Abstract
Background
Information is limited regarding the longitudinal tracking of left ventricular (LV) mass over the adult life-course and the determinants of such change.
Methods and Results
We used multi-level modeling to evaluate the correlates of LV mass prospectively over a 16-year period in 4217 Framingham study participants (mean age 45 years, 53% women) using up to 4 serial routine echocardiographic observations on each individual (11,762 observations). Age, sex, body mass index (BMI), systolic blood pressure (BP), antihypertensive treatment, smoking, and diabetes were related to longitudinal measures of LV mass. Women and participants with diabetes experienced a steeper increase in LV mass with advancing age (compared to men, and those without diabetes; p for interactions <0.0001and 0.0003, respectively). Women also displayed greater increments in LV mass with increasing BMI (compared to men, p=0.04 for interaction). Participants with optimal values of these risk factors experienced lesser increases in LV mass over time. Analyses evaluating short-term (4-year) changes in LV mass (2605 unique individuals providing 4494 observations) identified the same key determinants that influenced its long-term trajectory, i.e., BMI, sex, systolic BP, antihypertensive treatment, and smoking.
Conclusions
Our longitudinal observations on a large community-based sample identified higher blood pressure, excess adiposity, smoking and diabetes as fundamental determinants of LV mass tracking over the adult life course. These observations are consistent with the notion that maintenance of optimal levels of these risk factors in midlife will reduce the burden of LV hypertrophy, and possibly heart failure, in older age.
Keywords: cardiac mass, change, serial measurements, hypertrophy, epidemiology, multi-level modeling, echocardiography
Introduction
Clinically overt heart failure is the endpoint of a long disease continuum characterized by progressive structural and functional changes of the heart, a dynamic process referred to as cardiac remodeling.1 Given the current burden of heart failure and the projected increase over the next 5 decades,2 it is important to understand factors that influence cardiac remodeling over the adult life-course. LV mass is an important cardiac remodeling trait that is an intermediate phenotype for heart failure,3 and is also associated with increased risk for various cardiovascular disease (CVD) outcomes.4 Epidemiological studies have identified age, sex, blood pressure (BP), and adiposity as the key correlates of LV mass cross-sectionally.5 Yet, data on the clinical determinants of tracking of LV mass over the adult life course and correlates of short-term change in LV mass are sparse and limited to observations in selected patient groups (e. g. hypertensives6) or in adolescents and young adults.7-9 An investigation of the tracking of LV mass through mid-adulthood would be fundamental to elucidate the evolution of sub-clinical LV remodeling and Stage B heart failure10 in the community, which antedate overt heart disease by years to decades.
We hypothesized that key cardiovascular risk factors i.e., age, sex, BP, antihypertensive treatment, smoking, adiposity, and diabetes, which are correlates of LV mass in cross-sectional studies, are also the critical determinants of longitudinal changes in LV mass during short- and long-term follow-up. Further, we postulated that the maintenance of optimal levels of these risk factors in midlife would be associated with a favorable trajectory of LV mass over the adult life course. To test these hypotheses, we investigated a large sample from the community. First, we evaluated the clinical correlates of LV mass longitudinally over a period of 16 years using multi-level modeling. Second, we analyzed correlates of short-term (4 years) change in LV mass (Figure 1).
Figure 1.
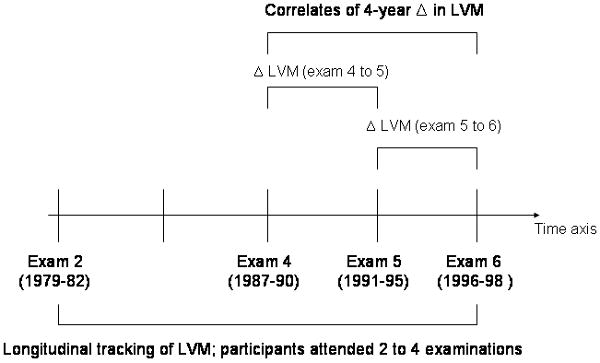
Overview of study design. Δ indicates change; LVM, left ventricular mass
Methods
Study sample
The Framingham Offspring Study started in 1971 enrolling 5,124 children of the original Framingham cohort and the spouses of these children.11 Participants are examined at each Heart Study clinic visit, which take place approximately every 4 to 8 years.11 The Heart Study examination includes a medical history focusing on new onset cardiovascular events since the previous examination, a targeted physical examination including anthropometry and laboratory assessment of cardiovascular risk factors. The study protocols were approved by the Boston University Medical Center Institutional Review Board. All participants provided written informed consent.
Echocardiographic measurements
Routine transthoracic echocardiography was performed on Offspring cohort attendees at examination cycles 2 (1979-82), 4 (1987-90), 5 (1991-95) and 6 (1996-98). The echocardiographic equipment used differed across the examinations: Hoffrel 201 ultrasound receiver (+Aerotech transducer) at examination cycle 2; Hewlett Packard (model 77020AC) ultrasound machine at examination cycles 4 and 5; Sonos 1000 Hewlett-Packard machine at examination cycle 6. All echocardiograms were evaluated by an experienced technician or cardiologist using a standardized reading protocol. End-diastolic LV septal (SWT) and posterior wall (PWT) thickness and LV internal dimensions (LVID) at the end of diastole and systole were obtained using a leading-edge technique.12 LV mass was calculated as 0.8[1.04(LVID+SWT+PWT)3−(LVID)3]+0.6.13 The reproducibility of echocardiographic measurements was good.14
Of 4337 unique attendees (12,351 observations) at exams 2, 4, 5, or 6 (where echocardiograms were performed), 103 individuals (375 person-exams) were excluded for prevalent or incident myocardial infarction or heart failure; both conditions affect LV mass measurements based on M-mode echocardiography. We also excluded observations if people were <25 or >75 years old at the time of that specific observation, leading to the exclusion of 214 observations and 17 individuals (who had none of their echocardiograms performed within the age range of interest). Thus, 4217 unique individuals providing 11,762 observations were included in the analyses on longitudinal tracking of LV mass (Figure 1).
Short-term change in LV mass was evaluated in 2605 unique participants who attended at least two consecutive examinations at which echocardiography was performed. To maximize the number of observations, data on the change in LV mass from examination cycles 4 to 5 and from cycles 5 to 6 were pooled (n=4,494 participants-observations; Figure 1).
Statistical Analyses
Modeling of individual growth curves for Log-LV mass (multi-level modeling)
Multi-level statistical modeling allows the analysis of data that vary at multiple levels. It is applicable to longitudinal data where repeated measurements are obtained on different occasions (level 1) within the same individual (level 2). Compared to traditional regression models, this analytical approach has the advantage of accommodating participants with missing data at some of the serial examinations and facilitates analyses using the maximum number of observations in a longitudinal investigation.
LV mass was natural-logarithmically-transformed to normalize its distribution and stabilize its variance in men and women permitting pooled sex analysis. We estimated growth curves for LV mass using multi-level statistical modeling (SAS PROC MIXED; using a compound symmetry matrix) and elucidated the associations of LV mass with the following clinical covariates, using a direct entry procedure: age, sex, BMI, systolic BP, antihypertensive treatment, smoking status and diabetes. These variables were chosen based on their cross-sectional association with LV mass in the published reports.5
The examination cycle was included to adjust for variation in LV mass across examinations due to variation in the instrumentation used. Random intercepts were fitted for all models to reflect a different starting value of LV mass for each participant.
We examined age as a random effect and also non-linear effects of age. Neither the quadratic nor the random effects of age were statistically significant. We fit a series of clinically pre-specified models, with direct entry of candidate variables. Statistical interactions between age, sex, and other clinical risk factors were also investigated. Regression coefficients, their respective standard errors and the corresponding p-values for all significant predictor variables and interaction terms are provided in the online supplement. To facilitate interpretation of the data, percent changes in LV mass per clinically useful increments of the predictor variables are provided for the correlates of longitudinal LV mass measures as well as its short term change. Growth curves were generated for men and women to display the tracking of LV mass over time.
Finally, for illustrative purposes we describe the influence of an individual's risk factor burden on long-term LV mass tracking by modeling growth curves for LV mass for the following 4 subgroups (assuming the same risk factor profile at different ages, and using regression coefficients shown in the online supplement): women with a low CVD risk factor burden (non-smoker, non-hypertensive [systolic BP of 130 mm Hg and not on antihypertensive treatment], free of diabetes, BMI of 25 kg/m²); women with a high CVD risk factor burden (smoker, hypertensive [systolic BP of 150 mm Hg and on antihypertensive treatment], with diabetes, BMI of 30 kg/m²); men with a low CVD risk factor burden (non-smoker, non-hypertensive [systolic BP of 130 mm Hg and not on antihypertensive treatment], free of diabetes, BMI of 27.5 kg/m²); and men with a high CVD risk factor burden (smoker, hypertensive [systolic BP of 150 mm Hg and on antihypertensive treatment], with diabetes, BMI of 30 kg/m²).
Analyses of correlates of short-term changes in LV mass
GEE (generalized estimation equation) regression models, which account for relatedness among participants, were used to determine clinical correlates of change in LV mass during a mean follow-up period of 4 years. As in the above-mentioned analysis, LV mass was logarithmically-transformed to harmonize the SD in men and women. Multivariable models incorporated the same set of covariates used for the analyses of longitudinal tracking of LV mass (see above). We also incorporated the interaction terms that were statistically significant in the analyses of long-term LV mass tracking.
All analyses were performed using SAS software. S-PLUS and Microsoft Excel were used to create the graphical displays.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
The baseline characteristics of the two samples that were used to investigate the correlates of longitudinal tracking of LV mass over 16 years and its short-term changes (over 4-years) are displayed in Table 1.
Table 1.
Clinical and echocardiographic characteristics of the study samples used to characterize clinical correlates of short-term (mean follow-up 4 years) change in and long-term (maximum 16 year-period) tracking of LV mass.
| Sample for correlates of long-term LV mass tracking | Sample for correlates of short-term LV mass change | |||
|---|---|---|---|---|
| Variable | Men (n=1973) |
Women (n=2244) |
Men (n=1094) |
Women (n=1511) |
| Clinical features | ||||
| Age, years | 45±10 | 45±10 | 49±10 | 50±10 |
| Systolic BP, mm Hg | 126±16 | 119±17 | 127±17 | 122± 19 |
| Diastolic BP, mm Hg | 81±9 | 75±9 | 81±10 | 76±10 |
| Antihypertensive treatment, % | 11.3 | 9.6 | 15.0 | 11.7 |
| Hypertension, % | 29.4 | 18.9 | 34.9 | 25.2 |
| Height, inches | 69±3 | 64±2 | 69±3 | 64±2 |
| Weight, lbs | 182±28 | 144±29 | 185±27 | 147±28 |
| BMI, kg/m² | 26.8±3.7 | 24.8±4.9 | 27.2±3.5 | 25.3±4.7 |
| Smoking*, % | 33.8 | 35.3 | 20.4 | 21.4 |
| Diabetes, % | 4.4 | 2.3 | 4.8 | 2.9 |
| Echocardiographic features | ||||
| Baseline LV mass, crude, g | 187±41 | 131±30 | 188±35 | 145±29 |
| Follow-up LV mass, crude, g | N/A | N/A | 187±37 | 143±30 |
| Baseline Log-LV mass, g | 5.2+0.2 | 4.8+0.2 | 5.2±0.2 | 5.0±0.2 |
| Follow-up Log-LV mass, g | N/A | N/A | 5.2±0.2 | 4.9±0.2 |
Values are mean ± SD or percentages.
Abbreviations: BP, blood pressure; LV, left ventricular N/A= not applicable.
For the sample evaluating long-term tracking of LV mass, characteristics are from the first eligible examination.
We observed a temporal trend for smoking prevalence in our data set. The prevalences are substantially higher at earlier examination cycles (which have been used to define smoking for the long-term analyses; first exam with echocardiography attended by each participant) than at the later examination cycles which were used for the short-term analyses (see Figure 1 for overview of the study design).
Clinical correlates of longitudinal tracking of LV mass
Age, sex, BMI, systolic BP, antihypertensive treatment, smoking, and diabetes were related to tracking of LV mass over the 16-year observation period (Table 2; online supplementary Table 1). We noted a statistically significant interaction between age and sex (p<0.0001), with women having a steeper increase in LV mass over time relative to men (Figure 2; Table 2; online supplementary Table 1). We also observed a statistically significant interaction between sex and BMI (online supplementary Table 1, p = 0.04), indicating that the association of BMI with LV mass over time is of a larger magnitude in women relative to men (Table 2).
Table 2.
Clinical correlates of longitudinal tracking of LV mass over a 16-year period.
| Predictor variable | % Change in LVM | 95% Confidence Interval |
|---|---|---|
| Men* | 31.83% | (30.51%, 33.17%) |
| Age (men, without diabetes, 10 units increase) | -0.55% | (-1.19%,0.10%) |
| Age (men, with diabetes, 10 units increase) | 2.87% | (0.99%,4.80%) |
| Age (women, without diabetes, 10 units increase) | 2.09% | (1.46%,2.74%) |
| Age (women, with diabetes, 10 units increase) | 5.60% | (3.65%,7.60%) |
| BMI (men, 5 units increase) | 7.51% | (6.61%,8.43%) |
| BMI (women, 5 units increase) | 8.64% | (8.01%,9.29%) |
| Systolic blood pressure (10 units increase) | 1.39% | (1.14%,1.63%) |
| Antihypertensive treatment | 2.35% | (1.24%,3.47%) |
| Smoking | 1.17% | (0.23%,2.12%) |
| Diabetes** | -1.61% | (-3.80%,0.62%) |
The table shows the percent change in LV mass per increment of the predictor variable as indicated. There was a significant interaction of age and sex, age and diabetes, and BMI and sex (see online supplementary Table 1 for regression coefficients and p-values). Therefore, the effects of age and BMI are provided in the appropriate subgroups (men and women, and with and without diabetes).
The effect of men (as compared to women) is for participants with an age of 50 years (mean age of all participants at all exams) and a BMI of 25 kg/m².
The effect of diabetes is for participants at age 50 years.
Figure 2.
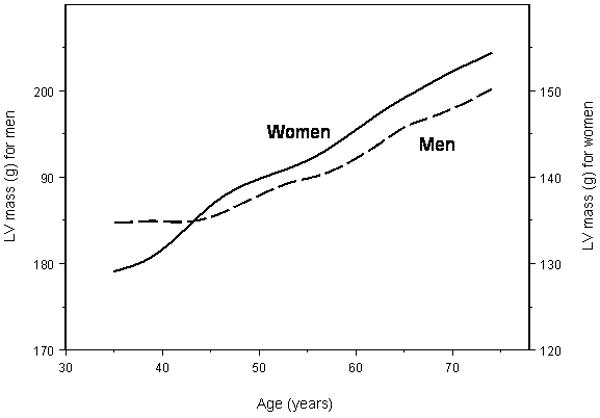
Unadjusted mean LV mass with increasing age for men (dashed line) and women (solid line). Left Y-axis, LV mass scale for men; right Y-axis, LV mass scale for women.
The association of diabetes with LV mass was statistically significant (β=0.03, p=0.0016) in a model that did not incorporate BMI, the BMI*sex or diabetes*age interactions. However, the association of diabetes was rendered statistically non-significant once BMI was added to the multivariable model. These observations suggest that BMI may capture some of the association between diabetes and LV mass.
The interaction between age and diabetes was statistically significant (online supplementary Table 1, p=0.0003), indicating that the effect of age on LV mass varies according to diabetes status. In men and women free of diabetes, there was only a slight increase in LV mass in women and a slight decrease in men over time (Table 2). However, in participants with diabetes, we observed a much steeper increase in women and men, adjusting for all other covariates in the model (Table 2).
Impact of cumulative risk factor burden on LV mass progression
Women and men with a higher CVD risk factor burden had higher baseline LV mass and a greater increase over time compared to participants with a lower CVD risk factor burden (Figure 3).
Figure 3. Long-term LV mass tracking in individuals with low and high CVD risk factor burden.
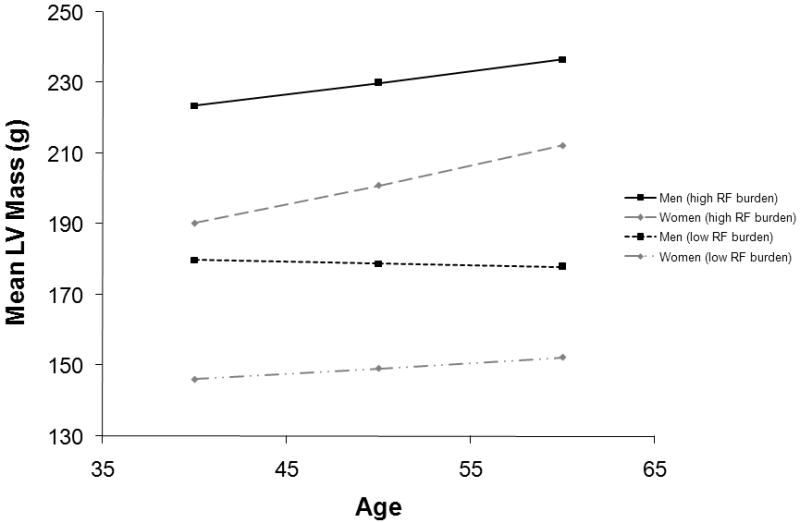
Specifically: in women (◊) with a low CVD risk factor burden (non-smoker, free of hypertension [systolic BP of 130 mm Hg and not on antihypertensive treatment], free of diabetes, BMI of 25 kg/m²; grey dotted line); in women (◊) with a high CVD risk factor burden (smoker, with hypertension [systolic BP of 150 mm Hg and on antihypertensive treatment], with diabetes, BMI of 30 kg/m²; grey dashed line); in men (□) with a low CVD risk factor burden (non-smoker, free of hypertension [systolic blood pressure of 130 mm Hg and not on antihypertensive treatment], free of diabetes, BMI of 27.5 kg/m²; black dotted line); and in men (□) with a high CVD risk factor burden (smoker, with hypertension [systolic BP of 150 mm Hg and on antihypertensive treatment], with diabetes, BMI of 30 kg/m²; black solid line). Regression coefficients from online supplementary Table 1 were used for these estimations.
Clinical correlates of short-term changes in LV mass
Paralleling the analyses of longitudinal tracking of LV mass over a 16-year period, analyses of short-term (4-year) change in LV mass identified the following correlates: sex, BMI, systolic BP, antihypertensive treatment, smoking and the age*diabetes interaction term were significantly associated with delta LV mass (online supplementary Table 2;Table 3). However, the strongest correlate of change in LV mass was baseline LV mass, being inversely associated with delta LV mass. In the absence of interaction terms (specifically interactions with age), the main effect of age was statistically significant (β: 0.008, p=0.015). However, in the presence of interaction terms, neither age nor the age*sex and sex*BMI interaction terms were significantly associated with short-term change in LV mass (online supplementary Table 2; Table 3).
Table 3.
Correlates of short term (4 years) change in Log-LV mass in individuals who attended at least two consecutive exams where echocardiograms were performed.
| Predictor variable | % Change in LVM | 95% Confidence Interval |
|---|---|---|
| Men* | 18.28% | (16.67%,19.92%) |
| Age (men, without diabetes, 10 units increase) | 1.31% | (0.35%,2.27%) |
| Age (men, with diabetes, 10 units increase) | 4.39% | (1.33%,7.55%) |
| Age (women, without diabetes, 10 units increase) | 0.30% | (-0.48%,1.09%) |
| Age (women, with diabetes, 10 units increase) | 3.36% | (0.36%,6.44%) |
| BMI (men, 5 units increase) | 4.08% | (2.88%,5.30%) |
| BMI (women, 5 units increase) | 4.60% | (3.79%,5.43%) |
| Systolic blood pressure (10 units increase) | 1.01% | (0.61%,1.40%) |
| Antihypertensive treatment | 2.43% | (0.76%,4.13%) |
| Smoking | 1.41% | (0.05%,2.79%) |
| Diabetes** | -2.08% | (-5.29%,1.24%) |
The table shows the percent change in LV mass per increment of the predictor variable as indicated. (See online supplementary Table 2 for regression coefficients and p-values).
The effect for men (as compared to women) is for participants with an age of 50 years (mean age of all participants at all exams) and a BMI of 25 kg/m².
The effect of diabetes is for participants at age 50 years.
Discussion
Given the aging of the United States population and the rising burden of heart failure, it is important to understand determinants of cardiac remodeling over the adult life-course. We have previously reported that midlife BP and BMI are powerful determinants of heart failure risk in older age,15 and we postulated that the effect of these risk factors on LV mass may be a contributory mechanism. In this context, we evaluated clinical correlates of longitudinal tracking of LV mass over a 16-year period using 11,762 observations from 4,217 Framingham Heart Study participants. In addition, we analyzed determinants of change in LV mass during a short-term follow-up (4 years) using 4,494 participant observations.
Principal findings
Overall, we observed a remarkable consistency in the correlates of LV mass in analyses conducted using multiple observations over a 16-year period versus evaluation of a 4-year change in LV mass. In addition to sex, age, adiposity (BMI) and BP, LV mass over the adult life-course was also related to antihypertensive treatment, smoking and diabetes. We observed interesting sex-related differences in the evolution of LV mass with age and increasing adiposity in our long-term analyses, a finding that was not discernible when evaluated over the short-term. Women had a greater and steeper increase in LV mass with increasing age and with higher BMI. Participants with diabetes displayed a steeper longitudinal trajectory of LV mass compared to participants without diabetes, the effect being more pronounced in women with diabetes compared to men with the condition. In addition, baseline LV mass was the strongest predictor of short-term change in LV mass. Finally, cumulative burden of cardiovascular risk factors significantly influenced the LV mass progression over time: participants with a greater burden of risk factors displayed higher LV mass values at baseline and experienced a steeper increase over time.
Comparison with the published literature
Effect of age, blood pressure and body composition on long-term LV mass
Age, BP, and excess adiposity are major determinants of LV mass in cross-sectional studies based on single-occasion measurements.16,17 In the present analyses, we demonstrate that these covariates are also significant correlates of long-term tracking of LV mass over a 16 year period in adulthood, and also determine short-term change in LV mass. This is in agreement with prior studies on LV mass progression in children and young adults.7-9 In addition, LV mass at baseline is an important predictor of future changes in LV mass in our investigation and in previous studies.7,18 Of note, baseline LV mass was inversely associated with change in LV mass on short-term follow-up. Thus, higher values for baseline LV mass are associated with a smaller change in LV mass on short-term follow-up. This is biologically reasonable because of the phenomenon of regression to the mean.19
Sexual dimorphism in LV mass tracking
In a previous cross-sectional analyses of Framingham Heart Study data, a greater increase in LV mass with age was observed in women (compared to men).20 Similar findings were noted when a binary LV mass trait (LV hypertrophy) was used; a 10-year increment was associated with a 15% increased risk for LV hypertrophy in men but with a 67% increased risk in women.16 Similar findings were reported from the Mayo Clinic and in an autopsy study.21,22 Consistent with these results, we observed a steeper increase in LV mass in women (relative to men) over a 16-year time interval.
The reasons for the greater increase in LV mass in women are not entirely clear. In a report that focused on healthy individuals free of hypertension, overweight and prior cardiovascular disease, Dannenberg et al. observed only a minor increase in LV mass cross-sectionally in women between the ages of 20 and 90 years, and noted a minor decrease in LV mass in men within the corresponding age range.23 This is consistent with the notion that the increase in LV mass over time may be mainly driven by a higher prevalence of risk factors with increasing age.23 However, we observed effect modification by sex of the age-LV mass relations in multivariable-adjusted analyses. It is conceivable, therefore, that additional factors may mediate the observed interaction (see below).
Sex hormones might also partly account for the observed sex-differences in trajectories of LV mass.24 Estrogen affects cardiac remodeling in many ways and probably has an inverse net-association with LV mass.25 In an small series of hypertensive women, pre-menopausal participants had lower LV mass than men with the same level of blood pressure, whereas these sex differences were no longer observed in postmenopausal women.26 Experimental data also suggest a beneficial role of estrogens on LV remodeling25, and genetic variants in genes encoding the estrogen receptor alpha have been associated with age-related changes in LV mass.27 A decline in estrogen levels with age may, therefore, account for the steeper increase in LV mass with age in women. Another mechanism that may explain the age-associated increase in LV mass in women may be the greater pulsatile vascular load in women compared to men.28
We also observed a steeper rise in LV mass in women with increasing BMI. These longitudinal observations confirm cross-sectional findings of a greater sensitivity of cardiac mass in women to the effects of excess adiposity.29
Effect of smoking on LV mass progression
Positive associations between smoking and LV mass and LV wall thickness were observed in cross-sectional analyses in different community-based samples.30-32 Recently, Payne and colleagues reported an association of smoking with exercise-induced LV growth in young army recruits undergoing an intense 12-week training program.33 In the present analyses, we observed that smoking was positively related to changes in LV mass during short-term follow-up and to tracking of LV mass over a 16-year time interval.
It is well established that smoking modulates vascular remodeling34, and increasing evidence indicates that it also affects cardiac remodeling.35,36 For example, chronic carbon monoxide exposure increased myocardial endothelin-1 expression and induced cardiac hypertrophy in a rat model.36
Effect of diabetes on LV mass progression
Previous cross-sectional analyses of population-based cohorts have reported positive associations of type 2 diabetes with higher LV mass and wall thickness, and worse systolic function.32,37,38 In our analyses, we observed that diabetes modified the association of age with long-term LV mass tracking and with short-term changes in LV mass. Participants with diabetes had a steeper increase in LV mass over time compared to those without the condition. The steeper trajectory of LV mass in women as compared to men might explain the greater incidence of heart failure in women with diabetes (as compared to men with the condition) in a post-myocardial infarction sample,39 and in community-based investigations.40
Strengths and limitations
The strengths of the present study include the large sample size, the community-based longitudinal design, the use of multi-level modeling that facilitates evaluation of serial echocardiograms, and the evaluation of both long-term tracking and short-term changes in LV mass. However, some limitations should be acknowledged. The change in instrumentation across examinations raises issues of comparability of measurements across examinations. For this reason, we adjusted for the ‘examination cycle’ as a covariate in our analyses. Furthermore, the Framingham laboratory has had a limited number of readers over the years and adheres to a rigorous quality control image acquisition and measurement protocol. Any differences in determination of LV mass across the examination cycles are likely to result in random misclassification and such measurement errors would bias us towards the null hypothesis of no association between the covariates evaluated and LV mass.
Of all participants who had echocardiographic data available at baseline, a total of 280 participants died during the follow-up period of 16 years. Those who died had a worse cardiovascular risk profile, including higher unadjusted LV mass, as compared to participants who did not die during the follow-up period. However, after adjustment for age and sex, the difference in LV mass was rendered statistically non-significant, indicating that our results have not been substantively altered because of informative missingness related to greater mortality of those with higher LV mass.
We focused our analyses on covariates that were identified as key correlates of LV mass in previous cross-sectional analyses and that were available at all 4 examinations when echocardiograms were obtained. We, therefore, did not analyze other variables including physical activity and additional measures of body composition that could yield additional insights regarding the longitudinal correlates of LV mass.
Although our statistical analysis takes into account longitudinal measures of exposure variables and covariates (all variables were measured at each examination), it does not specifically account for the possibility that some time-dependent covariates also act as mediators (e. g., diabetes), which could result in an underestimation of the true effects of some covariates. Additional studies are warranted that appropriately analyze the effect of time-dependent covariates that are also mediators, such as marginal structural models41 and G-estimation methods.42
Lastly, our participants are middle-aged to elderly and almost exclusively white and of European ancestry. Thus, the generalizability of our results to other age groups or ethnicities is unknown.
Conclusion
Given the increasing burden of heart failure in the United States, it is important to understand the determinants of cardiac remodeling over the adult life-course. In the present investigation, we identified higher BP, excess adiposity, smoking, and diabetes as fundamental determinants of LV mass over both the short- and long-term. These findings are consistent with the notion that maintenance of optimal levels of these risk factors in midlife will reduce the burden of sub-clinical LV hypertrophy, and presumably heart failure, in the elderly.
Supplementary Material
Acknowledgments
none
Funding sources: This work was supported by NHLBI Contract N01-HC-25195; grants: 6R01-NS 17950, 2 K24 HL04334, RO1HL080124 (RSV).
Footnotes
Clinical summary: Clinically overt heart failure is the endpoint of a long disease continuum characterized by progressive structural and functional changes of the heart, a dynamic process referred to as cardiac remodeling. Considering the current burden of heart failure and the projected increase over the next 50 years, it is critical to understand factors that influence cardiac remodeling over the adult life-course. Using longitudinal data from the Framingham Offspring cohort (up to 4 serial routine echocardiographic observations on each individual; in total 11,762 observations) and multi-level statistical modeling, we identified age, sex, body mass index (BMI), systolic blood pressure (BP), antihypertensive treatment, smoking, and diabetes as key correlates of longitudinal tracking of left ventricular mass, an important cardiac remodeling phenotype. Women and participants with diabetes experienced a steeper increase in left ventricular mass with advancing age (compared to men, and those without diabetes). Women also displayed a greater increase in left ventricular mass with increasing BMI (compared to men). Participants with optimal values of these cardiovascular risk factors experienced lesser increases in left ventricular mass over time. Analyses evaluating short-term (4-year) changes in left ventricular mass (2605 unique individuals providing 4494 observations) identified the same key determinants that influenced its long-term trajectory, i.e., BMI, sex, systolic BP, antihypertensive treatment, and smoking. These observations are consistent with the notion that maintenance of optimal levels of these risk factors in young-midlife will reduce the burden of left ventricular hypertrophy, and possibly heart failure, in older age.
Conflict of Interest Disclosures: none
Reference List
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 5.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, Wong ND, Manolio TA. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 6.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, De Simone G, Devereux RB, Porcellati C. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16:895–899. doi: 10.1016/s0895-7061(03)01018-5. [DOI] [PubMed] [Google Scholar]
- 7.Gardin JM, Brunner D, Schreiner PJ, Xie X, Reid CL, Ruth K, Bild DE, Gidding SS. Demographics and correlates of five-year change in echocardiographic left ventricular mass in young black and white adult men and women: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 2002;40:529–535. doi: 10.1016/s0735-1097(02)01973-3. [DOI] [PubMed] [Google Scholar]
- 8.Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJ, Snieder H. Growth of left ventricular mass in African American and European American youth. Hypertension. 2002;39:943–951. doi: 10.1161/01.hyp.0000015612.73413.91. [DOI] [PubMed] [Google Scholar]
- 9.Schieken RM, Schwartz PF, Goble MM. Tracking of left ventricular mass in children: race and sex comparisons: the MCV Twin Study. Medical College of Virginia. Circulation. 1998;97:1901–1906. doi: 10.1161/01.cir.97.19.1901. [DOI] [PubMed] [Google Scholar]
- 10.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC, Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 12.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 13.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 14.Sundstrom J, Sullivan L, Selhub J, Benjamin EJ, D'Agostino RB, Jacques PF, Rosenberg IH, Levy D, Wilson PW, Vasan RS. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J. 2004;25:523–530. doi: 10.1016/j.ehj.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, Kenchaiah S, Levy D, D'Agostino RB, Sr, Vasan RS. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–876. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108:7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 17.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 18.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Regression towards the mean. BMJ. 1994;308:1499. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study) Am J Cardiol. 1990;65:371–376. doi: 10.1016/0002-9149(90)90304-j. [DOI] [PubMed] [Google Scholar]
- 21.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 22.Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc. 1994;69:205–211. doi: 10.1016/s0025-6196(12)61058-1. [DOI] [PubMed] [Google Scholar]
- 23.Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study) Am J Cardiol. 1989;64:1066–1068. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- 24.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003;57:388–394. doi: 10.1016/s0008-6363(02)00705-8. [DOI] [PubMed] [Google Scholar]
- 26.Garavaglia GE, Messerli FH, Schmieder RE, Nunez BD, Oren S. Sex differences in cardiac adaptation to essential hypertension. Eur Heart J. 1989;10:1110–1114. doi: 10.1093/oxfordjournals.eurheartj.a059434. [DOI] [PubMed] [Google Scholar]
- 27.Peter I, Huggins GS, Shearman AM, Pollak A, Schmid CH, Cupples LA, Demissie S, Patten RD, Karas RH, Housman DE, Mendelsohn ME, Vasan RS, Benjamin EJ. Age-related changes in echocardiographic measurements: association with variation in the estrogen receptor-alpha gene. Hypertension. 2007;49:1000–1006. doi: 10.1161/HYPERTENSIONAHA.106.083790. [DOI] [PubMed] [Google Scholar]
- 28.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–1871. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 29.Marcus R, Krause L, Weder AB, Dominguez-Meja A, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Blood Pressure Study. Circulation. 1994;90:928–936. doi: 10.1161/01.cir.90.2.928. [DOI] [PubMed] [Google Scholar]
- 30.Gardin JM, Arnold A, Gottdiener JS, Wong ND, Fried LP, Klopfenstein HS, O'Leary DH, Tracy R, Kronmal R. Left ventricular mass in the elderly. The Cardiovascular Health Study. Hypertension. 1997;29:1095–1103. doi: 10.1161/01.hyp.29.5.1095. [DOI] [PubMed] [Google Scholar]
- 31.Gidding SS, Xie X, Liu K, Manolio T, Flack JM, Gardin JM. Cardiac function in smokers and nonsmokers: the CARDIA study. The Coronary Artery Risk Development in Young Adults Study. J Am Coll Cardiol. 1995;26:211–216. doi: 10.1016/0735-1097(95)00118-j. [DOI] [PubMed] [Google Scholar]
- 32.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne JR, Eleftheriou KI, James LE, Hawe E, Mann J, Stronge A, Kotwinski P, World M, Humphries SE, Pennell DJ, Montgomery HE. Left ventricular growth response to exercise and cigarette smoking: data from LARGE Heart. Heart. 2006;92:1784–1788. doi: 10.1136/hrt.2006.088294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med. 1998;3:21–28. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- 35.Villarreal FJ, Hong D, Omens J. Nicotine-modified postinfarction left ventricular remodeling. Am J Physiol. 1999;276:H1103–H1106. doi: 10.1152/ajpheart.1999.276.3.H1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loennechen JP, Beisvag V, Arbo I, Waldum HL, Sandvik AK, Knardahl S, Ellingsen O. Chronic carbon monoxide exposure in vivo induces myocardial endothelin-1 expression and hypertrophy in rat. Pharmacol Toxicol. 1999;85:192–197. doi: 10.1111/j.1600-0773.1999.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 37.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 38.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC, Devereux RB. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102–107. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 39.Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989;14:49–57. doi: 10.1016/0735-1097(89)90053-3. [DOI] [PubMed] [Google Scholar]
- 40.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 41.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Witteman JC, D'Agostino RB, Stijnen T, Kannel WB, Cobb JC, de Ridder MA, Hofman A, Robins JM. G-estimation of causal effects: isolated systolic hypertension and cardiovascular death in the Framingham Heart Study. Am J Epidemiol. 1998;148:390–401. doi: 10.1093/oxfordjournals.aje.a009658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


