Abstract
Aims
We sought to define the underlying mechanisms for atrial fibrillation (AF) during chronic heart failure (HF).
Methods and results
Preliminary studies showed that 4 months of HF resulted in irreversible systolic dysfunction (n = 9) and a substrate for sustained inducible AF (>3 months, n = 3). We used a chronic (4-month) canine model of tachypacing-induced HF (n = 10) to assess atrial electrophysiological remodelling, relative to controls (n = 5). Left ventricular fractional shortening was reduced from 37.2 ± 0.83 to 13.44 ± 2.63% (P < 0.05). Left atrial (LA) contractility (fractional area change) was reduced from 34.9 ± 7.9 to 27.9 ± 4.23% (P < 0.05). Action potential durations (APDs) at 50 and 90% repolarization were shortened by ∼60 and 40%, respectively, during HF (P < 0.05). HF-induced atrial remodelling included increased fibrosis, increased Ito, and decreased IK1, IKur, and IKs (P < 0.05). HF induced increases in LA Kv channel interacting protein 2 (P < 0.05), no change in Kv4.3, Kv1.5, or Kir2.3, and reduced Kir2.1 (P < 0.05). When ICa-L was elicited by action potential (AP) clamp, HF APs reduced the integral of ICa in control myocytes, with a larger reduction in HF myocytes (P < 0.05). ICaL measured with standard voltage clamp was unchanged by HF. Incubation of myocytes with N-acetylcysteine (a glutathione precursor) attenuated HF-induced electrophysiological alterations. LA angiotensin-1 receptor expression was increased in HF.
Conclusion
Chronic HF causes alterations in ion channel expression and ion currents, resulting in attenuation of the APD and atrial contractility and a substrate for persistent AF.
Keywords: Heart failure, Arrhythmia (mechanisms), Remodelling, K-channel
1. Introduction
Heart failure (HF) is a chronic irreversible syndrome and develops over months to years. HF is an increasingly prevalent disease and continues to result in substantial morbidity and mortality.1,2 A common co-morbidity during HF is atrial fibrillation (AF); HF increases the risk of developing AF by approximately four- to six-fold.3,4 Significantly, the development of AF in patients with advanced HF predicts a significantly increased risk of death.2,5
Although it has long been known that chronic HF increases the incidence of AF,4 the underlying causal mechanisms have not been well defined. Previous studies have evaluated HF-induced atrial remodelling after 2–6 weeks of HF induced by right ventricular (RV) tachypacing,6–8 and reported increased interstitial fibrosis, although myocytes from the right atrium had action potential (AP) prolongation accompanied by a modest decrement in ICa, with reductions in Ito and IKs.6,7 Inducible AF of seconds to minutes in duration occurs after this duration of HF.6,7
To examine the mechanisms whereby chronic HF induces a substrate for AF, we used a modification of a previously validated model of long-term, irreversible HF.9 Our data indicate that during chronic HF, atrial electrophysiological remodelling is distinct from that previously reported after 2–6 weeks of RV tachypacing. We report that this model results in a substrate for sustained AF, is accompanied by attenuation of atrial refractory period, and is attributable to a specific constellation of ion current abnormalities and shortening of the atrial AP duration (APD).
2. Methods
All procedures were approved by The Ohio State University Institutional Animal Care and Use Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). A total of 22 hound type dogs of either sex (2–3 years of age) had an RV pacemaker lead implanted in the RV apex as described previously.9 Following recovery from the pacemaker implant, the RV was paced at 180 bpm for 2 weeks; 200 bpm for the next 6 weeks, followed by 180 bpm for the duration of the protocol (modified Prevail 8086 pacemakers, Medtronic, Inc., Minneapolis, MN, USA). Sequential electrocardiograms, two-dimensional (2D) and M-mode echocardiograms, were performed at baseline and during brief periods of sinus rhythm during the pacing protocols, during butorphanol sedation (0.5 mg/kg, im).10
2.1. Pilot studies
Twelve dogs were used for in vivo pilot studies to assess reversibility of HF and inducibility of persistent AF. HF was induced as described above with a duration of 16–17 weeks of RV tachypacing. After 16–17 weeks of RV tachypacing, one subset (n = 9) had pacing terminated to assess reversibility of HF. A second subset (n = 3) was used for a pilot study (n = 3) to evaluate atrial arrhythmia inducibility using premature extrastimuli after 16 weeks of RV tachypacing; in this subset, persistent AF was induced in each animal. After AF induction, the RV pacemaker rate was reduced to 30 bpm, heart rhythm was assessed by electrocardiography daily for a week, and weekly thereafter for up to 3 months; ventricular rates were ∼140–170 bpm during AF.
2.2. Main study
On the basis of the results of the pilot studies, a group of RV tachypaced dogs (n = 10) were prepared to further assess mechanisms of atrial electrophysiological remodelling during chronic irreversible HF, using a duration (>16 weeks) when the pilot studies indicated the formation of a substrate for persistent AF. Atrial electrophysiology was assessed in vivo and in isolated left atrial (LA) appendage myocytes. Five age-matched dogs served as controls for these studies.
Atrial effective refractory periods (ERPs) and AF inducibility were assessed as described previously.10 To measure atrial contractility non-invasively, we adapted established methods to the canine LA.11,12 Images were obtained in the 2D parasternal long-axis view, and % fractional area change (FAC) calculated as FAC = (LA area end-systole − LA area end-diastole)/LA area end-systole × 100. Haemodynamic assessments were conducted in the main study just prior to euthanasia to evaluate end-diastolic pressure and peak left ventricular (LV) systolic pressure. Animals were pre-anaesthetized with butorphanol (0.4 mg/kg iv). Anaesthesia was sustained with isoflurane (1–1.5% with 100% oxygen). Body temperature was kept constant (37.0 ± 0.5°C) with a water-heated blanket. A 5 Fr catheter-tip manometers (model SPC-350, Millar Instruments Inc.) were inserted to the LV via carotid artery under the fluoroscopic guidance to obtain peak LV pressure (LVP) and end-diastolic pressure (EDP) in mmHg. ECG lead II was also obtained. All recordings were made on a physiological data acquisition system (MP100WSW, Biopac Systems, Inc., Santa Barbara, CA, USA), and measurements were obtained from the average of 10 consecutive beats.
After completion of the in vivo measurements, dogs were anaesthetized by intravenous injection of pentobarbital sodium;13 cardiac tissue was obtained from the LA for protein analyses, histology, and myocyte isolation. LA appendage myocytes were isolated using a modification of previously described methods14 by cannulation and perfusion of the left circumflex artery. After completion of the previously described method of perfusion with collagenase, the LA appendage was minced and placed into 5 mL of perfusate in a shaking water bath at 37°C for 5–15 min. Atrial myocytes were filtered and then centrifuged twice with incubation buffer (IB) to remove collagenase, prior to suspension in storage buffer. The myocytes were stored at room temperature in a standard IB solution containing (in mM): 118 NaCl, 4.8 KCl, 1.2 MgCl2, 1.2 KH2PO4, 0.68 glutamine, 10 glucose, 5 pyruvate, 1 CaCl2, along with 1 µmol/L insulin and 1% BSA until used. This typically yielded 40–60% rod-shaped myocytes with staircase ends and sharp margins. In some experiments, myocytes were stored in IB containing 10 mM N-acetylcysteine (NAC), a membrane-permeable precursor of glutathione. All myocyte experiments were conducted within 10 h of isolation.
2.3. Electrophysiological protocols
Data acquisition was performed with Clampex version 8.0 or 9.0 software (Axon Instruments, Union City, CA, USA) and Axopatch 200A patch clamp amplifiers (Axon Instruments Inc.). Perforated whole-cell patch-clamp (using amphotericin B) was used to minimize alterations in intracellular milieu during AP, AP clamp, and potassium current recordings. Myocytes were placed in a laminin-coated cell chamber (Cell Microcontrols, Norfolk, VA, USA) and superfused with solution containing (in mM): 135 NaCl, 5 MgCl2, 5 KCl, 10 glucose, 1 CaCl2, 5 HEPES, pH adjusted to 7.40 with NaOH, at a temperature of 36 ± 0.5°C. For AP recordings, the concentration of CaCl2 in the bath solution was increased to 1.8 mM. During potassium current recordings, L-type calcium current was blocked by the addition of 2 µM nifedipine to the superfusate. Borosilicate glass micropipettes (1.5–3 MΩ) were filled with pipette solution containing (in mM): 100 K+-aspartate, 40 KCl, 5 MgCl2, 5 EGTA, 5 HEPES, pH adjusted to 7.2 with KOH.
APs were recorded with perforated whole-cell patch techniques. APDs were measured from the average of the last 10 (steady-state) APs, obtained during a train of 25 APs at each stimulation rate.
For voltage-clamp experiments, only recordings with an access resistance <20 MΩ were included in the analyses; for measurements of drug-sensitive currents, only cells with less than a 20% change in access resistance were included in the analyses. Series resistance compensation (40–70%) was used for current recordings. All drug-sensitive currents were recorded after 3–5 min of drug superfusion, which in our pilot experiments resulted in steady-state current blockade.
Transient outward potassium current (Ito) was elicited by a series of 100 ms test potentials from −50 to +50 mV, from a holding potential of −60 mV as described previously.13 The steady-state current was used to define the sustained outward K+ current. Rapid (IKr) and slow (IKs) components of the delayed rectifier current were separated using d-sotalol (a selective IKr blocker) as described previously;13 4-aminopyridine (50 µM) was added to prevent potential contamination with IKur. IK1 was elicited as described previously15,16 and measured as the 2 mM barium-sensitive current.17
IKur was defined as the 4-aminopyridine-sensitive sustained outward potassium current and elicited from a holding potential of −40 mV using 10 mV voltage steps from −20 to +50 mV. A −40 mV holding potential with an 80 ms pre-pulse to +30 mV was used to inactivate Ito.13 The sustained 4-aminopyridine-sensitive IKur was measured as the steady-state difference current, recorded after a minimum of 4 min of superfusion with 4-aminopyridine.
2.3.1. L-type calcium current
Conventional whole-cell patch-clamp techniques were used for the measurement of calcium currents (ICa-L). ICa-L was measured using standard voltage step protocols, as described previously.18 Due to the significant alterations in the AP waveform, we observed during HF, AP clamp with both control and HF AP waveforms was used to elicit ICa-L; APs with the median APD90 were selected as the stimulation waveform. AP clamp with a train of 10 APs was used to elicit steady-state ICa-L. The pipette solution contained (mM): 125 CsCl, 20 tetraethylammonium chloride, 5 MgATP, 3.6 creatine phosphate, 10 HEPES, and 50 µM EGTA, pH 7.2. The bath solution (35°C) contained (mM): 157 tetraethylammonium chloride, 1 CaCl2, 0.5 MgCl2, 10 HEPES, pH 7.4. ICa-L recordings began 3 min after patch rupture. Preliminary experiments with nifedipine confirmed that ICa-L was isolated by these methods, and the current was quantified by integrating the area under the elicited current curve (ClampFit v.10, Axon Instruments).
2.4. Western blots
K+ channel subunits were assessed by immunoblot analysis. Twenty micrograms of protein from tissue homogenates were subjected to 4–20% SDS–PAGE, blotted onto nitrocellulose membranes (Bio-Rad Labs, Hercules, CA, USA). Anti-Kv1.5, -Kv channel interacting protein 2 (KChIP2), and -Kir2.1 antibodies were from Santa Cruz Biotech (Santa Cruz, CA, USA); anti-Kv4.3, -Kir2.3 and -angiotensin-1 (AT-1) receptor antibodies were from Alomone Laboratories (Jerusalem, Israel). Each sample was normalized to a protein control (GAPDH; Abcam, Cambridge, MA, USA) to normalize loading of each sample. Blots were developed with Super Signal West Pico (Pierce) and quantified using ImageJ (NIH, USA) and Origin 7 (OriginLab, Northampton, MA, USA) software.
2.5. Measurement of fibrosis
Atrial sections from canine heart were prepared as described previously19 and stained with Masson's trichrome to quantify fibrosis. The tissue sections were visualized using a Nikon microscope (TE2000-U, Japan) and areas occupied by blue pixels (fibrosis) were quantified using MetaMorph image analysis software (Molecular Devices, CA, USA).
2.6. Solutions and chemicals
All chemicals for buffer and stock solution preparation were purchased from Fisher Scientific (USA), Sigma Aldrich (St Louis, MO, USA), and Invitrogen Inc. (Carlsbad, CA, USA). Stock solutions of nifedipine, amphotericin B, and 4-aminopyridine were prepared fresh daily. Isoproterenol solutions were prepared daily from commercially available injectable solutions (Sanofi Winthrop Pharmaceuticals, New York, NY, USA). d-Sotalol was obtained from Merck Research Laboratories (West Point, PA, USA). All nifedipine, isoproterenol, and amphotericin B solutions were protected from exposure to light.
2.7. Statistical analysis
Acquired data were analysed using Clampfit 8.0 (Axon Instruments) and Origin 6.1 (OriginLab). Currents were normalized to cell capacitance and are expressed as pA/pF. Action potential durations (APDs) and current densities were analysed by ANOVA with post hoc least significant difference testing as appropriate (SAS for Windows v9.1, Cary, NC, USA). All data are presented as mean ± SE.
3. Results
3.1. Pilot studies
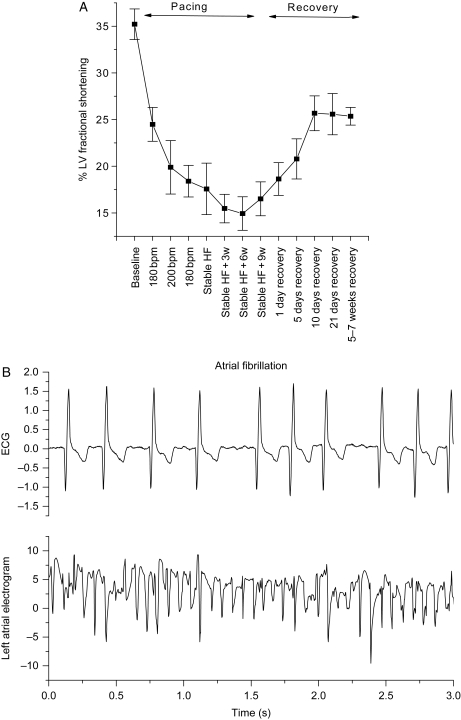
In pilot studies, we observed irreversibility of the fractional shortening induced by 16 weeks of RV tachypacing (Figure 1). Compared with baseline, RV tachypacing produced significant reductions in fractional shortening, and when tachypacing was stopped after 16 weeks, fractional shortening remained significantly reduced from baseline for 5–7 weeks (P < 0.05). Thus, our model of RV tachypacing-induced HF results in irreversible systolic dysfunction. At the same time point (16 weeks of RV tachypacing), AF was induced by atrial premature stimulation and was sustained for 3 months in all three animals tested (Figure 1).
Figure 1.
Four months of continuous RV tachypacing results in irreversible systolic dysfunction and a substrate for inducible AF. (A) Fractional shortening (%) data obtained from nine dogs, monitored during the tachypacing period (HF, 16 weeks) and during recovery after cessation of tachypacing for up to 7 weeks. (B) Representative ECG and atrial electrogram recorded simultaneously during persistent AF induced by programmed electrical stimulation.
3.2. Main study
3.2.1. Cardiac structure and function
After 4 months of RV tachypacing, there were significant alterations in ventricular function and dimensions (Table 1, P < 0.05), accompanied by significant reductions in LA emptying (FAC, P < 0.05). Haemodynamic alterations were consistent with HF (Table 1).
Table 1.
Echocardiographic and haemodynamic parameters in HF animals
| Baseline | Heart failure | |
|---|---|---|
| Echocardiographic parameters | ||
| Ventricular | ||
| Left ventricular dimension (cm) | ||
| Diastole | 3.37 ± 0.17 | 3.84 ± 0.51* |
| Systole | 2.40 ± 0.07 | 3.37 ± 0.45* |
| Fractional shortening (%) | 37.2 ± 0.83 | 13.44 ± 2.63* |
| Atrial | ||
| LA area (cm2) | ||
| End-diastole | 4.68 ± 1.16 | 5.31 ± 1.32 |
| End-systole | 7.16 ± 1.53 | 7.34 ± 1.62 |
| Fractional area change (%) | 34.9 ± 7.9 | 27.9 ± 4.23* |
| Control | Heart failure | |
| Haemodynamic parameters (mmHg) | ||
| End-diastolic pressure | 6.9 ± 0.49 | 10.4 ± 1.53* |
| Peak left ventricular pressure | 123.5 ± 3.66 | 97.3 ± 6.56* |
*P < 0.05.
There was a significant HF-induced increase in LA interstitial fibrosis from 0.54 ± 0.03% in controls to 9.3 ± 0.28% in HF (P < 0.05). The distribution of the fibrosis in HF samples was diffuse, with focal regions of endocardial fibrosis (see Supplementary material online, Figure S1). Myocytes from the LA appendage of the HF group were hypertrophied; capacitance increased from 95 ± 4 pF in controls to 146 ± 11 pF in HF (P < 0.05).
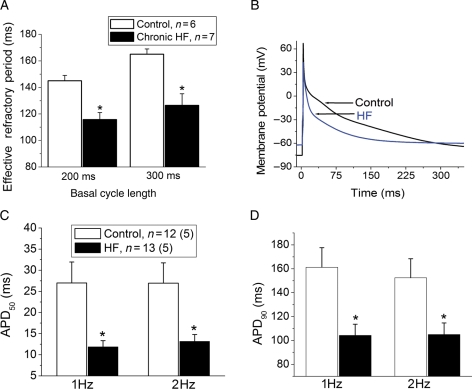
3.2.2. Atrial refractoriness and atrial myocyte APs
In vivo atrial ERPs were significantly shorter in the HF group compared with controls (Figure 2A, P < 0.05), which was paralleled by a reduction in atrial myocyte APDs (Figure 2B). Depolarization of the resting potential was seen in the HF atria group compared with controls (HF atria: −68 ± 3 mV vs. control: −74 ± 2 mV, P < 0.05). The APDs at 50 and 90% repolarization (APD50 and APD90, respectively) were significantly shorter in the HF atrial myocytes compared with controls (Figure 2C and D).
Figure 2.
Four-month tachypacing induced HF shortens in vivo atrial ERP and myocyte APD. (A) Reduced atrial ERP in HF group after 16–18 weeks of RV tachypacing (n = 7 animals) vs. control (n = 6 animals), at two basal cycle lengths of 300 and 200 ms respectively. (B) Representative AP traces in control (black) and HF (blue) myocytes, respectively. Note the depolarization in resting membrane potential in HF myocyte compared with control. (C and D) Average APD at 50% (APD50) and 90% (APD90) repolarization, respectively, where n is the number of myocytes, and (n) is the number of animals in each group. (*P < 0.05 vs. control).
3.2.3. Potassium currents and channels
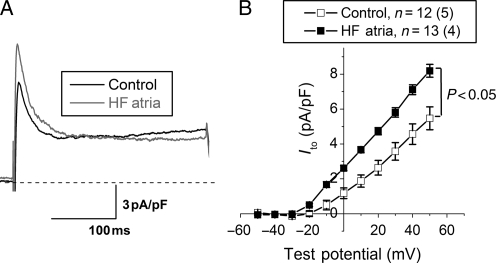
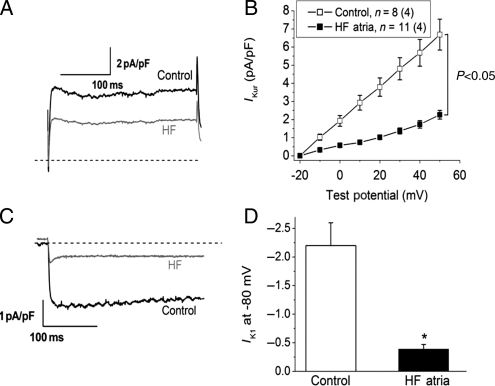
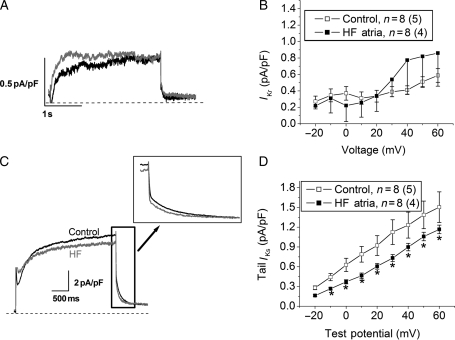
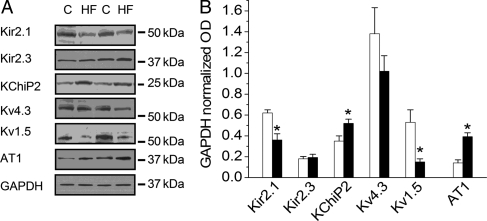
Ito was increased in myocytes from the HF atria group (Figure 3, P < 0.05 vs. control). HF significantly reduced IKur in atrial myocytes relative to controls (Figure 4A and B, P < 0.05). The inward component of the inward rectifier current (IK1) was reduced in HF (Figure 4C and D), whereas outward IK1 at −60 mV did not differ between the two groups (0.4 ± 0.02 vs. 0.39 ± 0.03 pA/pF in control and HF, respectively). Measurements of IKr and IKs revealed a significant HF-induced reduction in IKs, with no effect on IKr (Figure 5). Alterations in ion currents were further assessed by measurement of ion channel subunits. HF induced no change in Kv4.3, but significantly increased KChIP2 expression (Figure 6). Both Kv1.5 and Kir2.1 were significantly reduced during HF, whereas Kir2.3 was preserved.
Figure 3.
HF results in increased transient outward K+ current (Ito). (A) Transient outward K+ current traces from the two groups recorded in response to a depolarizing voltage step to +50 mV from a holding potential of −60 mV. The average current–voltage relationship in the two groups is shown in (B) (P < 0.05 vs. control). n indicates the number of myocytes, and (n) the number of animals in each group.
Figure 4.
HF causes reductions in atrial ultra rapid (IKur) and inward rectifier current (IK1) densities. (A) IKur traces from control (black) and HF (grey), respectively, elicited by a depolarizing voltage step to +50 mV from a holding potential of −40 mV. (B) Average current density of 4-aminopyridine-sensitive IKur in the two groups (P < 0.05 vs. control). (C) Inward rectifier current trace (barium-sensitive current) recorded in response to a hyperpolarizing voltage step to −80 mV from −40 mV. (D) Average inward IK1 density at −80 mV in the two groups (*P < 0.05 vs. control). n indicates the number of myocytes, and (n) the number of animals in each group.
Figure 5.
HF reduces the slow component of the delayed rectifier current (IKs), whereas the rapid component (IKr) is unchanged. (A) IKr traces elicited from control (black) and HF (grey) myocytes. (B) Average IKr density in the two groups (P = NS). (C) IKs traces from the two groups, elicited by a depolarizing voltage step to +50 mV from −50 mV. (D) Average tail IKs density in the two groups (*P < 0.05 vs. control).
Figure 6.
LA potassium channel subunit expression is altered, and AT-1 receptor expression is increased during HF. (A) Representative western blots. (B) Pooled data normalized to GAPDH for Kir2.1, Kir2.3, K+ channel interacting protein (KChiP2), Kv4.3, Kv1.5, and AT-1 receptors. (*P < 0.05 vs. control, n = 6–9).
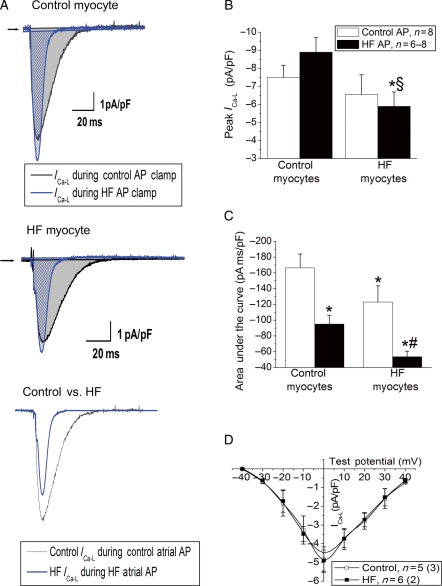
3.2.4. Calcium current
Since the APD during HF was significantly attenuated, we hypothesized that this would alter sarcolemmal ICa. Therefore, L-type calcium current was recorded using representative (median) AP waveforms (shown in Figure 2B) to assess whether HF-induced attenuations of the APD reduced sarcolemmal calcium current. Application of each AP waveform on the matching myocyte type revealed significant differences in the morphology of ICa-L (Figure 7A). In control myocytes, there was no significant change in the peak amplitude of ICa-L with the two AP waveforms. However, application of the HF AP to the HF myocytes significantly attenuated peak ICa-L relative to the control AP in HF myocytes (Figure 7B, P < 0.05). Measuring the integral of the area under the peak ICa-L trace (Figure 7C) showed that the HF AP waveform significantly reduced the overall sarcolemmal calcium flux in both control and HF myocytes, but to a significantly greater extent in the HF myocytes (Figure 7, P < 0.05). There was no difference in ICa-L measured with standard voltage step protocols (Figure 7D).
Figure 7.
AP clamp reveals abnormal atrial calcium current during HF. (A) L-type calcium current elicited from a control and HF myocyte, recorded during a control and HF atrial AP clamp. (Top) Steady-state ICa in a control myocyte elicited by either control (black) or HF (blue) AP. (Centre) ICa in an HF myocyte elicited by either control (black) or HF (blue) AP. (Bottom) Comparison of ICa elicited in a control myocyte with control AP stimulation (black) to ICa elicited in an HF myocyte by HF AP stimulation. (B) Peak ICa from the two groups in response to the two different AP clamp stimuli (*P < 0.05 vs. control AP on control myocyte, §P < 0.05 vs. HF AP clamp on control myocyte). (C) Area under the ICa trace in the two groups (*P < 0.05 vs. control AP on control myocyte, #P < 0.05 vs. HF AP on control myocyte). (D) Current density–voltage relationship for L-type calcium current elicited by voltage step protocol, where n is the number of myocytes, and (n) is the number of animals in each group.
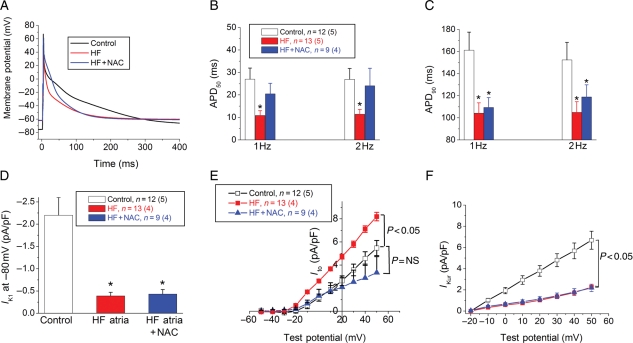
3.2.5. Redox modulation
Oxidative stress has been implicated in some forms of atrial electrophysiological remodelling.10,20 Therefore, we treated aliquots of myocytes with NAC to assess whether electrophysiology was altered by improving redox balance. Treatment with NAC did not alter APD50, APD90, Ito, or the sustained outward K+ current in control myocytes (n = 6). In HF myocytes, incubation with NAC restored APD50, whereas APD90 was unchanged (Figure 8). To further investigate the potential current alterations by which NAC incubation partially restored the APD in HF myocytes, IK1, Ito, and IKur were measured after incubation of HF myocytes in NAC (Figure 8D–F). The HF-induced increase in Ito density was restored back towards control values by NAC incubation (P = NS vs. control). NAC did not have any effect on IK1 or IKur density. Angiotensin signalling has been implicated in atrial oxidative stress,21 and we observed an increase in AT-1 receptor expression during HF.
Figure 8.
NAC partially restores atrial myocyte electrophysiology in HF myocytes. (A) APs from a control (black), HF (red), and HF myocyte incubated with NAC (blue). (B) Average APD50 in the three groups at 1 and 2 Hz, respectively. (C) Average APD90 values in the three groups at 1 and 2 Hz, respectively. (D) Inward rectifier (IK1) current density in the three groups (*P < 0.05 vs. control). (E) Transient outward K+ current (Ito) current–voltage relationship in the three groups. (F) IKur current density in control, HF, and HF + NAC myocytes, respectively. n indicates the number of myocytes, and (n) the number of animals in each group.
4. Discussion
HF and AF are increasingly common diseases, with the prevalence projected to continue increasing in the coming decades.1,22,23 Both diseases result in significant morbidity and mortality. Importantly, the addition of one of these diseases to the other increases the risk of death. Potential mechanisms through which HF may promote AF include increased atrial interstitial fibrosis and/or electrophysiological alterations. A key finding of our study is that as previously reported for AF-induced AF,24 chronic HF induces shortening of the atrial AP (and ERP) and results in a substrate for persistent AF.
We report that compared with short-term HF, chronic HF results in a distinct form of atrial electrophysiological remodelling and provides a substrate for inducible sustained AF. Notably, the underlying ion current alterations differ when comparing chronic AF and short-term HF. Previous studies of HF-induced atrial remodelling have utilized short-term canine ventricular tachypacing. Of note, Nattel and co-workers6,7 evaluated canine atrial structural and electrophysiological changes following 5 weeks of ventricular tachypacing to induce HF, and on average, inducible AF was minutes in duration. The primary electrophysiological changes in this model were slowed electrical conduction, atrial APD prolongation accompanied by a modest decrement in ICa, and significant reductions in Ito and IKs. There is also evidence in this canine HF model of increased atrial interstitial fibrosis, which may also contribute to the substrate for AF.25–27 Our model resulted in a substrate for persistent AF; spontaneous AF was not observed, which is consistent with the previous reports in shorter-term tachypaced canine HF.
We hypothesized that chronic, largely irreversible HF would provide a substrate for persistent AF; this was evident in our pilot studies. This suggests that fundamental changes in the myocardium occurring during chronic HF are associated with the development of an atrial substrate for persistent arrhythmias. The major differences between our results and those of the preceding studies in short-term HF are: the ability to induce sustained persistent AF in some animals and the lack of reversion to normal LV systolic dysfunction after termination of RV tachypacing. All animals completed the 4-month period of RV tachypacing, in contrast to the higher rates of death or technical failure reported with faster stimulation rates for shorter durations.28 The extent of atrial fibrosis was similar when comparing our results to the earlier reports,6 suggesting that increased atrial fibrosis occurs early and remains a stable feature during HF.
In our model of chronic HF, we observed increases in atrial Ito and reciprocal shortening of the APD, accompanied by decreases in IK1, IKur, and IKs. Furthermore, the HF-induced decrements in APD resulted in reduced calcium flux. The differences in the observed direction of change in the atrial APD (and the underlying differences in ion currents), compared with that reported earlier,7 suggest that there may be HF duration-dependent atrial remodelling. Further experimentation is required to systematically address this possibility.
Intriguingly, the shortening atrial refractoriness which we observed is consistent with the ‘first factor’ of atrial remodelling thought to promote the perpetuation of AF.29 The seminal studies which provided the evidence for the ‘first factor’ are models of sustained AF where continuous high-rate activity (lone AF models) significantly reduced the duration of the atrial ERP24,30,31 providing a positive feedback loop, whereby transient AF becomes persistent.24 However, although the direction of change in atrial repolarization and refractoriness we observed is similar to reports utilizing either lone AF models32 or myocytes from humans with chronic AF,33,34 the underlying mechanisms are not entirely consistent. For example, Ito is reduced in studies of AF-induced atrial remodelling, IKur (IKsus) is variably altered, and ICa-L is also reduced during AF-induced atrial remodelling.32,35–39 In contrast, our findings of a large increase in Ito with an associated reduction in APD suggest that in chronic HF, electrophysiological mechanisms distinct from AF-induced atrial remodelling result in the same AP phenotype: attenuation of atrial refractoriness. Unlike the reductions in ICa described in AF-induced atrial remodelling, our data suggest that in chronic HF, the intrinsic properties of sarcolemmal ICa are maintained (Figure 7). However, AP clamp experiments indicate a reduction in ICa elicited during an HF AP. We suggest that the acceleration of early repolarization (e.g. an HF-induced reduction in APD50 from ∼27 to 11 ms) limits sarcolemmal ICa flux, which could itself contribute to further shortening of the AP. A recent paper describing right atrial myocyte electrophysiology in cells from humans with LV systolic dysfunction reports a decrease in refractoriness and APD90, with associated reductions in Ito and preserved ICa-L (measured by voltage step protocol) and IK1.40 Collectively, this suggests the possibility of an AP phenotype (i.e. shorter atrial ERP and APD) which may be common to the disease-induced (i.e. AF or chronic HF) atrial substrate for the perpetuation of persistent AF.
The reductions in APD90 and the atrial ERP were substantial and could potentially shorten the ‘wavelength of re-entry’ thereby increasing the number of arrhythmia wavelets during AF, as has been postulated to occur in AF-induced atrial remodelling.41,42 Thus, the persistence of AF which we observed, relative to other shorter duration HF studies, may have resulted from the attenuated refractoriness of the atria during HF. The shortened APD and atrial ERP in HF may have modulated not only arrhythmia persistence, but also atrial contractility. The AP shortening and shortening of the atrial ERP are consistent with the observed attenuation of atrial contractility. APD attenuation also resulted in reduced sarcolemmal calcium flux during HF; this was directly assessed using AP clamp, and in vivo we observed an associated reduction in atrial contractile function. In addition to the reduced ICa which we observed, it has also been reported that there are abnormalities in calcium handling which also contribute to atrial contractile dysfunction during HF,43,44 and thus multiple factors may contribute to atrial contractile dysfunction during HF. Hypothetically, this suggests that any intervention attenuating atrial electrophysiological remodelling has the potential to reduce atrial arrhythmia persistence as well as improving atrial contractility.
Our western blot analyses provide some insights into the HF-induced alterations in ion currents. We observed increased Ito, and examination of the channel subunits indicates that Kv4.3 (pore-forming subunit) expression was unchanged, whereas the expression of the regulatory β-subunit KChIP2 was significantly increased. KChIP2 is known to increase Kv4-mediated ion currents45,46 and contributes to regional differences in Ito in canine myocardium.47 Thus, our data suggest that increased KChIP2 contributes, in part, to the increased atrial Ito observed during chronic HF. However, the restoration of Ito with NAC incubation also suggests a role for redox modulation of Ito amplitude during chronic HF.
We observed a reduction in IKur and the putative channel subunit, Kv1.5, was also reduced. Interestingly, KChIP2 has been shown to modulate Kv1.5 trafficking.48 Reduced channel expression, alone or in combination with reduced trafficking to the membrane (as would be predicted from the increased KChIP2 expression), may have resulted in reduced atrial IKur during HF. The reduction in inward IK1 observed in our data indicates altered current rectification. The reduced expression of the pore-forming channel subunit, Kir2.1, with preserved Kir2.3 expression may have contributed to the observed reduction in IK1 rectification.49 Thus, many of the observed alterations in ion channels are consistent with HF-induced alterations in ion channel subunit expression.
Oxidative stress has been implicated in some forms of atrial electrophysiological remodelling.10,20 Atrial remodelling due to high-rate activity of the atrium results in mitochondrial dysregulation and oxidative stress.10,21,50,51 Angiotensin-II is increased in HF and has been suggested to contribute to adverse remodelling of the atria through profibrotic, pro-oxidant, and possibly electrophysiological effects21,50,52,53 (reviewed by Ehrlich et al.54); notably, we observed an HF-mediated increase in LA AT-1 receptor expression.
A recent report indicates that glutathione is reduced in LA samples from patients with chronic HF, suggesting a role for abnormal redox balance in human HF.55 We used NAC, a glutathione precursor,56 to examine whether there was redox modulation of atrial electrophysiology during chronic HF. We observed a partial normalization of atrial electrophysiology with NAC. We recently showed that glutathione depletion results in reduced ICa and contractility in the LA, which was attenuated by NAC treatment.18 The precise mechanisms for the present observations of improved electrophysiology with NAC treatment remain undefined at the present time and further experimentation will be required to address the underlying mechanisms.
4.1. Limitations
Our model of HF is a non-ischaemic model of cardiomyopathy, and HF patients have multiple aetiologies of disease. Our animals were not treated with beta-blockers, ACE-inhibitors, or diuretics, as would occur in human HF. We studied a single duration of HF which is unlikely to reflect time-variant atrial remodelling. We only studied myocytes from the LA appendage and cannot rule out the contributions of other regions of the atria or pulmonary vein sleeves to the aetiology of AF during chronic HF.
Focal mechanisms appear to contribute to the initiation of AF during canine tachypacing-induced HF.8,43,57 Our studies addressed the substrate for persistence of AF and did not address mechanisms of initiation of AF. Although we observed reduced in vivo atrial contractility, we did not evaluate specific abnormalities in calcium handling or basic contractile properties, as contributors to impaired contractile function.
5. Summary and conclusions
Chronic RV tachypacing results in irreversible HF and atrial ion current remodelling that is distinct from that reported in short-term HF studies. Chronic HF results in shortening of atrial ERP, and the APD, which is attributable in part to increased Ito. AP remodelling in HF reduces sarcolemmal ICa-L flux and was associated with impaired atrial contractility. We suggest that the duration of HF is a critical modulator of atrial remodelling processes and ultimately of the persistence of AF.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the American Heart Association (0635086N to D.T. and 0725619B to Y.N.) and National Institutes of Health Grants (HL089836 to C.A.C. and HL074045 to S.G.).
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869–875. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 4.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, et al. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 8.Stambler BS, Fenelon G, Shepard RK, Clemo HF, Guiraudon CM. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:499–507. doi: 10.1046/j.1540-8167.2003.02519.x. [DOI] [PubMed] [Google Scholar]
- 9.Nishijima Y, Feldman DS, Bonagura JD, Ozkanlar Y, Jenkins PJ, Lacombe VA, et al. Canine nonischemic left ventricular dysfunction: a model of chronic human cardiomyopathy. J Card Fail. 2005;11:638–644. doi: 10.1016/j.cardfail.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 11.Gurlertop Y, Yilmaz M, Acikel M, Bozkurt E, Erol MK, Gundogdu F, et al. The use of anatomic M-mode echocardiography to determine the left atrial appendage functions in patients with sinus rhythm. Echocardiography. 2005;22:99–103. doi: 10.1111/j.0742-2822.2005.03131.x. [DOI] [PubMed] [Google Scholar]
- 12.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) Am J Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 13.Sridhar A, da Cunha DN, Lacombe VA, Zhou Q, Fox JJ, Hamlin RL, et al. The plateau outward current in canine ventricle, sensitive to 4-aminopyridine, is a constitutive contributor to ventricular repolarization. Br J Pharmacol. 2007;152:870–879. doi: 10.1038/sj.bjp.0707403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnes CA, Muir WW, III, Van Wagoner DR. Effect of intravenous anesthetics on inward rectifier potassium current in rat and human ventricular myocytes. Anesthesiology. 1997;87:327–334. doi: 10.1097/00000542-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Carnes CA, Dech SJ. Dihydrotestosterone and cardiac inward rectifier potassium current. Int J Androl. 2002;25:210–214. doi: 10.1046/j.1365-2605.2002.00349.x. [DOI] [PubMed] [Google Scholar]
- 17.Sridhar A, Dech SJ, Lacombe VA, Elton TS, McCune SA, Altschuld RA, et al. Abnormal diastolic currents in ventricular myocytes from spontaneous hypertensive and heart failure (SHHF) rats. Am J Physiol Heart Circ Physiol. 2006;291:H2192–H2198. doi: 10.1152/ajpheart.01146.2005. [DOI] [PubMed] [Google Scholar]
- 18.Carnes CA, Janssen PM, Ruehr ML, Nakayama H, Nakayama T, Haase H, et al. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–28073. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 19.Nishijima Y, Sridhar A, Viatchenko-Karpinski S, Shaw C, Bonagura JD, Abraham WT, et al. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci. 2007;81:1152–1159. doi: 10.1016/j.lfs.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 21.Goette A, Bukowska A, Dobrev D, Pfeiffenberger J, Morawietz H, Strugala D, et al. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30:1411–1420. doi: 10.1093/eurheartj/ehp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 24.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien DW, Fu Y, Parker HR, Chan SY, Idikio H, Scott PG, et al. Differential morphometric and ultrastructural remodelling in the left atrium and left ventricle in rapid ventricular pacing-induced heart failure. Can J Cardiol. 2000;16:1411–1419. [PubMed] [Google Scholar]
- 26.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, et al. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, et al. The spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 28.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–2678. doi: 10.1161/01.cir.0000016826.62813.f5. [DOI] [PubMed] [Google Scholar]
- 29.Schotten U, Neuberger HR, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol. 2003;82:151–162. doi: 10.1016/s0079-6107(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 30.Attuel P, Childers R, Cauchemez B, Poveda J, Mugica J, Coumel P. Failure in the rate adaptation of the atrial refractory period: its relationship to vulnerability. Int J Cardiol. 1982;2:179–197. doi: 10.1016/0167-5273(82)90032-8. [DOI] [PubMed] [Google Scholar]
- 31.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 32.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 33.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 34.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 35.Cha TJ, Ehrlich JR, Zhang L, Nattel S. Atrial ionic remodeling induced by atrial tachycardia in the presence of congestive heart failure. Circulation. 2004;110:1520–1526. doi: 10.1161/01.CIR.0000142052.03565.87. [DOI] [PubMed] [Google Scholar]
- 36.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 37.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 38.Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001;37:926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 39.Brundel BJ, Henning RH, Kampinga HH, Van Gelder IC, Crijns HJ. Molecular mechanisms of remodeling in human atrial fibrillation. Cardiovasc Res. 2002;54:315–324. doi: 10.1016/s0008-6363(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 40.Workman AJ, Pau D, Redpath CJ, Marshall GE, Russell JA, Norrie J, et al. Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF. Heart Rhythm. 2009;6:445–451. doi: 10.1016/j.hrthm.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 42.Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 43.Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythmia Electrophysiol. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 44.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythmia Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 45.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 46.Deschenes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- 47.Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Guo W, Mellor RL, Nerbonne JM. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K(+) channels. J Mol Cell Cardiol. 2005;39:121–132. doi: 10.1016/j.yjmcc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Panama BK, Lopatin AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol. 2006;571:287–302. doi: 10.1113/jphysiol.2005.097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bukowska A, Schild L, Keilhoff G, Hirte D, Neumann M, Gardemann A, et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp Biol Med (Maywood) 2008;233:558–574. doi: 10.3181/0706-RM-155. [DOI] [PubMed] [Google Scholar]
- 51.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 52.Choudhury A, Varughese GI, Lip GY. Targeting the renin-angiotensin-aldosterone-system in atrial fibrillation: a shift from electrical to structural therapy? Expert Opin Pharmacother. 2005;6:2193–2207. doi: 10.1517/14656566.6.13.2193. [DOI] [PubMed] [Google Scholar]
- 53.Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456–461. doi: 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 54.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 55.Damy T, Kirsch M, Khouzami L, Caramelle P, Le Corvoisier P, Roudot-Thoraval F, et al. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS ONE. 2009;4:e4871. doi: 10.1371/journal.pone.0004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozanski GJ, Xu Z. Glutathione and K(+) channel remodeling in postinfarction rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H2346–H2355. doi: 10.1152/ajpheart.00894.2001. [DOI] [PubMed] [Google Scholar]
- 57.Fenelon G, Shepard RK, Stambler BS. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:1093–1102. doi: 10.1046/j.1540-8167.2003.03110.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.