Abstract
Aims
Although transforming growth factor-beta (TGF-β) is believed to stimulate intimal hyperplasia after arterial injury, its role in remodelling remains unclear. We investigate whether Smad3, a TGF-β signalling protein, might facilitate its effect on remodelling.
Methods and results
Using the rat carotid angioplasty model, we assess Smad3 expression following arterial injury. We then test the effect of arterial Smad3 overexpression on the response to injury, and use a conditioned media experimental design to confirm an Smad3-dependent soluble factor that mediates this response. We use small interfering RNA (siRNA) to identify this factor as connective tissue growth factor (CTGF). Finally, we attempt to replicate the effect of medial Smad3 overexpression through adventitial application of recombinant CTGF. Injury induced medial expression of Smad3; overexpression of Smad3 caused neointimal thickening and luminal expansion, suggesting adaptive remodelling. Smad3 overexpression, though exclusively medial, caused adventitial changes: myofibroblast transformation, proliferation, and collagen production, all of which are associated with adaptive remodelling. Supporting the hypothesis that Smad3 initiated remodelling and these adventitial changes via a secreted product of medial smooth muscle cells (SMCs), we found that media conditioned by Smad3-expressing recombinant adenoviral vector (AdSmad3)-infected SMCs stimulated adventitial fibroblast transformation, proliferation, and collagen production in vitro. This effect was attenuated by pre-treatment of SMCs with siRNA specific for CTGF, abundantly produced by AdSmad3-infected SMCs, and significantly up-regulated in Smad3-overexpressing arteries. Moreover, periadventitial administration of CTGF replicated the effect of medial Smad3 overexpression on adaptive remodelling and neointimal hyperplasia.
Conclusion
Medial gene transfer of Smad3 promotes adaptive remodelling by indirectly influencing the behaviour of adventitial fibroblasts. This arterial cell–cell communication is likely to be mediated by Smad3-dependent production of CTGF.
Keywords: TGF-β, Smad3, Connective tissue growth factor, Restenosis, Remodelling, Adventitia
1. Introduction
Atherosclerosis, the leading cause of death in the USA, is currently addressed through several treatment modalities, including angioplasty, endarterectomy, and bypass. The success of each of these therapies is, however, limited by the development of intimal hyperplasia as well as constrictive remodelling.1,2 Significant progress has been made towards the limitation of neointimal hyperplasia using drug-eluting stents, but therapeutic manipulation of the phenomenon of remodelling remains unexplored. Although neointimal hyperplasia is a significant contributor to restenosis, the patency of the angioplastied artery is ultimately determined by alterations in vessel geometry, collectively termed remodelling.3–5 ‘Compensatory’ enlargement of the vessel wall, as measured by changes in external elastic lamina (EEL) area, often accompanies neointimal thickening.6 This growth, termed adaptive remodelling, prevents the neointimal lesion from impinging on the lumen.7,8 Occasionally, the vessel actually shrinks and exacerbates occlusion, a process called constrictive remodelling.
The adventitia is thought to be central to both adaptive and constrictive remodelling. Almost immediately after injury, adventitial fibroblasts transform into myofibroblasts and demonstrate increased proliferation and expression of both smooth muscle α-actin (SMA) and collagen.9–11 Although the increases in fibroblast proliferation and SMA expression correspond to an increase in neointimal thickness, the causal relationship between adventitial fibroblasts and arterial remodelling remains unclear. Similarly, controversial reports exist regarding the role of adventitial collagen. Increased collagen has been reported to be associated with constrictive as well as adaptive remodelling.12–15
Transforming growth factor-beta (TGF-β) is known to play a role in the development of restenosis.16–19 Both clinical and experimental studies demonstrate that TGF-β is up-regulated at the site of vascular injury.16,17 The effect of TGF-β on the arterial wall geometry is complex and not entirely clear. In animal models, administration of exogenous TGF-β at the time of arterial injury has been found to stimulate neointimal formation, although Kingston et al.13 found that TGF-β1 inhibited the hyperplastic response. Inhibition of TGF-β via a number of different mechanisms decreases intimal hyperplasia, suggesting that the primary role for TGF-β in injured arteries is to promote the development of intimal hyperplasia.18,19 In contrast, inhibition of the TGF-β receptor enhances arterial lumen. Specifically, Smith et al.19 showed that blocking the TGF-β signal by using a soluble TGF-β receptor inhibitor led to a significant increase in lumen area as well as EEL length, suggesting TGF-β produces constrictive remodelling. Kingston et al.13 reported, however, that adenovirus-mediated expression of TGF-β3 led to increased luminal area as well as collagen accumulation. These authors suggested that TGF-β3 may promote adaptive remodelling through the induction of collagen. We felt that exploring the role of specific TGF-β signalling proteins in arterial remodelling might shed further light on the complex role of TGF-β and its effects following arterial injury.
The most commonly studied TGF-β pathway is that involving Smad proteins, classified as receptor-activated Smads (RSmads: Smad2 and Smad3), a common Smad (coSmad: Smad4), and inhibitory Smads (iSmads: Smad6 and Smad7).20,21 TGF-β receptor activation leads to RSmad phosphorylation. The phosphorylated RSmad then complexes with Smad4. The complex enters the nucleus and regulates transcription of target genes through the Smad-binding elements in the DNA.22
In an effort to define the role of TGF-β in remodelling, we first ectopically expressed Smad3 in the rat carotid angioplasty model of restenosis. Our findings confirm that TGF-β/Smad-mediated processes cause adaptive remodelling through a novel pathway involving connective tissue growth factor (CTGF).
2. Methods
2.1. Cell culture
Aortic smooth muscle cells (SMCs) were isolated from Sprague–Dawley rat aortas as described by Clowes et al.23 Aortic fibroblasts were isolated as described previously by Pagano et al.24 as well as in the Supplementary material online. A rat fibroblast cell line, NRK-49F, was obtained for use in collagen-luciferase assays.
2.2. Proliferation assay
Proliferation was assayed by measuring DNA synthesis as described previously. Adventitial fibroblasts were seeded onto 24-well plates (10 000 cells/well) in 10% foetal bovine serum media overnight, then starved in 0.5% serum for 48 h, followed by incubation with conditioned media or agonists as indicated. During the final 6 h of the assay, tritiated thymidine was added to each well; at the conclusion of the assay, incorporation of 3H-thymidine was quantified.25
2.3. Collagen promoter activation assay
A luciferase construct containing the human α2(I)-procollagen (COL1A2, −772 → +38 region) gene promoter was constructed as described previously.26 For purposes of transfection efficacy, a fibroblast cell line, NRK-49, was used. Following transfection with this construct, cells were starved for 48 h and treated with conditioned media or agonists for 72 h. Luciferase activity in the cell lysate, indicating collagen promoter activity, was quantified with a Dual-Luciferase™ Assay (Promega, Fitchburg, WI, USA).
2.4. Collagen synthesis assay
Collagen synthesis was assayed by measuring incorporation of tritiated proline as described previously. Adventitial fibroblasts were seeded onto six-well plates, starved for 48 h, and treated with conditioned media or agonists for 72 h. During the final 24 h of the incubation with agonists, tritiated proline was added and samples were processed as described previously.27
2.5. Small interfering RNA transfection
Transfection of cultured aortic SMCs was performed using Lipofectamine™ 2000 and according to the manufacturer's instructions. Confirmation of small interfering RNA (siRNA) effectiveness in decreasing CTGF expression was confirmed with western blot.
2.6. Balloon injury model and in vivo gene delivery
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, NIH Publication No. 85-23, 1996 revision. Approval from the Institutional Animal Care and Use Committee was granted (Protocol 0508-398A). Male Sprague–Dawley rats underwent angioplasty of the left common carotid artery with viral perfusion as described elsewhere.23 Immediately following the removal of the catheter, adenovirus suspended in phosphate-buffered saline (200 µL of 1 × 1010 pfu/mL) was administered intraluminally and allowed to dwell for 20 min. Construction of an Smad3-expressing recombinant adenoviral vector (AdSmad3) was described previously.28 An empty viral vector Adnull was included as a control. The ability of Adsmad3 to respond to TGF-β with enhanced phosphorylation was confirmed in cultured SMCs.29
For in vivo studies of periadventitial CTGF, 80 ng of recombinant CTGF suspended in 200 µL of pluronic gel was applied to the adventitial surface of the artery immediately after injury. Control animals were subjected to application of pluronic gel alone. Fourteen days after injury, carotid was performed as harvested utilizing perfusion fixation at a physiological pressure of 100 mmHg.
2.7. Morphometric analysis and immunohistochemistry
Paraffin-embedded arteries were cut into 5 µm sections for analysis. Morphometric analysis was carried out on elastic-stained arteries. For each animal, 10 sections were selected for analysis using digital software for Dell PC computers (ImageJ and Photoshop V.7). The areas encompassed by the lumen surface (luminal area), internal elastic lamina–lumen surface (neointimal area), EEL–internal elastic lamina (medial area), and EEL length, total vessel area, and total vessel area–EEL (adventitial area) were measured. For evaluation of the remodelling index, the EEL was measured and compared with that of controls, as described previously.5 Immunostaining and quantification was performed as described by Lehr et al.30 (for details, please see Supplementary material online). Picrosirius Red analysis was performed as described by Kingston et al.18
2.8. Statistical analysis
Values were expressed as fold increase or means ± SE. For in vivo studies, the differences between AdSmad3- and AdNull-treated arteries or CTGF-treated and control arteries were analysed using unpaired Student's t-test. For in vitro studies, a one-way ANOVA with multiple comparison tests using the Bonferroni correction was performed. All experiments were repeated at least three times.
3. Results
3.1. Intraluminal gene transfer of Smad3
We have previously shown that endogenous Smad3 expression is significantly up-regulated following injury.29 We hypothesized that this up-regulation may influence intimal hyperplasia, arterial remodelling, or both. To test this, we enhanced Smad3 expression in injured arteries via adenovirus-mediated gene transfer.
As shown in the Supplementary material online, we first used an adenoviral vector bearing β-galactosidase to demonstrate that transgene expression, evident at 3 days post-injury and persisting for 14 days, was limited to medial and neointimal cells. Such localized expression of transgene has been previously observed when similar arterial gene transfer techniques were used.31 It is speculated that the EEL works as a physical barrier to prevent adenovirus from diffusing to the adventitia. Thus, any adventitial effect of Smad3 overexpression would be indirect, mediated by infected medial or neointimal cells.
We next used virus encoding either Smad3 (AdSmad3) or empty vector (AdNull) to infect injured arteries. AdSmad3-infected arteries exhibited significantly greater medial Smad3 expression than AdNull controls 3 and 7 days post-injury and significantly greater neointimal Smad3 expression 7 and 14 days post-injury (see Supplementary material online, Figure S2).
These results confirmed that intraluminal gene delivery results in a significant Smad3 induction in the neointimal and medial portions of the arterial wall.
3.2. Effects of Smad3 gene transfer on arterial geometry
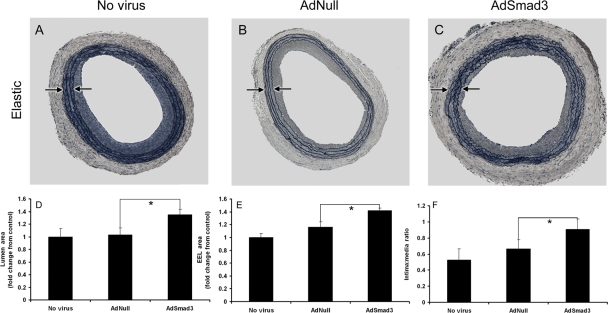
We evaluated AdSmad3's effect on arterial geometry through analysis of arteries harvested 14-day post-injury. Smad3 overexpression significantly increased the intima-to-media ratio(AdNull: 0.67 ± 0.06; AdSmad3: 0.89 ± 0.07; Figure 1A–C and F). Despite neointimal enlargement, luminal area was significantly increased compared with AdNull controls (1.31-fold ± 0.38, P < 0.05; Figure 1A–D). This resulted from an Smad3-induced, 1.22-fold expansion of the EEL (P < 0.05; Figure 1A–C and E). AdSmad3-treated arteries exhibited thicker neointima but with compensatory remodelling, ultimately leading to luminal enlargement.
Figure 1.
Effects of AdSmad3 on the arterial injury response. (A–C) Representative arterial sections stained with Verhoeff's Elastin Stain 14 days post-injury and treatment with no virus (A), AdNull (B), or AdSmad3 (C). Arrows: medial boundaries. ×40. (D–F) Morphometric analyses of injured arteries. The luminal area (D), EEL area (E), and intima/media ratio (F) were determined by microscopic examination of sections from AdSmad3-treated arteries and compared with those of AdNull controls (n = 6, *P < 0.05).
3.3. Effect of Smad3 gene transfer on the arterial adventitia
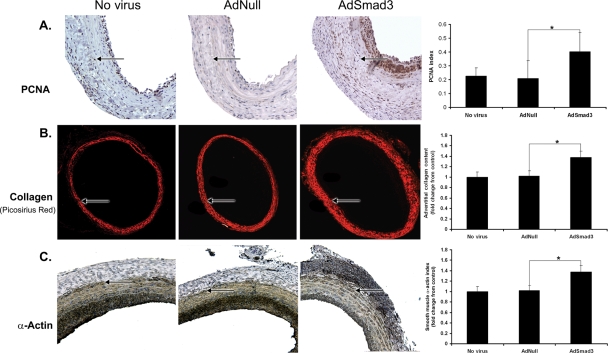
We next investigated the Smad3-induced adventitial changes that corresponded to the observed adaptive remodelling. AdSmad3-infected arterial adventitia demonstrated PCNA positivity almost double that of AdNull controls (1.94 ± 0.4-fold, P < 0.05), indicating significantly greater proliferation (Figure 2A). Using Picrosirius Red staining, we found that Smad3 gene transfer resulted in a 40% increase in adventitial collagen (1.38 ± 0.09, P < 0.05, Figure 2B). Associated with Smad3 gene transfer, there was also a significant increase in the number of adventitial cells that stained positive for SMA (Figure 2C). Such SMA-positive cells are referred to in the literature as myofibroblasts, an adventitial cell population believed to be result from fibroblast transformation in response to arterial injury.19
Figure 2.
Effect of AdSmad3 on the adventitia. Sections of uninfected, AdNull-, and AdSmad3-infected arteries harvested 14 days post-injury were assessed for proliferation (A), expressed as the fraction of adventitial nuclei that stained positively for PCNA. Picrosirius staining (B) was used to determine the relative amounts of adventitial collagen. Sections were also immunostained for SMA (C), a marker of myofibroblast transformation, expressed as fraction of adventitial area staining positively (n = 6; *P < 0.05). Arrows: EEL. (A) ×200, (B) ×40, and (C) ×200.
3.4. Smad3 gene transfer produces an indirect effect on the arterial adventitia
Consistent with previous reports, we found that gene transfer in the rat balloon angioplasty model is confined to the intima and media.31 We postulated that the adventitial changes observed with Smad3 overexpression were indirectly induced by Smad3-infected medial SMCs. We hypothesized that Smad3 overexpression in medial SMCs led to the production of a soluble factor capable of stimulating fibroblasts. We tested this hypothesis in vitro using cultured medial SMCs and adventitial fibroblasts. Both types of primary cells were isolated from rat aortas and their identities were confirmed by positive staining for smooth muscle-specific myosin heavy chain and Thy 1-1, respectively. Vascular SMCs were infected with either AdSmad3 or AdNull and stimulated with TGF-β (5 ng/mL). The resulting conditioned media was then applied to fibroblast cultures. Several functions of fibroblasts that could potentially contribute to adaptive remodelling were evaluated (see Supplementary material online, Figure S6).
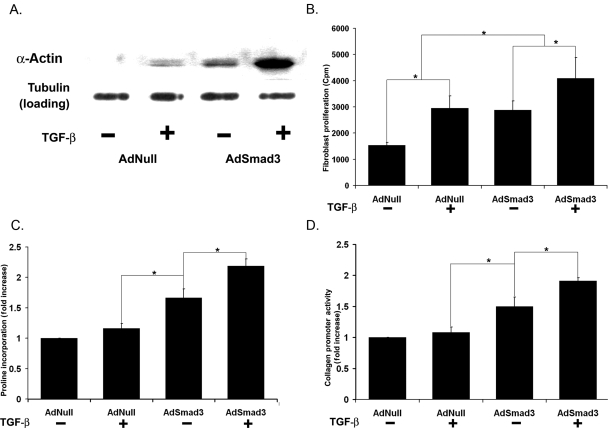
We first examined myofibroblast transformation through SMA expression. As shown in Figure 3A, conditioned media from AdSmad3 SMCs and TGF-β + AdSmad3 SMCs increased SMA expression. Next, we tested fibroblast proliferation as measured by tritiated thymidine incorporation. Media conditioned by AdSmad3 SMCs significantly increased fibroblast proliferation (Figure 3B).
Figure 3.
Effects on fibroblast function by media conditioned by SMC in vitro. (A) Western blot for SMA in cell lysate of fibroblasts stimulated by conditioned media taken from SMCs treated as indicated. (B) Proliferation of primary fibroblasts as measured by 3H-thymidine incorporation assay. (C) Collagen production in primary adventitial fibroblasts in response to conditioned media as measured by 3H-proline incorporation assay. (D) COL1A2 promoter activity in an immortalized fibroblast cell line in response to conditioned media, as measured with a luciferase reporter construct (n = 4, *P < 0.05).
As AdSmad3-infected carotid arteries exhibited increased adventitial collagen, we next tested the ability of conditioned media to stimulate fibroblast collagen production. A tritiated proline assay demonstrated that fibroblasts increased collagen synthesis when cultured in media derived from AdSmad3 or TGF-β + AdSmad3 SMCs (Figure 3C). To confirm this, we examined type I collagen gene expression using a luciferase reporter containing the proximal promoter of the human IA2 collagen gene (COL1A2).26 Fibroblasts were first transfected with the reporter and then stimulated with conditioned media. Conditioned media from AdSmad3 SMCs significantly increased collagen promoter activity (TGF-β–AdSmad3 over TGF-β–AdNull: 1.49 ± 0.12-fold, P < 0.05; Figure 3D).
3.5. SMCs influence fibroblast function through CTGF
We have shown that medial SMCs can influence adventitial fibroblast behaviour through a TGF-β/Smad3-dependent soluble factor. This factor may be CTGF, previously demonstrated to stimulate myofibroblast transformation, proliferation, and collagen production.32 An abundance of CTGF is evident in conditioned media derived from Smad3-overexpressing SMCs. Furthermore, in vitro fibroblast studies revealed that recombinant CTGF (100 ng/mL) mimicked the effects of conditioned media, increasing SMA expression, proliferation, and collagen synthesis (see Supplementary material online, Figure S4).
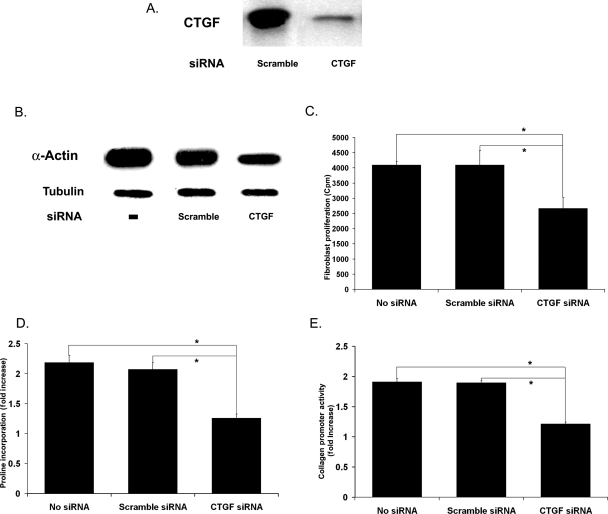
To demonstrate this role of CTGF, we used an siRNA to block CTGF production in SMCs. Compared with scrambled control, CTGF siRNA significantly reduced the CTGF detected in conditioned media (Figure 4A). More importantly, CTGF siRNA effectively blocked the ability of AdSmad3-infected SMCs to condition media that stimulated fibroblast SMA expression, proliferation, and collagen synthesis (Figure 4B–E).
Figure 4.
Effect of CTGF siRNA on SMC–fibroblast communication. SMCs were infected with AdNull or AdSmad3, allowed to recover for 24 h, and then incubated with siRNA (33 pM) of either a scrambled control or CTGF-specific sequence for 6 h before TGF-β1 stimulation (5 ng/mL, 72 h) (A) Western blot for CTGF in media conditioned by SMCs incubated with a scrambled control siRNA or CTGF-specific siRNA. All SMCs were infected with AdSmad3 and stimulated with TGF-β1. (B–D) Primary fibroblasts were treated with media conditioned by AdSmad3-infected, TGF-β1-stimulated SMCs incubated with no siRNA, scrambled control, and CTGF-specific sequences. Their SMA expression (B), proliferation (C), and collagen production (D) were then evaluated. (E) Activity of COL1A2 promoter in a fibroblast cell line in response to the same media as in (B–D) as measured with a luciferase reporter construct (n = 4, *P < 0.05).
3.6. Gene transfer of Smad3 increases CTGF in arterial wall
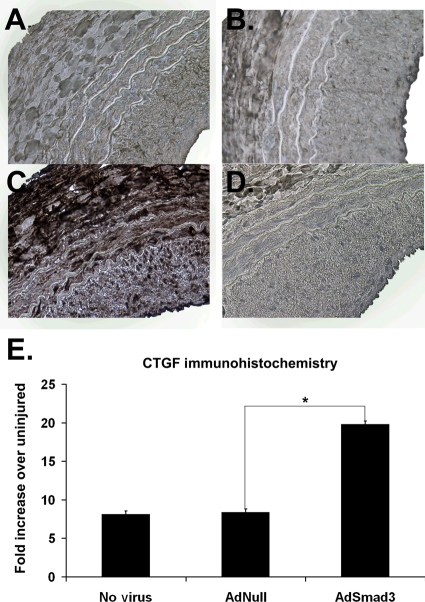
Having established the role of CTGF in Smad3-induced SMC communication with adventitial fibroblasts, we returned to the arterial injury model to find if AdSmad3 infection caused significant CTGF expression. Immunohistochemistry using a CTGF-specific antibody demonstrated increased CTGF in injured, compared with non-injured, arteries. Moreover, AdSmad3 infection further enhanced arterial CTGF expression (Figure 5A–E).
Figure 5.
Effects of AdSmad3 on expression of endogenous CTGF. (A–D) Representative sections, harvested 14 days following balloon angioplasty with or without viral infection, immunostained for CTGF. (A) No virus, (B) AdNull, (C) AdSmad3, and (D) AdSmad3 stained with secondary antibody alone (negative control). (E) Quantification of CTGF-positive immunostaining expressed as the fold increase from uninjured control (n = 4, *P < 0.05). ×200.
We next tested whether periadventitial administration of recombinant CTGF could reproduce the effects of intraluminal gene transfer of Smad3. Immediately following balloon angioplasty, a pluronic gel suspension of recombinant CTGF (400 ng/mL) was applied periadventitially to the injured artery, which was harvested 14 days later.
CTGF's effect on vessel morphometry was largely identical to that of AdSmad3. As with AdSmad3, CTGF significantly increased neointimal thickening (2.32 ± 0.17-fold over control, P < 0.05). The EEL of CTGF-treated arteries was significantly larger than that of control arteries treated with pluronic gel alone (1.36 ± 0.10-fold, P = 0.0027) (Figure 6A). Thus, periadventitial administration of CTGF resulted in adaptive remodelling; unlike AdSmad3-treated arteries, however, CTGF-treated arterial luminal area was not significantly different from control.
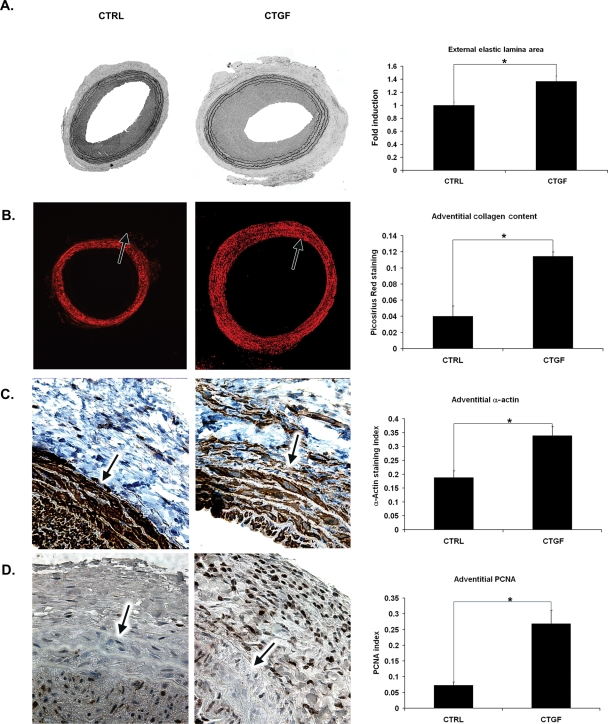
Figure 6.
Effect of periadventitial application of CTGF on the adventitia. Immediately after injury, 400 ng/mL recombinant human CTGF suspended in F127 pluronic gel was applied periadventitially. Fourteen days post-injury, the injured segments of the CTGF-treated arteries were analysed for (A) change in EEL area compared with controls (×40), (B) adventitial collagen content, as measured by Picrosirius Red staining (×40), (C) adventitial SMA expression as measured by immunohistochemistry (×200), and (D) cellular proliferation as measured by PCNA immunostaining. Representative sections of six experiments are shown (×200). Arrows: EEL (n = 6, *P < 0.05).
Immunohistochemistry also revealed similarities between the adventitia of AdSmad3-infected and CTGF-treated arteries. As with AdSmad3, the adventitia of CTGF-treated arteries showed a 2.7 ± 0.15-fold increase in collagen compared with control (Figure 6B). Additionally, CTGF stimulated adventitial myofibroblast transformation and proliferation (1.80 ± 0.04 and 2.46 ± 0.16, respectively; P < 0.05) (Figure 6C and D).
4. Discussion
TGF-β is recognized for its ability to enhance intimal hyperplasia following arterial injury, but conflicting evidence exists regarding its effect on adaptive remodelling.11,13,18,19 Smad3 is a signalling protein known to mediate many of TGF-β's effects, and in these studies, we evaluated its role in arterial response to injury—specifically, in adaptive remodelling.
Using the rat carotid balloon angioplasty model, we first found that arterial injury leads to significant induction of endogenous Smad3. We also found that Smad3 overexpression via gene transfer resulted in increased luminal area, despite enhanced neointimal hyperplasia. Our findings thus demonstrate that TGF-β/Smad3 pathway activation stimulates intimal hyperplasia as well as adaptive remodelling that compensates for that hyperplasia. Results from PCNA immunostaining revealed a significant increase in the number of proliferating cells in the intima, media, and adventitia of AdSmad3-treated arteries. The increased neointimal formation may be related to enhanced SMC proliferation from a direct effect of Smad3 on medial or neointimal SMCs. Moreover, proliferating myofibroblasts from the adventitia may also migrate into and enhance the formation of neointima.
Kobayashi et al.33 also recently addressed the role of TGF-β/Smad3 in arterial injury. Using a photochemical femoral artery injury model in an Smad3-null mouse, they found that Smad3 gene deficiency, compared with controls, leads to a significantly greater intima:media ratio, luminal loss, and arterial occlusion. These findings would appear to conflict with ours, in that our data suggest that Smad3 overexpression increases intimal hyperplasia. There are several important differences between the two studies.
Kobayashi's group used a different arterial injury model than ours; possibly different injury response mechanisms are elicited by a photochemical vs. the mechanical injury associated with balloon angioplasty. Moreover, the use of an Smad3-null mouse results in a global deficiency of the protein affecting all cells including white blood, endothelial, SMCs, and adventitial cells. Our local gene transfer resulted in the local overexpression of Smad3, limited just to the media. However, overexpression may not always reproduce the true function of an endogenous protein. Future studies employing targeted gene deletion or ‘knock-down’ of Smad3 expression in the arterial wall will be necessary to further clarify the role of endogenous Smad3 in vascular remodelling as well as intimal hyperplasia.
Since gene transfer of Smad3 produced similar effects on arterial remodelling as TGF-β3, as has been previously demonstrated by Kingston et al.,13 it is possible that TGF-β3 actives the Smad3 pathway more profoundly in an injured artery compared with other TGF-β isoforms. However, this hypothesis requires validation.
Our findings, furthermore, offer persuasive evidence that TGF-β/Smad3-induced adaptive remodelling is mediated by medial SMC production of CTGF. Support for this includes the following: (i) both in vitro and in vivo, Smad3 overexpression in medial SMCs leads to increased CTGF production; (ii) blocking CTGF synthesis with siRNA attenuates the crosstalk between SMCs and adventitial fibroblasts; (iii) application of recombinant CTGF to the injured arterial adventitia causes adaptive remodelling and mimics the effect of Smad3 gene transfer.
It has previously been shown that TGF-β stimulates CTGF production via Smad3.34 Moreover, an Smad-binding element has been identified in the CTGF promoter.34 TGF-β plays a profibrotic role in a number of disease processes in which CTGF mediates TGF-β's effects.24,34,35 A strong relationship between TGF-β, CTGF, and human disease is therefore pre-existent. Fibroblasts have been shown to mediate these pathological effects of TGF-β and CTGF.36 CTGF has also been shown to stimulate myofibroblast transformation, proliferation, and matrix protein in skin, lung, and kidney fibroblasts.24,32,35
In the in vitro fibroblast study, medium conditioned by AdNull-infected TGF-β-treated SMCs stimulated both α-actin expression and proliferation of fibroblasts compared with medium conditioned by non-TGF-β-treated SMCs. This stimulatory effect of conditioned medium is potentially mediated by the endogenous Smad3 pathway within SMCs. Consistently, Adnull-infected SMCs responded to TGF-β with enhanced production of CTGF, presumably mediated by endogenous Smad3. Alternatively, TGF-β that used to stimulate SMCs would have remained in the conditioned media as it was too small to be eliminated by filtering. The residual TGF-β could directly act on fibroblasts.
Of note, we observed that endogenous CTGF is up-regulated in the intima, media, and adventitia of AdSmad3-treated arteries. Since CTGF can be synthesized by medial SMCs and secreted into the extracellular space, it is not entirely unexpected that we would detect this soluble factor in all three layers of the arteries. It should also be pointed out that our data do not exclude intimal cells or fibroblasts as sources of CTGF synthesis. However, the fact that only medial SMCs were infected with AdSmad3 in this study suggests that Smad3-expressing cells are the major source of CTGF up-regulation in AdSmad3-infected arteries.
Jiang et al.37 have recently suggested a role for CTGF in the vein graft remodelling. Using a rabbit vein graft model, these authors found that increased intramural wall stress following vein graft implantation resulted in an up-regulation of both TGF-β and CTGF. They also observed that TGF-β and CTGF levels were inversely correlated with the magnitude of outward remodelling of the vein graft. They found, as we did, that CTGF production was associated with myofibroblast transformation and cell migration. Their data and ours confirm an important role for TGF-β/CTGF in vessel wall remodelling. Contrary to our findings, however, these authors have concluded that the effect of CTGF is to limit, rather than enhance, outward vessel remodelling. Admittedly, the models are quite different; these authors have studied an arteriovenous vein graft fistula, and our studies are after arterial injury. They found an association between elevated CTGF and diminished outward remodelling but did not demonstrate a causal relationship; we have shown that direct application of CTGF to the arterial adventitia produces adaptive remodelling. Nevertheless, taken together, both studies suggest that CTGF, released in response to TGF-β, plays a pivotal role in the architectural changes that follow vessel injury.
Both Smad3 gene transfer and CTGF application led to similar cellular changes in the adventitia: increased cell proliferation, SMA expression, and collagen accumulation. We have assumed that it is through these effects that CTGF produces adaptive remodelling. However, the exact mechanisms that lead to adaptive remodelling remain elusive. Although medial gene transfer of Smad3 and adventitial application of CTGF produced very similar effects on the arterial injury response, the link between Smad3 and CTGF that we observed in cell culture remains to be directly tested in vivo. Our future experiments will focus on using CTGF-specific siRNA to test the ability of Smad3 to regulate arterial remodelling in the absence of CTGF induction.
Myofibroblast transformation, proliferation, and collagen production appear to be involved, but precisely how is not clear. Although excess collagen was originally thought to promote constrictive remodelling, there is increasing evidence, including our current data, suggesting that excess collagen may lead to adaptive remodelling. We found an increase in adventitial collagen in AdSmad3- and CTGF-treated arteries, both of which exhibited adaptive remodelling.
Our observations in vivo regarding increased collagen deposition in the adventitia of arteries exhibiting adaptive remodelling as well as our in vitro evidence of increased collagen synthesis by fibroblasts imply that collagen turnover is integral to adaptive remodelling. To this end, matrix metalloproteases (MMPs), specifically MMP-2 and MMP-9, which have been implicated by others in the arterial response to injury, may play an important role in this process. In future studies, we will test this hypothesis using this and similar models.38–40 TGF-β has been shown to induce the expression of MMP-9 in human meningeal cells and in a human head and neck squamous cell carcinoma cell line.41,42 Moreover, using human endometrial carcinoma cell lines, Van Themsche et al.43 demonstrated that TGF-β3 (not β1 or β2) increases the invasiveness of tumour cells through production of MMP-9. It is possible that TGF-β3 through Smad3 up-regulates the expression of MMPs in the injured arterial wall which subsequently promotes arterial remodelling. In future studies, we will test this hypothesis in various arterial injury models.
Our finding that CTGF stimulates both intimal hyperplasia and adaptive remodelling is of interest. We speculate that the expanded intimal hyperplasia in CTGF-treated arteries is, in part, caused by myofibroblast migration into the neointima. It is also possible that exogenous CTGF may diffuse from the adventitia to the media and directly affect medial SMCs. CTGF has been reported to directly stimulate vascular SMC proliferation in vitro.44,45 Stimuli that produce intimal hyperplasia presumably simultaneously initiate compensatory expansion. In further support of this concept is the observation of significantly increased neointimal hyperplasia in the Smad3-overexpressing arteries displaying adaptive remodelling.
A limitation of the current study is our inability to detect phosphorylated Smad3 in vivo. Phosphorylation of Smad3 by the TGF-β receptor is a key step in the transduction of TGF-β signal. Although it would be ideal to prove in vivo that Smad3 gene transfer leads to an increase in phosphorylated Smad3, for a number of reasons, we feel confident that Smad3 was activated. First, compared with null virus, in vivo Smad3 expression resulted in a significant change in arterial morphology. Smad3 would not be able to produce an effect without being phosphorylated and consequently activated. Also, it is well established that the rat carotid injury model produces high local levels of TGF-β, which is the direct activator of Smad3. Lastly, we have shown, in vitro, that SMCs overexpressing Smad3 and stimulated with TGF-β produce increased levels of phosphorylated Smad3.
In conclusion, we have established that TGF-β, through an Smad3 pathway, can induce adaptive remodelling following arterial injury. We have established a novel mechanism for this effect. TGF-β activates Smad3 in medial SMCs, causing them to secrete CTGF, which in turn stimulates adventitial fibroblasts to migrate, proliferate, produce collagen, and transform into myofibroblasts. It is presumably through these events that changes in the arterial architecture occur. This process is complex, and our data does not indicate an obvious target that can be manipulated to enhance adaptive remodelling. For example, accentuation of Smad3 or CTGF in the arterial wall does produce adaptive remodelling, but this event is accompanied by the synchronous stimulation of intimal hyperplasia. In the case of Smad3, there is a modest increase in luminal diameter, and with CTGF, there is no net change. It is our hope, however, that these studies will open the door for further investigations of the precise mechanisms of this process, with the goal of eventually defining targets that will specifically enhance adaptive remodelling and diminish the deleterious effects of restenosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by a Public Health Service Grant R01-HL-68673 (K.C.K. and B.L.) from the National Heart, Lung, and Blood Institute, an American Heart Association Grant-In-Aid 0455859T (B.L.), a Ruth L. Kirschstein National Research Service Award F32-HL086222-01 (R.K.), and National Institute of Health Training Grants T32-CA68971-07 (S.T.H.) and T32-GM008466 (E.J.R. and S.T.).
Acknowledgements
The authors would like to thank Ms Sophia Chu for adenovirus preparation and Steven Doty of the Hospital for Special Surgery for technical assistance.
Conflict of interest: none declared.
References
- 1.Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89:2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- 2.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–1191. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 3.Kakuta T, Currier JW, Haudenschild CC, Ryan TJ, Faxon DP. Differences in compensatory vessel enlargement, not intimal formation, account for restenosis after angioplasty in the hypercholesterolemic rabbit model. Circulation. 1994;89:2809–2815. doi: 10.1161/01.cir.89.6.2809. [DOI] [PubMed] [Google Scholar]
- 4.Losordo DW, Rosenfield K, Kaufman J, Pieczek A, Isner JM. Focal compensatory enlargement of human arteries in response to progressive atherosclerosis. In vivo documentation using intravascular ultrasound. Circulation. 1994;89:2570–2577. doi: 10.1161/01.cir.89.6.2570. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Zhao H, Yutani C, Imakita M, Ishibashi-Ueda H. Morphometric and histologic assessment of remodeling associated with restenosis after percutaneous transluminal coronary angioplasty. Cardiology. 1998;90:115–121. doi: 10.1159/000006829. [DOI] [PubMed] [Google Scholar]
- 6.Mekontso-Dessap A, Kirsch M, Guignambert C, Zadigue P, Adnot S, Loisance D, et al. Vascular-wall remodeling of 3 human bypass vessels: organ culture and smooth muscle cell properties. J Thorac Cardiovasc Surg. 2006;131:651–658. doi: 10.1016/j.jtcvs.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 8.Bond MG, Adams MR, Bullock BC. Complicating factors in evaluating coronary artery atherosclerosis. Artery. 1981;9:21–29. [PubMed] [Google Scholar]
- 9.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 10.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, O'Brien JE, Jr, Fard A, Zalewski A. Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–1305. doi: 10.1161/01.atv.16.10.1298. [DOI] [PubMed] [Google Scholar]
- 12.Mallawaarachchi CM, Weissberg PL, Siow RC. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2005;25:1383–1387. doi: 10.1161/01.ATV.0000168415.33812.51. [DOI] [PubMed] [Google Scholar]
- 13.Kingston PA, Sinha S, Appleby CE, David A, Verakis T, Castro MG, et al. Adenovirus-mediated gene transfer of transforming growth factor-beta3, but not transforming growth factor-beta1, inhibits constrictive remodeling and reduces luminal loss after coronary angioplasty. Circulation. 2003;108:2819–2825. doi: 10.1161/01.CIR.0000097068.49080.A0. [DOI] [PubMed] [Google Scholar]
- 14.Brasselet C, Durand E, Addad F, Al Haj Zen A, Smeets MB, Laurent-Maquin D, et al. Collagen and elastin cross-linking: a mechanism of constrictive remodeling after arterial injury. Am J Physiol Heart Circ Physiol. 2005;289:H2228–H2233. doi: 10.1152/ajpheart.00410.2005. [DOI] [PubMed] [Google Scholar]
- 15.Staab ME, Srivatsa SS, Lerman A, Sangiorgi G, Jeong MH, Edwards WD, et al. Arterial remodeling after experimental percutaneous injury is highly dependent on adventitial injury and histopathology. Int J Cardiol. 1997;58:31–40. doi: 10.1016/s0167-5273(96)02844-6. [DOI] [PubMed] [Google Scholar]
- 16.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 17.Nikol S, Isner JM, Pickering JG, Kearney M, Leclerc G, Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992;90:1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingston PA, Sinha S, David A, Castro MG, Lowenstein PR, Heagerty AM. Adenovirus-mediated gene transfer of a secreted transforming growth factor-beta type II receptor inhibits luminal loss and constrictive remodeling after coronary angioplasty and enhances adventitial collagen deposition. Circulation. 2001;104:2595–2601. doi: 10.1161/hc4601.099405. [DOI] [PubMed] [Google Scholar]
- 19.Smith JD, Bryant SR, Couper LL, Vary CP, Gotwals PJ, Koteliansky VE, et al. Soluble transforming growth factor-beta type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res. 1999;84:1212–1222. doi: 10.1161/01.res.84.10.1212. [DOI] [PubMed] [Google Scholar]
- 20.Roberts AB, Russo A, Felici A, Flanders KC. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann N Y Acad Sci. 2003;995:1–10. doi: 10.1111/j.1749-6632.2003.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 22.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 23.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 24.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Itoh H, Louie O, Kubota K, Kent KC. The signaling protein Rho is necessary for vascular smooth muscle migration and survival but not for proliferation. Surgery. 2002;132:317–325. doi: 10.1067/msy.2002.125786. [DOI] [PubMed] [Google Scholar]
- 26.Kubota K, Okazaki J, Louie O, Kent KC, Liu B. TGF-beta stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J Surg Res. 2003;109:43–50. doi: 10.1016/s0022-4804(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, et al. Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J Clin Invest. 1995;96:2667–2675. doi: 10.1172/JCI118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryer EJ, Hom RP, Sakakibara K, Nakayama KI, Nakayama K, Faries PL, et al. PKCdelta is necessary for Smad3 expression and transforming growth factor beta-induced fibronectin synthesis in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26:780–786. doi: 10.1161/01.ATV.0000209517.00220.cd. [DOI] [PubMed] [Google Scholar]
- 29.Tsai S, Hollenbech S, Ryer EJ, Wang C, Yamanouchi D, Liu B, et al. TGF-b through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neoinitmal formation. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.91478.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehr HA, Mankoff DA, Corwin D, Santeusanio G, Gown AM. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J Histochem Cytochem. 1997;45:1559–1565. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
- 31.Steg PG, Feldman LJ, Scoazec JY, Tahlil O, Barry JJ, Boulechfar S, et al. Arterial gene transfer to rabbit endothelial and smooth muscle cells using percutaneous delivery of an adenoviral vector. Circulation. 1994;90:1648–1656. doi: 10.1161/01.cir.90.4.1648. [DOI] [PubMed] [Google Scholar]
- 32.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Yokote K, Fujimoto M, Yamashita K, Sakamoto A, Kitahara M, et al. Targeted disruption of TGF-beta-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ Res. 2005;96:904–912. doi: 10.1161/01.RES.0000163980.55495.44. [DOI] [PubMed] [Google Scholar]
- 34.Louis H, Lacolley P, Kakou A, Cattan V, Daret D, Safar M, et al. Early activation of internal medial smooth muscle cells in the rabbit aorta after mechanical injury: relationship with intimal thickening and pharmacological applications. Clin Exp Pharmacol Physiol. 2006;33:131–138. doi: 10.1111/j.1440-1681.2006.04339.x. [DOI] [PubMed] [Google Scholar]
- 35.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Liu B, Ryer EJ, Kundi R, Kamiya K, Itoh H, Faries PL, et al. Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. J Vasc Surg. 2007;45:160–168. doi: 10.1016/j.jvs.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, et al. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H482–H488. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 38.Bendeck MP, Irvin C, Reidy MA. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78:38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 39.Zahradka P, Harding G, Litchie B, Thomas S, Werner JP, Wilson DP, et al. Activation of MMP-2 in response to vascular injury is mediated by phosphatidylinositol 3-kinase-dependent expression of MT1-MMP. Am J Physiol Heart Circ Physiol. 2004;287:H2861–H2870. doi: 10.1152/ajpheart.00230.2004. [DOI] [PubMed] [Google Scholar]
- 40.Furman C, Luo Z, Walsh K, Duverger N, Copin C, Fruchart JC, et al. Systemic tissue inhibitor of metalloproteinase-1 gene delivery reduces neointimal hyperplasia in balloon-injured rat carotid artery. FEBS Lett. 2002;531:122–126. doi: 10.1016/s0014-5793(02)03388-4. [DOI] [PubMed] [Google Scholar]
- 41.Kodama T, Kikuchi N, Satoh H, Ohtsuka M. Metacarpal bone metastasis from lung cancer. Onkologie. 2009;32:216–217. doi: 10.1159/000203347. [DOI] [PubMed] [Google Scholar]
- 42.Lamar JM, Iyer V, DiPersio CM. Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol. 2008;128:575–586. doi: 10.1038/sj.jid.5701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Themsche C, Mathieu I, Parent S, Asselin E. Transforming growth factor-beta3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase c-dependent induction of matrix metalloproteinase-9. J Biol Chem. 2007;282:4794–4802. doi: 10.1074/jbc.M608497200. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, LeMaire SA, Chen L, Shen YH, Gan Y, Bartsch H, et al. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation. 2006;114:I200–I205. doi: 10.1161/CIRCULATIONAHA.105.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000;79:915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.