–4 are the control, FK506, cADPR, and 8-Br-cADPR, respectively) whereas bound FKBP12.6 remained in the pellets in control conditions and in the presence of 8-Br-cADPR (upper panel, lanes 1–4 are the control, FK506, cADPR, and 8-Br-cADPR, respectively). FK506 released almost all of the FKBP12.6 from the SR fraction. Similar results were obtained with cADPR though amounts of dissociated FKBP12.6 found in the supernatants were lower. Here, 8-Br-cADPR was used as a negative control. (B) Summary data from densitometry analysis. These results are from three independent experiments. The molecular weight of this protein is ∼12 kDa. *P < 0.01.
By addition of FK506 almost all the FKBP12.6 was released from the SR fraction into the supernatant. A corresponding FKBP12.6 band was not detected in the pellet containing the SR fraction. Similar results were also observed after incubation of the SR fraction with cADPR. Most of the FKBP12.6 appeared in the supernatant and little remained associated with the SR fraction in the pellet. No obvious band was detected in the supernatant when the SR membrane was pre-treated with 8-Br-cADPR, a cADPR antagonist. These results suggest that cADPR significantly causes dissociation of FKBP12.6 from SR.
4. Discussion
Dissociation of FKBP12.6 protein from RYR2 caused by PKA phosphorylation is considered the leading mechanism underlying Ca2+ leakage in the failing heart.16 However, there is increasing evidence to challenge this mechanism.6,7,18–20,38 In the present study, we have used FKBP12.6 null mice to directly test the extent to which the dissociation of FKBP12.6 proteins mediates the Ca2+ sparks and inotropic action of ISO in cardiomyocytes. The present study shows that β-adrenergic stimulation increases Ca2+ spark frequency and significantly alters the kinetics of Ca2+ sparks in KO cardiomyocytes (Figure 1), suggesting that Ca2+ release from RYR2 does not occur through PKA phosphorylation-induced dissociation of FKBP12.6 from the RYR2 complex. This was further confirmed by the fact that ISO stimulation increased the magnitude of contraction, accelerated the rate of stress development and increased twitch relaxation time of left ventricular papillary muscles from FKBP12.6 KO mice (Figure 4). Thus, results from the present and earlier studies6,7,16,19–21 do not support the hypothesis that phosphorylation of RYR2 by PKA causes dissociation of FKBP12.6 from the RYR2 complex and is followed by Ca2+ leakage.39 Our later experiments showed that alteration of Ca2+ spark properties induced by ISO was prevented in the presence of thapsigargin, and a PLB antibody (Figures 2 and 3), suggesting that the modulation of Ca2+ release induced by PKA phosphorylation was caused by affecting PLB and the function of SERCA.18,40 Taken together, our data do not support the theory that phosphorylation of RYR2 by PKA causes dissociation of FKBP12.6 from the RYR2 complex resulting in Ca2+ leakage.
cADPR is capable of inducing Ca2+ release from the SR by activation of RYRs in different tissues and cells.27–30,41 There is growing evidence showing that cADPR induces Ca2+ release by causing the dissociation of FKBP12.6 from RYRs in pancreatic β-cells, smooth muscle and vascular endothelial cells,20,30 however, that cADPR mediates Ca2+ release in cardiac myocytes is highly controversial. Prakash et al.26 reported that cADPR induces Ca2+ release from the SR both through RYR channels and via a mechanism independent of RYR channels, and Lukyanenko et al.27 have demonstrated that cADPR enhancement of Ca2+ sparks is mediated indirectly by increased accumulation of Ca2+ in the SR and subsequent luminal Ca2+-dependent activation of RYRs. Here we demonstrated that cADPR altered Ca2+ spark properties in WT cardiomyocytes (Figure 5A) but not in FKBP12.6 null cells (Figure 5B), and that this effect of cADPR on Ca2+ spark frequency, amplitude, RT as well as FWHM was not significantly affected by either thapsigargin (Figure 6) or the PLB antibody, 2D12 (data not shown), suggesting that the effect of cADPR on Ca2+ spark properties in mouse cardiomyocytes is related to the dissociation of FKBP12.6 proteins from the RYR2 complex but not to PLB phosphorylation. One might argue that the increase in Ca2+ spark frequency/amplitude by cADPR may be by enhancing SR Ca2+ ATPase activity. However, experiments conducted in voltage-clamped myocytes demonstrated that cADPR increased peak Ca2+ without affecting RT and half-time decay of sparks (Figure 7A), suggesting that the effect of cADPR on Ca2+ spark properties was not mediated by affecting SR Ca2+ ATPase activity.
Indeed, this was further confirmed by experiments investigating FKBP12.6 release by cADPR (Figure 8) and by our recent experiments that JTV-519, an RYR2 complex stabilizer, completely abrogated the alteration of Ca2+ spark properties induced by cADPR (data not shown) as well as by the observations that cADPR can directly bind to FKBP12.6 in islet microsomes31 and that FKBP12.6 colocalizes with RYRs.32 Further studies demonstrated that Ca2+ influx significantly decreased colocalization, and that this colocalization was reversed by 8-Br-cADPR, suggesting cADPR involvement in the dissociation of FKBP12.6 from RYRs.31 Although it has been reported that the loss of FKBP12.6 has no significant effect on the conduction and activation of RYR2 or the propensity for spontaneous Ca2+ release,42 the majority of studies have confirmed that FKBP12.6 is a RYR2 regulatory protein which closely associates with and regulates RYR2 Ca2+ release channels, and that the dissociation or deletion of FKBP12.6 from the RYR2 complex causes significant alteration in Ca2+ spark properties.13–15,29,30,43,44
In summary, our data demonstrate that (i) β-adrenergic stimulation of Ca2+ release and contractility does not require dissociation of FKBP12.6 from the RYR2 complex and (ii) cADPR alters Ca2+ spark properties in mouse cardiomyocytes by causing dissociation of FKBP12.6 proteins from the RYR2 complex.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Basic Research Program of China [007CB512100 to G.J.], NIH grants [HL45239 and DK65992 to M.K.]; F2HL[76999 to Y.T.]; the Knowledge Innovation Program of the Chinese Academy of Sciences [KSCX2-YW-R-50 to G.J.]; the National Foundation of Science and Technology [#30670505 to G.J.]; the ‘863’ research program [2006AA02A106 to G.J.]; and the National Natural Science Foundation of China (30770791) and the State Key Development Program for Basic Research of China (2006CB503806 and 2007CB1203).
Acknowledgements
We thank Dr Peace Cheng for critical reading of the manuscript.
Conflict of interest: none declared.
References
- 1.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 2.Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H, et al. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. J Physiol. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder SH, Sabatini DM. Immunophilins and the nervous system. Nat Med. 1995;1:32–37. doi: 10.1038/nm0195-32. [DOI] [PubMed] [Google Scholar]
- 4.Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, et al. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- 5.Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 2004;35:621–628. doi: 10.1016/j.ceca.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 7.Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 8.Witcher DR, Strifler BA, Jones LR. Cardiac-specific phosphorylation site for multifunctional Ca2+/calmodulin-dependent protein kinase is conserved in the brain ryanodine receptor. J Biol Chem. 1992;267:4963–4967. [PubMed] [Google Scholar]
- 9.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, et al. Ca2+/calmodulin kinase II-dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ Res. 2007;100:399–407. doi: 10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- 11.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 12.Xin HB, Rogers K, Qi Y, Kanematsu T, Fleischer S. Three amino acid residues determine selective binding of FK506-binding protein 12.6 to the cardiac ryanodine receptor. J Biol Chem. 1999;274:15315–15319. doi: 10.1074/jbc.274.22.15315. [DOI] [PubMed] [Google Scholar]
- 13.Ji GJ, Feldman ME, Greene KU, Sorrentino V, Xin HB, Kotlikoff MI. RYR2 proteins contribute to the formation of Ca2+ sparks in smooth muscle. J Gen Physiol. 2004;123:377–386. doi: 10.1085/jgp.200308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- 15.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 16.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 17.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 19.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 20.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 21.Meng X, Xiao B, Cai S, Huang X, Li F, Bolstad J, et al. Three-dimensional localization of serine 2808, a phosphorylation site in cardiac ryanodine receptor. J Biol Chem. 2007;282:25929–25939. doi: 10.1074/jbc.M704474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones PP, Meng X, Xiao B, Cai S, Bolstad J, Wagenknecht T, et al. SR localization of PKA phosphorylation site, Ser(2030), in the three-dimensional structure of cardiac ryanodine receptor. Biochem J. 2008;410:261–270. doi: 10.1042/BJ20071257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhlang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger-cyclic ADP-ribose. J Cell Med. 2006;10:407–422. doi: 10.1111/j.1582-4934.2006.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HC, Aarhus R, Graeff RM. Sensitization of calcium-induced calcium release by cyclic ADP-ribose and calmodulin. J Biol Chem. 1995;270:9060–9066. doi: 10.1074/jbc.270.16.9060. [DOI] [PubMed] [Google Scholar]
- 25.Meszaros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of a non-skeletal type ryanodine receptor Ca2+ channel. Nature. 1993;364:76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- 26.Prakash YS, Kannan MS, Walseth TF, Sieck GC. cADP ribose and [Ca2+]I regulation in rat cardiac myocytes. Am J Physiol Heart Circ Phyisiol. 2000;279:H1482–H1489. doi: 10.1152/ajpheart.2000.279.4.H1482. [DOI] [PubMed] [Google Scholar]
- 27.Lukyanenko V, Gyorke I, Wiesner TF, Gyorke S. Potentiation of Ca2+ release by cADPR-ribose in the heart is mediated by enhanced SR Ca2+ uptake into the sarcoplasmic reticulum. Circ Res. 2001;89:614–622. doi: 10.1161/hh1901.098066. [DOI] [PubMed] [Google Scholar]
- 28.Macgoregor AT, Rakovic S, Galioone A, Terrar DA. Dual effects of cyclic ADP-ribose on sarcoplasmic reticular Ca2+ release and storage in cardiac myocytes isolated from ginea-pig and rat ventricle. Cell Calcium. 2007;41:537–546. doi: 10.1016/j.ceca.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, et al. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol. 2004;286:538–546. doi: 10.1152/ajpcell.00106.2003. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi N, Takaswa S, Nata K, Tohgo A, Kato I, Ikehata F, et al. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem. 1997;272:3133–3136. doi: 10.1074/jbc.272.6.3133. [DOI] [PubMed] [Google Scholar]
- 31.Teggatz EG, Zhlang G, Zhang AY, YI F, Li N, Zou AP, et al. Role of cyclic ADP-ribose in Ca2+-induced Ca2+ release and vasoconstriction in small renal arteries. Microvasc Res. 2005;70:65–75. doi: 10.1016/j.mvr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Copello JA, Qi Y, Jeyakumar LH, Ogunbunmi E, Fleischer S. Lack of effect of cADP-ribose and NAADP on the activity of skeletal muscle and heart ryanodine receptors. Cell Calcium. 2001;30:269–284. doi: 10.1054/ceca.2001.0235. [DOI] [PubMed] [Google Scholar]
- 33.Fruen BR, Mickelson JR, Shomer NH, Velez P, Louis CF. Cyclic ADP-ribose does not affect cardiac or skeletal muscle ryanodine receptors. FEBS Lett. 1994;352:123126. doi: 10.1016/0014-5793(94)00931-7. [DOI] [PubMed] [Google Scholar]
- 34.Guo X, Laflamme MA, Becker PL. Cyclic ADP-ribose does not regulate sarcoplasmic reticulum Ca2+ release in intact cardiac myocytes. Circ Res. 1996;79:147–151. doi: 10.1161/01.res.79.1.147. [DOI] [PubMed] [Google Scholar]
- 35.Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166–1171. doi: 10.2337/diabetes.51.4.1166. [DOI] [PubMed] [Google Scholar]
- 36.Sham JS, Jones LR, Morad M. Phospholamban mediates the beta-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am J Physiol. 1991;261:H1344–H1349. doi: 10.1152/ajpheart.1991.261.4.H1344. [DOI] [PubMed] [Google Scholar]
- 37.Iino S, Cui Y, Galinoe A, Terrar DA. Actions of cADP-ribose and its antagonists on contraction in guinea pig isolated ventricular myocytes. Influence of temperature. Circ Res. 1997;81:879–884. doi: 10.1161/01.res.81.5.879. [DOI] [PubMed] [Google Scholar]
- 38.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts: role of phosphatases and response to isoproterenol. J Biol Chem. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 39.Wehrens XH, Lehanart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 40.Galione A, Lee HC, Busa WB. Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-robose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- 41.Prakash YS, Kannan M, Walseth TF, Sieck GC. cADPR ribose and [Ca2+]i regulation in rat cardiac myocytes. Am J Phuysiol Heart Circ Physiol. 2000;279:1482–1489. doi: 10.1152/ajpheart.2000.279.4.H1482. [DOI] [PubMed] [Google Scholar]
- 42.Xiao J, Tian X, Jones PP, Bolstad J, Kong H, Wang R, et al. Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J Biol Chem. 2007;282:34828–34838. doi: 10.1074/jbc.M707423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L, et al. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ Res. 2001;88:188–194. doi: 10.1161/01.res.88.2.188. [DOI] [PubMed] [Google Scholar]
- 44.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.