Abstract
Testicular cancers respond favorably to chemotherapy with the platinum-containing drug cis-diamminedichloroplatinum(II) (cisplatin). One factor that could explain the efficacy of cisplatin is the low frequency of p53 mutations observed in this tumor type. The present study examines the p53-mediated responses in murine testicular teratocarcinoma cells exposed to the drug. Cisplatin treatment of teratocarcinoma cells with a wild-type p53 gene resulted in accumulation of the p53 protein through posttranscriptional mechanisms; induction of p53-target genes was also observed. Drug treatment resulted in rapid apoptosis in p53-wild-type cells but not in p53−/− teratocarcinoma cells. In the latter cells, cisplatin exposure caused prolonged cell cycle arrest accompanied by induction of the p21 gene. Clonogenic assays demonstrated that the p53 mutation did not confer resistance to cisplatin. These experiments suggest that cisplatin inhibits cellular proliferation of testicular teratocarcinoma cells by two possible mechanisms, p53-dependent apoptosis and p53-independent cell cycle arrest.
Chemotherapeutic agents and radiation are often used in the treatment of cancer to augment surgical procedures, particularly to address the complications of metastatic spread. In most cases, however, chemotherapy has limited success in conferring long-term patient survival. One notable exception is in the management of testicular cancer, which is the most common malignancy found in men <35 years of age (1, 2). Several recent changes in the treatment of this disease have caused the patient mortality rate to diminish to <10%, making it one of the most curable solid neoplasms (1). In particular, the largest effect in the disease response to treatment was afforded by the addition of the platinum compound cis-diamminedichloroplatinum(II) (cisplatin) to the chemotherapeutic regimen (1, 2).
Cisplatin is used to treat a wide variety of cancers in addition to testicular tumors (3, 4), although its effectiveness is often limited by its inherently poor activity against many tumor types and by the development of resistance (5). The anticancer mechanism of cisplatin is not clearly understood, but it is generally accepted that it acts through the formation of DNA adducts (6). The fact that some cells are killed by the DNA damage while others are resistant is most likely a consequence of several cellular pathways (7, 8), which must be unraveled to provide more comprehensive treatment strategies.
One response to genotoxic stress involves the p53 tumor suppressor gene product (9, 10). This nuclear phosphoprotein accumulates after DNA damage and controls cellular proliferation predominantly through its activity as a transcription factor. The expression of downstream genes contributes to tumor suppression either by activating cell cycle arrest, possibly to give the cell time to repair the damage and avoid genetic instability, or by initiating apoptosis in the injured cell. The fact that the loss of p53 activity promotes malignant transformation is confirmed by the high incidence of mutations in this gene in a wide spectrum of human cancers (11, 12). Moreover, a nonfunctional p53 might provide the ensuing tumors with resistance to chemotherapy by protecting the cells from drug-induced apoptosis. In this regard, the status of the p53 gene in a tumor can be an indicator of the clinical prognosis of a cancer patient (13).
Testicular tumors are atypical because the p53 gene is usually not mutated in this type of cancer (14–16). In addition, testicular tumors express high levels of the p53 protein, a characteristic that is usually a manifestation of a genetic mutation (17, 18). Recently, experiments with murine testicular teratocarcinoma cell lines demonstrated that the elevated levels of p53 protein did not result in increased transcriptional activity of p53-regulated genes (16), suggesting that the protein was predominantly inactive. Upon exposure of the cells to the DNA-damaging agent etoposide, however, their p53-mediated functions were restored, including activation of the programmed cell death pathway.
Although p53-dependent apoptosis suggests how DNA-damaging agents such as cisplatin kill neoplastic cells, the relationship between cisplatin sensitivity and p53 status remains unclear. In support of a role for p53 in the cytotoxic mechanism of cisplatin, several studies of ovarian carcinoma cell lines demonstrated that disruption of p53 function afforded drug resistance (19–21), although recent work has indicated that p53 is not a determinant of cisplatin cytotoxicity in ovarian cancer cells (22). In addition, non-small cell lung cancer cells were found to be sensitized to cisplatin-induced apoptosis after the introduction of a wild-type p53 gene (23), but cell lines that do not exhibit a predominant apoptotic response to chemotherapy, such as breast cancer or human foreskin fibroblast cell lines, were sensitized to cisplatin by the inactivation of p53 (24, 25). Furthermore, although a screen of human cancer cell lines revealed a trend in which the p53-mutants were more resistant to cisplatin, there was significant variability in drug response (26). Finally, while the present manuscript was in preparation, a report appeared indicating that the sensitivity of human testicular tumor cell lines to cisplatin-induced apoptosis was independent of p53 status (27).
These investigations reveal that the success of cisplatin-based chemotherapy may be due in part to apoptosis controlled by wild-type p53, but there is only limited information about the role of this protein in mediating cisplatin cytotoxicity in testicular cancer cells, the most responsive tumor type. Moreover, previous work (19–27) demonstrates the importance of cellular context and cell type in examining the effect of p53 on the cytotoxicity of cisplatin. In the present study, several mouse testicular teratocarcinoma cell lines, having both wild-type and mutant p53, were used to examine the response of p53-controlled pathways to cisplatin in a relevant cellular background. Experiments were also performed to determine whether or not p53 is essential for the cytotoxic activity of this drug. Our results clearly show that, whereas p53 is an important factor in the short-term response to drug treatment, wild-type p53 function is not an absolute requirement for cisplatin-induced cytotoxicity.
MATERIALS AND METHODS
Materials.
Cisplatin was a gift from Johnson-Matthey. Fresh aqueous stock solutions were prepared for each experiment. The [α-32P]dCTP, [35S]amino acids, and [125I]protein A were purchased from Dupont/NEN. Protease inhibitors were purchased from Boehringer Mannheim. Igepal CA-630, 4′,6-diamidino-2-phenylindole (DAPI), and cycloheximide were purchased from Sigma. Fixed Staphylococcus aureus (Staph A) was supplied by H. Ploegh (Massachusetts Institute of Technology). The anti-p53 mAb421 was obtained from A. J. Levine (Princeton, NJ).
Cells and Cell Culture.
F9 (ATCC CRL 1720) and Nulli-SCC1 (ATCC CRL 1566) cells were obtained from the American Type Culture Collection (Manassas, VA). The p53 mutant teratocarcinoma cell line (EB16) was a gift from A. J. Levine (16), and was derived from a testicular tumor of a p53 knockout mouse. The teratocarcinoma cell lines were propagated in DMEM with 2 mM l-glutamine and antibiotic antimycotics (Sigma) in addition to 10% fetal bovine serum (EB16), 15% fetal bovine serum (F9) or 10% calf serum (Nulli-SCC1). All plates were coated with a 0.1% (wt/vol) gelatin solution (Sigma) for 30 min and washed with PBS before use. The cells were grown at 37°C in a 5% CO2(g) atmosphere. Unless otherwise indicated, cisplatin was administered to cells growing in logarithmic phase on 10-cm plates in fresh media; continuous exposure means that the drug-treated media was not replaced.
p53 Protein Analysis.
At each time point, the cells were washed with cold PBS and then lysed by incubation for 30 min on ice in lysis buffer (50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/0.5% Igepal CA-630/1 mM DTT/10 μg/ml each of aprotinin, leupeptin, and pepstatin/0.5 mg/ml pefabloc). The cell lysate was centrifuged, and the supernatant was stored on ice until use. All subsequent steps were performed at 4°C with prechilled solutions.
Fixed Staph A was washed three times in NET buffer (50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/5 mM EDTA/0.5% Igepal CA-630). Samples containing equal amounts of protein in the same volume (1–1.5 ml) were precleared with 100 μl of Staph A and 6 μl each of normal mouse and rabbit sera (Sigma) for 1 h. After centrifugation the supernatant was incubated with 200 μl mAb421 for 12–16 h. Staph A (80 μl) was incubated for 45 min in the sample, followed by centrifugation. The pellet was washed three times in NET buffer, boiled for 5 min in SDS loading buffer (50 mM Tris⋅HCl, pH 6.8/100 mM DTT/2% SDS/10% glycerol/0.1% bromophenol blue), and loaded onto an SDS/8% polyacrylamide gel.
For Western blot analysis, the proteins were electroblotted onto Immobilon-P membrane (Millipore). The blot was blocked, probed with mAb421 diluted 20-fold with 0.05% Tween 20 in PBS containing 1% BSA, then probed with 10 μCi (1 Ci = 37 GBq) [125I]protein A in the same buffer, and exposed to film at −80°C with an intensifier screen. The data were quantitated by PhosphorImager analysis (Molecular Dynamics).
For metabolic labeling, either untreated or cisplatin-treated (24 h) cells were exposed to methionine/cysteine free media for 30 min and then pulse-labeled for 80 min with 0.3 mCi of [35S]methionine and cysteine. The cells were then washed and fed fresh media. Immunoprecipitations were performed as described above. After SDS/PAGE analysis, the gel was fixed for 30 min in 25% isopropanol, 10% acetic acid, then in Amplify (Amersham) for 30 min. The gel was vacuum dried on Whatman filter paper and exposed to film at −80°C with an intensifier screen. The data were quantitated as described above.
Northern Assay.
Total cellular RNA was isolated by using a single-step acid guanidinium thiocyanate-phenol-chloroform extraction (Ultraspec II, Biotecx Laboratories, Houston). The mRNA was resolved on agarose-formaldehyde gels, transferred to Hybond-N nylon membrane (Amersham), and cross-linked to the membrane with a Stratalinker (Stratagene). The blot was hybridized to randomly primed cDNA probes (Prime-It II, Stratagene) as described (28), and exposed to film at −80°C with an intensifier screen. The data were quantitated as described above.
DNA Degradation Assay.
The cells were treated with cisplatin for 24 h, harvested, and washed twice in cold TBS (100 mM Tris, pH 7.4/154 mM NaCl). DNA from 7.2 × 105 cells was isolated as described (16); the RNase treatment was extended to 2 h.
Apoptosis Assay.
Cells were treated continuously with cisplatin in 24-well plates (3–5 × 104 cells per well). At each time point, both attached and detached cells were collected and resuspended in PBS. To check for membrane perforation, an aliquot was stained in 0.2% trypan blue and counted on a hemacytometer. The remainder of the cells were fixed in cold methanol, stained with 1 μg/ml of DAPI in PBS, and mounted on a slide. Chromatin structure was observed under a fluorescent light microscope (Axioscope, Zeiss Germany).
Fluorescence-Activated Cell Sorter Analysis.
At each time point, both attached and detached cells were collected, washed with PBS, fixed by drop-wise addition of ethanol, and stored at 4°C. The cells were washed once with PBS followed by resuspension in PBS containing 50 μg/ml propidium iodide (Sigma) and 0.2 mg/ml RNase (Boehringer Mannheim). The samples were incubated at 37°C for 30–60 min and then analyzed on a Becton Dickinson FACScan. Quantitation of cell cycle distribution was performed with modfitlt software.
Clonogenic Assays.
Dilute cells were plated on 6-well plates (150–200 cells per well) the day before cisplatin treatment. Exposure to drug was either for 2 h, at which point the cells were washed with PBS and fed fresh media, or continuous. Colonies were allowed to grow for 5–7 days, stained with 1% methylene blue (Fluka) in 50% ethanol, and counted.
RESULTS
Cisplatin Induction of the p53 Protein.
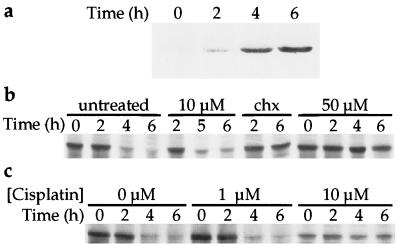
Previous reports have demonstrated that DNA-damaging agents induce the p53 protein in testicular tumor cells (16, 29, 30). In the present study, immunoprecipitation followed by Western blot analysis revealed that continuous treatment of the F9 teratocarcinoma cells with cisplatin resulted in a rapid (2 h) elevation (2–6-fold) in the steady-state levels of the p53 protein (Fig. 1a). The amount of cellular p53 protein increased over a 6 h period, reaching levels >10-fold higher than in untreated cells, followed by a decrease observed 4 h later (data not shown).
Figure 1.
Cisplatin induction and stabilization of p53 in teratocarcinoma cells. (a) F9 cells were treated with 10 μM cisplatin and whole protein extracts were prepared at the times indicated. Immunoprecipitation followed by Western blot analysis was performed as described in Materials and Methods. (b) The F9 cells were pulse-labeled as described in Materials and Methods, followed by exposure to cisplatin or cycloheximide (chx, 50 μg/ml) as indicated. (c) Cisplatin was added to the media 24 h before the pulse. Less total protein was immunoprecipitated for the time course of the cells treated with 10 μM cisplatin.
One possible explanation for the cellular accumulation of p53 protein is drug-induced protein stabilization (16, 31). To test whether such a mechanism is active in cisplatin-treated cells, the half-life of the p53 protein was determined by pulse-labeling the F9 cells before drug exposure (Fig. 1b). In untreated cells, the half-life of the p53 protein was 3–4 h, as reported (32), and the requirement for active protein synthesis in p53 degradation was demonstrated by stabilization of the protein after the addition of cycloheximide to the media (Fig. 1b). The 10 μM dose of cisplatin that produced a rapid increase in the steady-state levels of p53 did not significantly alter the half-life of the protein, although a higher dose (50 μM) did stabilize p53. When the cells were exposed to 10 μM cisplatin 24 h before the radioactive pulse, many of them died and detached from the plate, resulting in less total protein for immunoprecipitation (Fig. 1c). It is clear, however, that a 24-h dose of 10 μM, but not 1 μM, cisplatin stabilized the p53 protein. The mechanism of cisplatin-mediated p53 protein induction in the F9 cells was further examined by Northern blot analysis. No significant changes in the p53 mRNA levels were observed after exposure of the cells to the drug (Fig. 2).
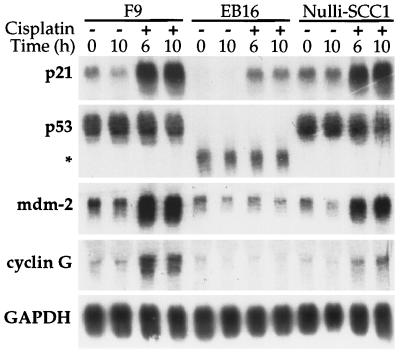
Figure 2.
Cisplatin induction of p21, but not cyclin G or mdm-2, in a p53-independent manner. The p53-wild-type (F9, Nulli-SCC1), and p53-mutant (EB16) teratocarcinoma cell lines were treated with 10 μM cisplatin. Total RNA was isolated at the indicated time points; 12 μg of RNA was resolved on a 1.1% agarose-formaldehyde gel, transferred to nitrocellulose, and probed with randomly primed cDNA. Two different blots were prepared from the same samples of RNA, one was probed with the p21, p53, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes, the other with the cyclin G and mdm-2 genes. The asterisk indicates the truncated p53 gene from the p53-mutant mouse.
Cisplatin Activation of p53 Activity.
To investigate whether the p53 protein in the F9 cells exposed to cisplatin was active, a northern blot containing total RNA was probed for the expression of p21, a transcriptional target of p53 (33). The p21 message was elevated after a 2 h treatment (data not shown) and continued to increase over the 10-h period of exposure (Fig. 2). In addition, the induction of two other p53-regulated genes, mdm-2 and cyclin G (34, 35), was observed. Similarly, the RNA of all three genes was induced by cisplatin in the p53-wild-type Nulli-SCC1 teratocarcinoma cell line. The blots were also analyzed for expression of the glyceraldehyde 3-phosphate dehydrogenase gene to control for gel loading and to demonstrate that cisplatin damage did not enhance gene expression nonspecifically.
Induction of p53-Dependent Apoptosis in Teratocarcinoma Cells.
One of the p53-regulated responses to genotoxic stress is initiation of the programmed cell death pathway (10), an effect that could be involved in the cytotoxic mechanism of cisplatin (36). To determine whether cisplatin kills the testicular teratocarcinoma cells through an apoptotic pathway, chromatin condensation, an early indicator of apoptosis (37), was evaluated in cells stained with DAPI. The nuclear change was observed in a significant number of the p53-normal cells after a 24-h exposure to 10 μM cisplatin (Fig. 3). After treatment for 2 days, most of the cells (>80%) were apoptotic and had detached from the plate. The results of the DAPI assays correlate with the loss of viable cells as determined by a trypan blue exclusion test (data not shown), suggesting that at these time points the teratocarcinoma cells were killed solely by apoptosis. The requirement for functional p53 in the apoptotic response was examined in a cell line derived from testicular tumors of mice with a defective p53 gene (16). After 48 h of drug treatment, the majority of these p53-mutant teratocarcinoma cells remained attached to the plate and exhibited very little of the altered chromatin structures (Fig. 3).
Figure 3.
Induction of p53-dependent apoptosis by cisplatin. Three murine testicular teratocarcinoma cell lines, the p53-normal F9 (○) and Nulli-SCC1 (◊) lines and the p53-mutant EB16 line (▪), were treated continuously with 10 μM cisplatin. At the time points indicated, the cells were collected and stained with DAPI to quantitate cells with condensed chromatin.
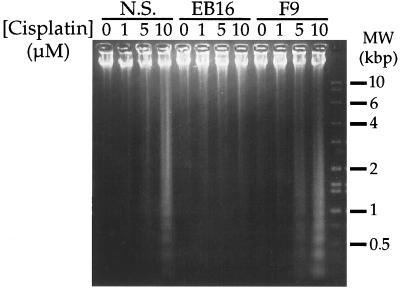
Another feature of programmed cell death is endonuclease digestion of genomic DNA (37). Resolution of the DNA from cisplatin-treated F9 and Nulli-SCC1 cells by agarose gel electrophoresis revealed the characteristic ladder of oligomers (Fig. 4). Such fragmented DNA was not present upon similar analysis of the p53-mutant EB16 cells.
Figure 4.
Cisplatin induction of p53-dependent DNA fragmentation. DNA from p53-wild-type (Nulli-SCC1 and F9) or p53-mutant (EB16) mouse teratocarcinoma cells, was isolated after 24 h continuous exposure to the concentrations of cisplatin indicated, and resolved on a 1.1% agarose gel.
Effect of Cisplatin on Cell Cycle.
To investigate further the response of the teratocarcinoma cells to cisplatin, flow cytometry was used to analyze the cell cycle distribution during exposure to the drug (Table 1 and data not shown). The use of a 1-μM dose of cisplatin allowed the effects of the drug on the cell cycle to be examined without immediately killing the cells. Treatment of F9 cells with this low dose for 24 h resulted in an accumulation of cells in the G2 phase. After 2 days of exposure, the cells were still arrested in G2, although some evidence for cell death (cellular debris) was observed. Significant cell death was apparent after a 1 day treatment with a higher dose (10 μM) of cisplatin and was complete after 48 h, in accord with the experiments described above (Fig. 3). The p53-wild-type Nulli-SCC1 cells exhibited similar cell cycle profiles, a low dose of cisplatin activated G2 arrest and some cell death, whereas a higher dose rapidly killed the cells.
Table 1.
Consequences of treating teratocarcinoma cells with cisplatin
| Dose, μM | Time, day | F9
|
Nulli-SCC1
|
EB16
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | S | G2/M | debris | G1 | S | G2/M | debris | G1 | S | G2/M | debris | ||
| 0 | 0 | 21 | 62 | 17 | 2 | 31 | 51 | 18 | 5 | 43 | 45 | 12 | 1 |
| 1 | 1 | 2 | 51 | 47 | 2 | 10 | 63 | 27 | 7 | 14 | 40 | 46 | 17 |
| 1 | 2 | 13 | 5 | 82 | 54 | 9 | 32 | 59 | 19 | 16 | 33 | 51 | 34 |
| 10 | 1 | 31 | 68 | 1 | 43 | 30 | 40 | 30 | 59 | 100* | 0 | 13 | |
| 10 | 2 | 49 | 38 | 13 | 95 | 48 | 29 | 23 | 75 | 100* | 0 | 26 | |
| 10 | 4 | 100* | 0 | 23 | |||||||||
Note. The p53 wild-type (F9 and Nulli-SCC1) and p53-mutant (EB16) murine teratocarcinoma cells lines were treated continuously with cisplatin. At the time points indicated, the cells were collected and analyzed by FACScan as described in Materials and Methods. *In analysis of the cell cycle of EB16 cells treated with 10 μM cisplatin, the fraction of cells of the G1 or S phase of the cell cycle could not be confidently determined.
Cell Cycle Response of p53-Mutant Cells.
The effect of cisplatin on the p53-mutant teratocarcinoma cells was also examined. A low dose of cisplatin caused accumulation of the cells in the G2 phase of the cell cycle (Table 1 and data not shown). In contrast, cell cycle analysis revealed that the EB16 cells treated with 10 μM cisplatin for 1 day accumulated in the G1/early S phase of the cell cycle. The block in the cell cycle was sustained through 4 days of continuous drug treatment and only a small amount of cellular debris was observed. This result was surprising because one of the mediators of G1 arrest is the p53-regulated p21 gene product (38–40). There is evidence, however, for a p53-independent pathway of p21 induction and cell cycle arrest (9, 41). In fact, in our system, Northern blot analysis revealed that the p21 message was induced during cisplatin treatment of the EB16 testicular teratocarcinoma cells, even in the absence of wild-type p53 (Fig. 2), providing a possible explanation for the observed G1 arrest. A similar p53-independent induction of two other p53-regulated genes, mdm-2 and cyclin G (34, 35), was not observed in the mutant cell line (Fig. 2).
Cisplatin Cytotoxicity.
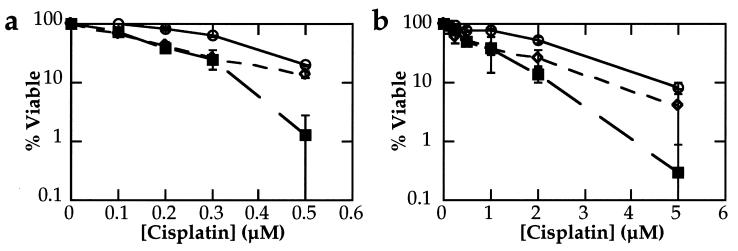
Colony forming assays were used to determine the overall sensitivity of the p53-wild-type and p53-mutant mouse testicular teratocarcinoma cell lines. This type of experiment differs from the apoptosis assays described above in that it only assesses viable cell division and does not distinguish between various mechanisms of cell death and/or cell cycle arrest. Such a limitation was noted in a recent study of human colon cancer cells in which a p21 mutation sensitized xenografts to γ-irradiation, an effect not observed by clonogenic assay (42). However, the response of cell lines tested by this type of in vitro assay does reflect the differential chemosensitivity of tumors treated in vivo (30). In the present study, the cells were either treated for 2 h or continuously exposed to cisplatin and grown until visible colonies had formed. Under both conditions, no significant difference was observed in the sensitivity of the different cell lines to cisplatin (Fig. 5), regardless of p53 status. Although the plating efficiencies of the three cell lines varied, for example in the experiments shown they ranged from 46–74% (Fig. 5a) and 13–49% (Fig. 5b), the p53 mutant cells did not have a consistently lower plating efficiency when compared with the other cell lines.
Figure 5.
Colony formation assays. The p53-wild-type F9 (○) and Nulli-SCC1 (◊) and p53-mutant EB16 (▪) teratocarcinoma cell lines were treated either continuously (a) or for 2 h (b) with cisplatin. After incubation for 5–7 days, visible colonies were stained with methylene blue and counted. For each cell line, the numbers were normalized to the untreated data point. Bars ± 1 esd.
DISCUSSION
Previous work investigated the relationship between the status of the p53 gene and cellular sensitivity to cisplatin, with contradictory results (19–27). The present study addresses the issue of cell type by investigating the p53 response to cisplatin in murine testicular teratocarcinoma cells. These cell lines resemble testicular cancer, for which cisplatin is a particularly effective treatment, in that the product of a wild-type p53 gene is overexpressed (32, 43).
Treatment of the p53+/+ teratocarcinoma cells lines with etoposide both induces and activates the p53 protein (16). Similarly, we report here that the p53 protein was elevated and transcriptionally active in the testicular teratocarcinoma cells after a 2 h exposure to cisplatin. One mechanism of p53 cellular accumulation is inhibition of the protein degradation pathway (28), and in support of this hypothesis the half-life of the p53 protein was prolonged in cisplatin-treated cells. In contrast to the rapid protein induction observed at the same drug concentration, however, protein stabilization was not immediately apparent and was only manifest after a much longer exposure to the drug (24 h). This result, coupled with the observation that the DNA-damaging agent did not result in increased levels of p53 mRNA, suggests that the rapid induction of the p53 protein by cisplatin occurs at least in part through a translational mechanism. Experiments with human leukemic blast cells also provided evidence for translational control of p53 expression (44). Cells collected from different patients with acute myelogenous leukemia displayed variable levels of p53 protein expression that did not correlate with the levels of mRNA. In addition, a comparison of acute leukemia cell lines demonstrated that the p53 mRNA was preferentially associated with the large polysomes in cell populations with larger ratios of p53 protein to mRNA. In fact, it is possible that p53 is involved in autoregulatory control of its expression, because the protein binds to, and inhibits the translation of, its own mRNA (45, 46).
In a similar fashion to other DNA-damaging agents, cisplatin activates the programmed cell death pathway in the mouse testicular teratocarcinoma cells. Immediate activation of apoptosis, however, was avoided by the use of a smaller dose of cisplatin, which permitted an investigation of the effects of the drug on the cell cycle. Both the F9 and Nulli-SCC1 cells accumulated in the G2 phase during drug treatment. A G2 block is a characteristic effect of cisplatin treatment and is considered to be a prerequisite for apoptosis induced by a low dose of cisplatin (36, 47, 48). In addition, there is evidence that cisplatin-induced apoptosis depends on the active cell cycle of proliferating cells, a requirement that was not observed for etoposide (49). Although there is support for p53 involvement in the G2/M checkpoint (9), it is unlikely that the protein participates in the cisplatin-mediated block of the cell cycle observed here, because some G2 accumulation was observed in the p53-mutant cells exposed to the low dose. The lack of a functional p53 may be more relevant to the fate of the cells after cell cycle arrest. Whereas the clonogenic experiments demonstrate that the cellular proliferation of all of the teratocarcinoma cells is inhibited by the low dose of cisplatin, preliminary studies suggest that, in contrast to the p53-wild type cells, the p53-mutant cells do not undergo programmed cell death (data not shown). Nonapoptotic death after G2 arrest would be consistent with a report suggesting that murine leukemia cells can die by a mechanism involving failure to overcome the cell cycle block when treated with a dose of cisplatin that does not immediately activate apoptosis (50).
The rapid drug-induced apoptosis observed in the F9 and Nulli-SCC1 cells depends on functional p53, because the characteristic chromatin condensation and DNA fragmentation were not observed in the p53-mutant cells. Instead, cell cycle analysis revealed a prolonged block in G1/early S phase in the mutant cells. This result was unexpected because an effector of the G1 checkpoint is the cyclin-dependent kinase inhibitor, p21 (38, 40), which is transcriptionally regulated by p53 (33), and is specifically induced in p53-dependent cellular responses to DNA damage (39). In the p53-mutant teratocarcinoma cells, however, exposure to cisplatin caused an elevation in p21 expression, a result that provides a possible explanation for the observed arrest in the cell cycle. Several reports have proposed the existence of p53-independent G1 arrest and p21 induction (9, 22, 41, 51). This pathway seems to be selectively activated by different DNA-damaging agents, depending on the cellular context. For example, UV light, but not ionizing radiation, induced p21, and G1 arrest in p53-deficient fibroblasts from Li-Fraumeni syndrome patients (51), but G1 accumulation was observed in irradiated p53−/− T lymphoma cells expressing Bcl-2 (41).
A p53-independent induction of p21 was not detected in human testicular tumor lines exposed to cisplatin (27). In that study, however, similar chemosensitivities of cells with both functional and nonfunctional p53 were observed and attributed to the activation of p53-independent apoptosis. Therefore, with respect to the lack of p53-involvement in ultimate sensitivity to drug treatment, the results from the human and mouse testicular tumor cell lines are consistent, but important differences in cellular mechanisms may exist. The p53-mutant mouse teratocarcinoma cells did not undergo cisplatin-induced apoptosis, although clonogenic assays revealed an overall sensitivity similar to that of the p53-wild-type cell lines. It is conceivable that the p53-mutant cells experience a permanent cell-cycle arrest mediated by p21, which has been linked with senescence (52, 53). Preliminary cell viability studies suggest that these cells eventually die (data not shown), however, making this possibility unlikely.
Whatever the reason for the loss of clonogenic capability, these experiments demonstrate that, despite the failure of cisplatin to induce programmed cell death in the p53-mutant cells, this deficiency does not confer resistance. In fact, it is possible that the p53-mutant cells are slightly more sensitive than the p53-normal cells in this assay (Fig. 5). A similar decrease in resistance was observed in fibroblast cells treated with paclitaxel, an anticancer drug that stabilizes cellular microtubules, when the cells were depleted of functional p53 (54). In contrast, preclinical trials demonstrated that paclitaxel was effective in the treatment of human tumor xenografts with and without p53 mutations (55). The inconsistency was explained by the observation that paclitaxel activates the release of tumor necrosis factor α by murine macrophages. Tumor necrosis factor α, which would have been induced in the in vivo experiments but not in the in vitro cell culture experiments, activates p53-independent apoptosis (55). These experiments and others (42) demonstrate that it is not always possible to extrapolate experimental data from cell culture to whole animals. Although the data presented here suggest that the unusual sensitivity of testicular cancer to cisplatin is not due solely to a wild-type p53 gene, it is impossible to make such a conclusion without in vivo evidence. A mutation in the p53-gene is an indicator of poor prognosis in patients with well or moderately differentiated ovarian cancer (56); however, overexpression of both wild-type and mutated p53 was found in advanced ovarian carcinomas refractory to cisplatin-based chemotherapy (57). In light of the results presented, both here and elsewhere (27), it would be informative to investigate whether the few testicular cancer patients who fail cisplatin-based chemotherapy have developed p53 mutations.
Acknowledgments
We are grateful to A. J. Levine for the p53−/− cell line and the anti-p53 antibody, and to H. Ploegh for the fixed Staph A. We thank L. Attardi, R. Shaw, and S. Lutzker for technical advice and many helpful discussions. This work was supported by grant CA 34992 from the National Cancer Institute. T.J. is an Associate Investigator and D.B.Z. a predoctoral fellow of the Howard Hughes Medical Institute.
ABBREVIATIONS
- chx
cycloheximide
- cisplatin
cis-diamminedichloroplatinum(II)
- DAPI
4′,6-diamidino-2-phenylindole
- Staph A
Staphylococcus aureus
References
- 1.Richie J P. In: Campbell’s Urology. Walsh P C, Retik A B, Stamey T A, Vaughan E D Jr, editors. Vol. 2. Philadelphia: Saunders; 1992. pp. 1222–1262. [Google Scholar]
- 2.Bosl G J, Motzer R J. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 3.Loehrer P J, Einhorn L H. Ann Intern Med. 1984;100:704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy G K. AHFS Drug Information 94. Bethesda, MD: Am. Soc. Hospital Pharmacists; 1994. [Google Scholar]
- 5.Young R C. In: Drug Resistance in Cancer Therapy. Ozols R F, editor. Boston: Kluwer Academic; 1989. pp. 1–12. [Google Scholar]
- 6.Zamble D B, Lippard S J. Trends Biochem Sci. 1995;20:435–439. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
- 7.Scanlon K J, Kashani-Sabet M, Tone T, Funato T. Pharmacol Ther. 1991;52:385–406. doi: 10.1016/0163-7258(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 8.Chu G. J Biol Chem. 1994;269:787–790. [PubMed] [Google Scholar]
- 9.Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 10.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 11.Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 12.Levine A J, Momand J, Finlay C A. Nature (London) 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 13.Lowe S W. Curr Opin Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y D, Jiafu Z, Xi Q S, Yongjiang M, Xiulong Z, Daizong L, Jianren G. J Urol. 1993;150:884–886. [Google Scholar]
- 15.Fleischhacker M, Strohmeyer T, Imai Y, Slamon D J, Koeffler H P. Mod Pathol. 1994;7:435–439. [PubMed] [Google Scholar]
- 16.Lutzker S G, Levine A J. Nat Med. 1996;2:804–810. doi: 10.1038/nm0796-804. [DOI] [PubMed] [Google Scholar]
- 17.Bártková J, Bártek J, Lukás J, Vojtěšek B, Stašková Z, Rejthar A, Kovařík J, Midgley C A, Lane D P. Int J Cancer. 1991;49:196–202. doi: 10.1002/ijc.2910490209. [DOI] [PubMed] [Google Scholar]
- 18.Riou G, Barrois M, Prost S, Terrier M J, Theodore C, Levine A J. Mol Carcinog. 1995;12:124–131. doi: 10.1002/mc.2940120303. [DOI] [PubMed] [Google Scholar]
- 19.Anthoney D A, McIlwrath A J, Gallagher W M, Edlin A R M, Brown R. Cancer Res. 1996;56:1374–1381. [PubMed] [Google Scholar]
- 20.Perego P, Giarola M, Righetti S C, Supino R, Caserini C, Delia D, Pierotti M A, Miyashita T, Reed J C, Zunino F. Cancer Res. 1996;56:556–562. [PubMed] [Google Scholar]
- 21.Gallagher W M, Cairney M, Schott B, Roninson I B, Brown R. Oncogene. 1997;14:185–193. doi: 10.1038/sj.onc.1200813. [DOI] [PubMed] [Google Scholar]
- 22.De Feudis P, Debernardis D, Beccaglia P, Valenti M, Graniela Siré E, Arzani D, Stanzione S, Parodi S, D’Incalci M, Russo P, et al. Br J Cancer. 1997;76:474–479. doi: 10.1038/bjc.1997.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiwara T, Grimm E A, Mukhopadhyay T, Zhang W-W, Owen-Schaub L B, Roth J A. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]
- 24.Fan S, Smith M L, Rivet II D J, Duba D, Zhan Q, Kohn K W, Fornace Jr A J, O’Connor P M. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 25.Hawkins D S, Demers G W, Galloway D A. Cancer Res. 1996;56:892–898. [PubMed] [Google Scholar]
- 26.O’Connor P M, Jackman J, Bae I, Myers T G, Fan S, Mutoh M, Scudiero D A, Monks A, Sausville E A, Weinstein J N, et al. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 27.Burger H, Nooter K, Boersma A W M, Kortland C J, Stoter G. Int J Cancer. 1997;73:592–599. doi: 10.1002/(sici)1097-0215(19971114)73:4<592::aid-ijc22>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Shackleford G M, Varmus H E. Cell. 1987;50:89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- 29.Küpper M, Köster M, Schmidt-Spaniol I, Wagner-Gillen I, Issinger O-G. Cell Mol Biol Res. 1994;40:587–592. [PubMed] [Google Scholar]
- 30.Chresta C M, Masters J R W, Hickman J A. Cancer Res. 1996;56:1834–1841. [PubMed] [Google Scholar]
- 31.Oren M, Maltzman W, Levine A J. Mol Cell Biol. 1981;1:101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reich N C, Oren M, Levine A J. Mol Cell Biol. 1983;3:2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Bayle J H, Olson D, Levine A J. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto K, Beach D. EMBO J. 1994;13:4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eastman A. Cancer Cells. 1990;2:275–280. [PubMed] [Google Scholar]
- 37.Wyllie A H. Nature (London) 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 38.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 39.El-Deiry W S, Harper J W, O’Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, et al. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 40.Waldman T, Kinzler K W, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 41.Strasser A, Harris A W, Jacks T, Cory S. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 42.Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J. Nat Med. 1997;3:1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- 43.Pennica D, Goeddel D V, Hayflick J S, Reich N C, Anderson C W, Levine A J. Virology. 1984;134:477–482. doi: 10.1016/0042-6822(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 44.Fu L, Minden M D, Benchimol S. EMBO J. 1996;15:4392–4401. [PMC free article] [PubMed] [Google Scholar]
- 45.Ewen M E, Miller S J. Biochim Biophys Acta. 1996;1242:181–184. doi: 10.1016/0304-419x(95)00010-d. [DOI] [PubMed] [Google Scholar]
- 46.Mosner J, Mummenbrauer T, Bauer C, Sczakiel G, Grosse F, Deppert W. EMBO J. 1995;14:4442–4449. doi: 10.1002/j.1460-2075.1995.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorenson C M, Eastman A. Cancer Res. 1988;48:6703–6707. [PubMed] [Google Scholar]
- 48.Demarcq C, Bunch R T, Creswell D, Eastman A. Cell Growth Differ. 1994;5:983–993. [PubMed] [Google Scholar]
- 49.Evans D L, Tilby M, Dive C. Cancer Res. 1994;54:1596–1603. [PubMed] [Google Scholar]
- 50.Ormerod M G, Orr R M, Peacock J H. Br J Cancer. 1994;69:93–100. doi: 10.1038/bjc.1994.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loignon M, Fetni R, Gordon A J E, Drobetsky E A. Cancer Res. 1997;57:3390–3394. [PubMed] [Google Scholar]
- 52.Di Leonardo A, Linke S P, Clarkin K, Wahl G M. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 53.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 54.Wahl A F, Donaldson K L, Fairchild C, Lee F Y F, Foster S A, Demers G W, Galloway D A. Nat Med. 1996;2:72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- 55.Lanni J S, Lowe S W, Licitra E J, Liu J O, Jacks T. Proc Natl Acad Sci USA. 1997;94:9679–9683. doi: 10.1073/pnas.94.18.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levesque M A, Katsaros D, Yu H, Zola P, Sismondi P, Giardina G, Diamandis E P. Cancer (Philadelphia) 1995;75:1327–1338. doi: 10.1002/1097-0142(19950315)75:6<1327::aid-cncr2820750615>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 57.Righetti S C, Della Torre G, Pilotti S, Menard S, Ottone F, Colnaghi M I, Pierotti M A, Lavarino C, Cornarotti M, Oriana S, Böhm S, Bresciani G L, Spatti G, Zunino F. Cancer Res. 1996;56:689–693. [PubMed] [Google Scholar]