Abstract
Tobacco dependence is highly prevalent in depressed patients. We assessed changes in [11C]-raclopride binding potential using positron emission tomography before and after the oral administration of d-amphetamine in healthy controls and unmedicated patients with current depression with and without current tobacco dependence. Over a single study day two [11C]-raclopride positron emission tomography scans were taken in thirty-eight subjects: at baseline and 2 hours following oral d-amphetamine 30mg. Twenty controls (9 smokers, 11 non-smokers) and 18 subjects with current major depressive episode (8 smokers, 10 non-smokers). Striatal [11C]-raclopride binding potential was measured before and after d-amphetamine. Depressed smokers had a lower baseline [11C]-raclopride binding potential compared to both control non-smokers (p < 0.007) and depressed non-smokers (p < 0.001). There was a main effect of smoking status on amphetamine-induced change in [11C]-raclopride binding potential (p <0.02), but no main effect of depression. This may be due to a floor effect because of the low BP (binding potential) at baseline. Depressed subjects reported significant increase of positive mood after d-amphetamine administration compared with controls (depressed smokers vs. control smokers: p < 0.05; depressed non-smokers vs. controls: p < 0.055). Tobacco dependence appears to decrease d-amphetamine-induced changes in [11C]-raclopride binding potential as measured by positron emission tomography. Comorbid major depression and tobacco dependence exacerbates this effect, suggesting an altered dopamine system in comorbid patients.
Keywords: tobacco dependence, comorbidity, dopamine, [11C]-raclopride
INTRODUCTION
Major Depressive Disorder (MDD) is a highly prevalent psychiatric illness accompanied by loss of productivity, important health care-related costs, and a heavy emotional burden for both the patients and families. It is estimated that 5% to 12% of men and 10% to 25% of women suffer from MDD at some point during their lifetime (Kessler et al., 2005).
Tobacco dependence is also highly prevalent. Worldwide, approximately 47% of men and 11% of women smoke daily (Anderson, 2006). In the United States, 20.9% of the population smoke regularly (Centers for Disease Control and Prevention, 2006). In Canada, a 2006 survey (Health Canada, 2007) indicates that 14% of the population report daily smoking.
Tobacco dependence and MDD are highly comorbid. MDD patients are two times more likely to smoke than non-depressed subjects (Fergusson et al., 2003). The odds for a smoker to be depressed are 2–3 times that of a non-smoker (John et al., 2004). In severely tobacco-dependent subjects, the odds ratio of MDD has been reported to range between 2.12 and 5.69 (Breslau and Johnson, 2000).
Smokers with comorbid MDD progress to a more severe level of dependence and to experience more severe tobacco withdrawal symptoms than smokers without MDD (Pomerleau et al., 2005). Futhermore, a history of depression (as either a tobacco withdrawal symptom or a psychiatric syndrome) predicts a poorer outcome in smoking cessation studies (Kinnunen et al., 1996).
Decreases in [11C]-raclopride binding potential (BP) in the ventral striatum have been demonstrated in smokers following cigarette smoking (Brody et al., 2006; Scott et al., 2007), but not consistently (Barrett et al., 2004). Intravenous nicotine studies have been shown to reduce the BP of [11C]-raclopride in animals but the only two human studies (one using nicotine gum and the other using nasal spray), found no overall change in [11C]-raclopride BP after nicotine administration in any of the striatal regions examined (Montgomery et al., 2007a; Takahashi et al., 2008).
Although there is considerable evidence to suggest a role for dopamine in MDD, PET studies in humans have reported no consistent changes in striatal D2 binding (D’Haenen and Bossuyt, 1994; Parsey et al., 2001). However, recent findings in drug-naïve patients with MDD do not support the involvement of striatal dopamine D2 receptors in the pathophysiology of MDD (Hirvonen et al., 2008; Montgomery et al., 2007b).
The objective of this study was to assess changes in [11C]-raclopride BP to striatal dopamine D2 receptors following an acute dose of d-amphetamine in four different subject groups: healthy controls and MDD subjects not currently taking antidepressants with or without tobacco dependence. We have previously reported studies using d-amphetamine challenge in MDD subjects, where there was a 2-fold increase in rewarding effects of the drug compared to controls, the magnitude of which correlated with depressive symptom severity (Cardenas et al., 2002; Tremblay et al., 2002), suggesting that depressed subjects have an altered DA system. Based on these studies, we hypothesized that: a) MDD groups would have a greater subjective response to d-amphetamine and that this would be reflected as a larger percent change from baseline in [11C]-raclopride BP; and b) tobacco dependence would further enhance this effect.
MATERIALS AND METHODS
Subjects
Healthy men and women, age 18 to 65, were recruited through word-of-mouth and newspaper advertisements in the Toronto Area. Potential subjects were contacted and systematically screened over the telephone. Subjects were assessed for nicotine dependence by using the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al., 1991) and severity of depression by using the 21-item Hamilton Depression Inventory (HAM-D) (Hamilton, 1960) to determine study suitability. Smoking subjects had to meet a FTND score ≥ 3; depressed subjects were not taking psychotropic medications and had a HAM-D score >15 at pre-screening interview (cut off for eligibility for full SCID interview), while controls were required to have a HAM-D score < 7. Potential study subjects then attended the research unit in person, underwent a Structured Clinical Interview for DSM Axis I Disorders (SCID-I) and a medical screening including a physical exam, ECG, standard blood tests and urine drug screen. Subjects were required to have a negative urine drug screen. Medical examinations were conducted to ensure the subjects were in good general health. Depressed subjects met criteria for a diagnosis of a current major depressive episode with no other Axis I disorder and controls did not report current or past diagnosis of any Axis I disorder.
Pharmacological Challenge
Oral d-amphetamine sulfate 30 mg was used as the pharmacological challenge. This dose was selected based on its safety and ability to produce reliable and measurable positive subjective effects (Cardenas et al., 2004; CPS, 1998). It is slightly higher than that used in some studies (Leyton et al., 2002), but lower than that used in others (Vollenweider et al., 1998).
Procedures
The study was single-blind, whereby subjects were told they could either receive d-amphetamine or placebo, but all subjects received the active drug. Subjects abstained from smoking from bedtime the evening prior to the study day (confirmed by an expired CO level of less than 10 ppm). Two [11C]-raclopride PET scans were performed in each subject. For each, a bolus injection of [11C]-raclopride (370 MBq) was administered and scanning continued for 60 minutes. At the end of the first scan (baseline), 30 mg of d-amphetamine was administered orally and 120 minutes later, the second scan was done. Subjects were permitted to smoke one cigarette approximately 30 minutes before entering the baseline PET scanner and up to 4 cigarettes during the entire study day.
Positron Emission Tomography (PET)
PET imaging data was collected using a GEMS PC2048–15B positron emission tomograph (4.5 × 4.5 × 4.5 mm FWHM in air, 15 slices, 6.5mm slice thickness). The subject’s head was aligned in the scanner with the aid of two orthogonal laser-positioning lines. The transaxial slices paralleled the orbitomeatal line. Head motion was minimized using a custom-fitted thermoplastic mask that also helped to reposition the subject’s head for subsequent scanning. A 10-minute transmission scan for attenuation correction was performed in each subject using a 68-Ge rotating pin source. Immediately after this scan, subjects received a bolus injection of 9.6 ±0.8 mCi of high-specific activity [11C]-raclopride (specific activity 1,184 ±320 mCi/μmole at the time of injection). Emission scans began synchronously with the bolus injection and continued for 60 minutes, with data acquisition of 1 minute for the first 5 frames, 2 minutes for the next 20 frames and then 5 minutes for the last 3 frames, producing at the end of the scan a total of 28 frames, with each time frame containing 15 trans-axial brain slices.
D-amphetamine Concentration and Visual Analogue Scales
A blood sample was taken at 120 minutes post drug administration to obtain d-amphetamine levels. Subjective effects of d-amphetamine and nicotine withdrawal symptoms were measured while subjects were scanned using computerized visual analogue scales (VAS) at baseline, and 120 and 180 minutes post-drug. VAS have been widely used to assess drug-mediated changes in affect (e.g., “high”, increased energy) (Fischman and Foltin, 1991).
PET Data Analysis
The resulting brain images were analyzed using ALICE Imaging Analysis, Version 3.0. The PET scan images come in two packages; one containing all the 420 individual slices (28 time frames by 15 slices per frame) (i.e. the dynamic scan), while the other package contains 15 sum images created by combining all 28 time frames of the same trans-axial plane throughout the scan.
D2 receptor availability in striatum was measured at baseline and 2 hours post-d-amphetamine by estimating the BP using the simplified reference tissue method as described in the literature (Lammertsma and Hume, 1996). Regions of interest (ROIs) were manually drawn on the sum images following the contour of the left and right striatum (including both ventral and dorsal striatum) as well as the cerebellum (reference tissue). After visual inspection, ROIs were overlaid and propagated onto the individual dynamic scans for calculation of the BP values for [11C]-raclopride throughout the scan. Left and right striatal ROIs were averaged to a single striatal value. The striatal and cerebellar BPs were then further processed in PMOD 2.55 (Biomedical Image Quantification Software by PMOD Technologies) by fitting the data to the simplified reference tissue, resulting in a single BP for each scan. This method is less susceptible to unwanted fluctuations in BPs from individual slices due to head movements during the scan (Lammertsma and Hume, 1996). Data obtained from ROIs selected from [11C]-raclopride PET images have been shown to be almost identical to those obtained from co-registered MRI images (Wang et al., 1996), therefore MRI co-registration was not deemed necessary. PET data was corrected for head movement and one subject from the MDD group was removed from PET analysis due to excessive head motion (detected by visual inspection of the PET images) during both scans.
Statistical analysis was done using SPSS version 13.0 software (SPSS Inc). Each subject had a [11C]-raclopride BP value for both scans. A logistic regression was performed first on the baseline scan with the independent variables group, age, and smoking status and on the post-drug scan with baseline scan values added to the model. The residual of the baseline scan was the regressed onto the post-drug scan in order to determine the variance in post-drug BP that was not accounted for by baseline differences.
The percentage of change in [11C]-raclopride BP was calculated as follows:
Subjective Effects Data Analysis
Data were analyzed with SPSS version 13.0, SPSS Inc. Chicago, IL. or Excel 2000, Microsoft Corporation. The “peak” subjective effects were defined as the highest magnitude post-drug VAS variable values (0–100mm) from t = 120–240 minutes after d-amphetamine administration. The baseline values were then subtracted to measure the peak minus baseline (change) scores.
All the VAS items’ change scores were subjected to a multivariate between-subjects analysis with smoking status and mood as fixed factors and age and gender as covariates to assess trends, then to independent samples Bonferroni post-hoc t-tests across the 4 main groupings and finally, to regression analyses vs. depression severity (HAM-D scores) to look for specific differences. The change scores of some of the dependent variables with similar trends were empirically grouped together as either negative or positive drug effects mean composite scales change scores. The positive and negative effects composite scale was derived as previously described (Cardenas et al., 2002; Tremblay et al., 2002). To validate pooling of the items, reliability analyses were first conducted on the change scores of the proposed groupings of variables of both composite measures. The Cronbach α for internal consistency was 0.86 for the positive effects composite scale, and 0.79 for the negative effects composite scale, thereby justifying our use of the pooling method.
Correlation analyses were then conducted on the negative and positive effects composite scores of the VAS comparing controls and MDD smokers and non-smokers groups across the entire range of the HAM-D scores. Significance of the difference in the correlation coefficients (R-values) for the independent groups was determined by a Z-test. Mean composite change scores were compared across all groups using ANOVAS and independent samples post-hoc t-tests. Subject characteristics and baseline measures before drug administration were analyzed with independent t-tests or Chi-Square analyses where appropriate.
Correlation analyses were also conducted on the negative and positive effects composite scores of the VAS against [11C]-raclopride BPs for each subject group. Significance of the difference in the correlation coefficients (R-values) for the independent groups was determined by a Z-test. Subjective effects data for 3 control non-smokers and 2 control smokers were lost due to hardware failure.
RESULTS
Subject Characteristics
Data were collected from 18 MDD (8 smokers, 10 non-smokers) and 20 control (9 smokers, 11 non-smokers) subjects (N = 38, 16 males, 22 females, ages 23–59 years). Two depressed subjects had a past history of suicide attempt (3 and 10 years before the study) and all were non-violent.
Table 1 shows subject characteristics and baseline measures. The MDD group had higher mean scores on measures of depression (HAM-D = 22.6 ± 7.6, BDI (Beck Depression Inventory) = 26.8 ± 8.6) than controls (HAM-D = 1.4 ± 2.7, BDI = 3.2 ± 3.8) (p < 0.001 for both HAM-D and BDI). As well, a significant age difference (p < 0.05) was found between the MDD smokers (38.8 ± 6.3) and control non-smokers (27.5 ± 4.0) (see Figure 1).
Table 1.
Subject demographics and characteristics
| Characteristic | Non-smokers | p-value | Smokers | p-value | ||
|---|---|---|---|---|---|---|
| Control (n=11) | Depressed (n=10) | Control (n=9) | Depressed (n=8) | |||
| Age (yrs) (SD) | 27.5 (4.0) | 36.3 (12.9) | NS | 36.8 (13) | 38.8 (6.3) | NS |
| Sex (M:F) | 3:8 | 2:8 | NS | 8:1 | 3:5 | 0.005 |
| FTND (SD) | 4.9 (1.2) | 5.2 (1.5) | NS | |||
| CPD (SD) | 15.2 (3.3) | 18.1 (5.1) | NS | |||
| Age 1st smoke (SD) | 20.1 (7.2) | 17.1 (4.3) | NS | |||
| HAM-D (SD) | 0.4 (1.2) | 23.2 (5.7) | 0.002 | 1.4 (2.7) | 22.6 (7.6) | 0.016 |
| BDI (SD) | 1.7 (2.3) | 24.4 (6.9) | 0.001 | 3.2 (3.8) | 26.8 (8.6) | 0.02 |
Note. FTND = Fagerstrom Test for Nicotine Dependence; CPD = cigarettes per day.
Fig 1. Correlation between baseline [11C]-raclopride BP and age for the four subject groups.
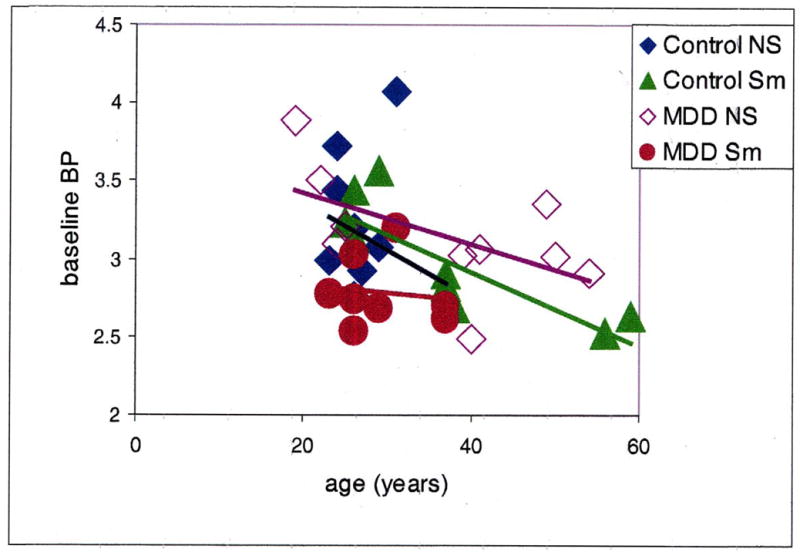
Control NS = control non-smokers, Control Sm = control smokers, MDD NS = depressed non-smokers, MDD Sm = depressed smokers. There was an overall significant correlation (r = −0.5; p < 0.05) and a significant correlation in the control smoker group only (r= −0.7; p < 0.01).
The male/female (M/F) ratio was not counterbalanced in the groups. However, these gender differences across the groups did not impact significantly in the overall results when age and gender were included in the multivariate analysis (see below).
D-amphetamine-Mediated Effects on [11C]-Raclopride Displacement
As expected, age overall was significantly correlated with baseline [11C]-raclopride BP (r = −0.5; p < 0.05), and was also correlated in the control smoker group (r = −0.7, p < 0.01) (Figure 1). Baseline and post-drug BPs were also highly intercorrelated, (r = 0.81, p < 0.0001) thus indicating trait differences in [11C]-raclopride BP. There were no significant differences among groups. In order to determine which variable best predicts post-amphetamine binding of [11C]-raclopride, the residuals from the logistic regression performed on scan 1 were regressed onto scan 2. The resulting output reflects the variance in scan 2 BPs that are not accounted for by baseline individual differences including difference due to age. Of the possible predictors of this variance only smoker status was significant, accounting for approximately 20% of the variance (p = 0.011). Figure 2 shows the percent change in [11C]-raclopride BP following amphetamine challenge in all four groups. A two-way ANOVA with age included as a covariate showed no main effect of MDD status (F = 0.667, p = 0.42). However, smoking status had a significant influence on the percentage change in [11C]-raclopride BP following amphetamine challenge (F = 10.6, p < 0.003) The interaction between the two factors approached significance (F = 3.34, p = 0.077).
Fig 2. [11C]-raclopride binding potential (BP) post-amphetamine administration expressed as a percentage of [11C]-raclopride BP at baseline among the four subject groups.
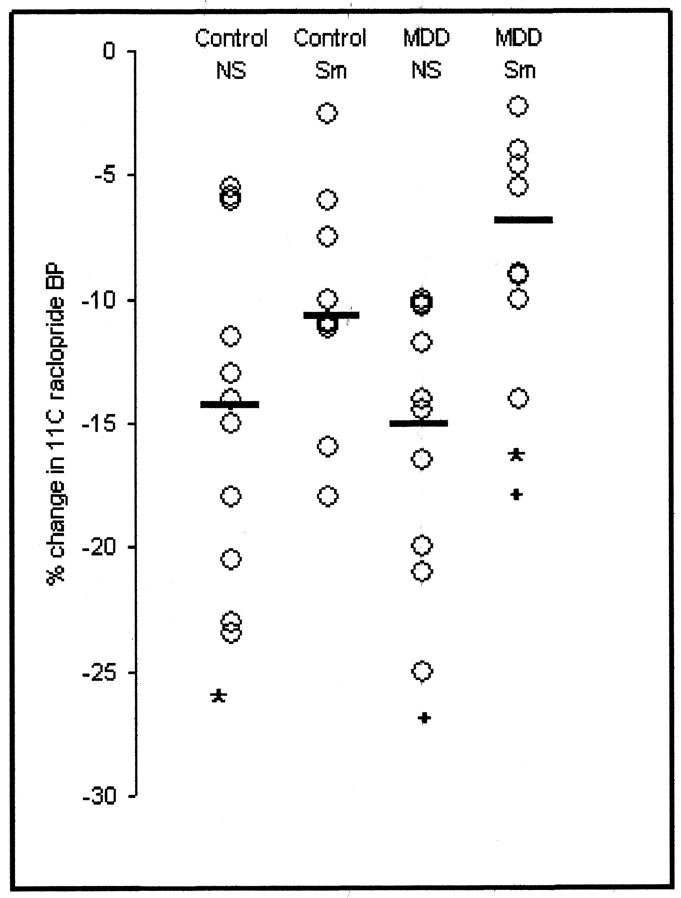
Control NS = control non-smokers, Control Sm = control smokers, MDD NS = depressed non-smokers, MDD Sm = depressed smokers.
* denotes a significant difference (p=0.03) between control non-smokers and depressed smokers and + denotes a significant difference (p=0.01) between depressed non-smokers and depressed smokers. The ⚊ denotes the mean.
Table 2 shows the [11C]-raclopride BP for each group BP pre- and post-d-amphetamine administration as well as the percent change in BP. Mean plasma concentrations (N = 24) of d-amphetamine at 2 hours post-drug administration were similar among groups at 333.5 ± 125 nmol/L (range 62 nmol/L to 602 nmol/L). The percent change in [11C]-raclopride BP was not correlated with plasma d-amphetamine concentrations (r = −0.34, p = 0.11).
Table 2.
[11C]-raclopride BP for each group BP pre- and post-d-amphetamine administration
| Group | Baseline BP | Post-amphetamine BP | % change from baseline |
|---|---|---|---|
| Control Non- smokers (n=11) | 3.14 ± 0.3 | 2.69 ± 0.4 | −14.2 ± 3.9* |
| Control Smokers (n=9) | 2.99 ± 0.2 | 2.66 ± 0.3 | −11.1 ± 3.6 |
| MDD Non-smokers (n=10) | 3.13 ± 0.2 | 2.63 ± 0.3 | −15.6 ± 2.9 
|
| MDD Smokers (n=8) | 2.79 ± 0.2 | 2.59 ± 0.2 | −6.8 ± 2.9* 
|
Subjective Effects of Dextroamphetamine
The primary outcome variables for subjective effects of d-amphetamine were the positive and negative effects composite scales. Positive and negative effects composite scores for all groups are summarized in Figure 3.
Fig 3. Subjective effects of d-amphetamine measured using visual analog scales (VAS).
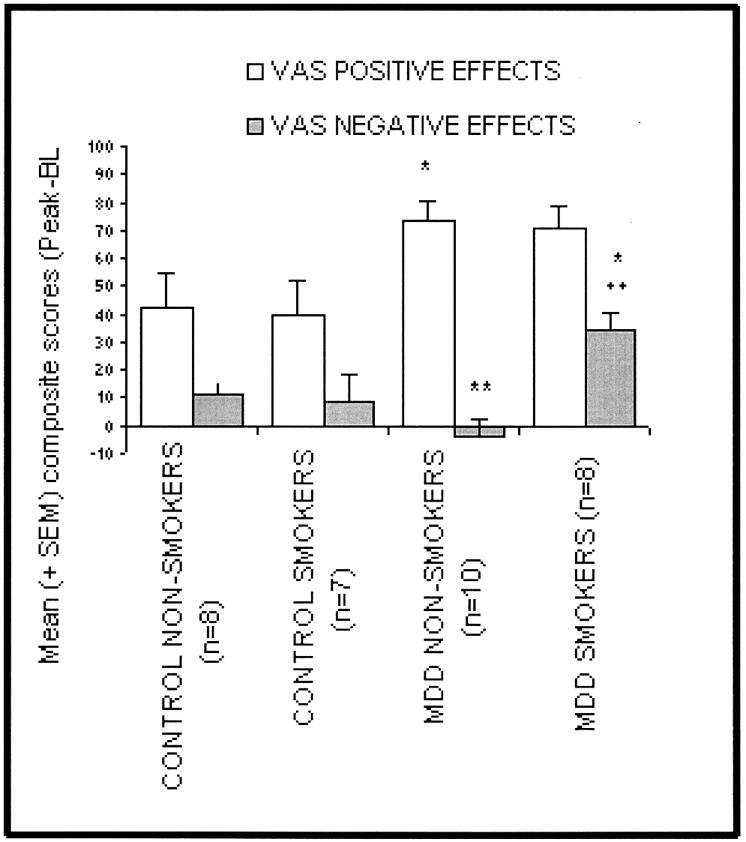
Data were consolidated into positive drug effects (e.g., “high”, alert) and negative drug effects (e.g., irritable, restless) and are presented as the mean of the peak score minus the baseline score.
* = significantly different from control non-smokers (p < 0.05),
** = significantly different from MDD non-smokers (p < 0.01)
The MDD subjects (smokers and non-smokers) showed significant elevations of positive mood following d-amphetamine administration relative to their control counterparts (73.7 vs. 42.0, p = 0.01) and (70.4 vs. 39.9, p = 0.055) respectively. The MDD smokers have significant increases in the negative effects change scores relative to the other three groups [MDD non-smokers, control smokers (p < 0.05) and control non-smokers (p < 0.01)].
A correlation analysis of the dependent variables positive effects composite change scores across the entire range of independent HAM-D scores showed that the severity of MDD correlated significantly with increased reports of positive subjective effects after peak (Cmax) d-amphetamine effects (e.g. “high”, energy) for smokers (r = 0.567, p < 0.05).
A separate correlation analysis of negative effects composite scores also correlated with the severity of MDD in smokers (r = 0.62, p < 0.05), but not in non-smokers (r = 0.43, p = NS). A separate MDD group correlation analysis of the negative effects composite change scores vs. the HAM-D scores shows a similar trend for the MDD smokers (r = 0.541; p < 0.05). Thus, the magnitude of negative amphetamine effects experienced by smokers also correlates with the severity of depression.
A significant correlation was observed between peak d-amphetamine-mediated negative effects as assessed with the VAS and d-amphetamine-induced changes in [11C]-raclopride BP in MDD smokers (r2 = 0.63, p < 0.03), whereby an increase in negative effects score was positively correlated to the decrease in [11C]-raclopride binding post-amphetamine. All other correlations between subjective effects and binding for the other subject groups were non-significant.
DISCUSSION
This is the first [11C]-raclopride PET study in patients with comorbid tobacco dependence and MDD. The primary findings were: subjects with comorbid tobacco dependence and MDD had a significantly lower [11C]-raclopride BP at baseline as well as lower amphetamine-induced changes in [11C]-raclopride binding, as compared to non-smokers either with or without MDD; and d-amphetamine-induced decreases in [11C]-raclopride BP were significantly lower in smokers compared to non-smokers. Thus, smokers in general appear to have a decreased dopaminergic tone and depressed smokers have more serious alterations of dopamine pathways than those patients with either disorder alone. These findings may have clinical implications since low DA function in comorbid patients might exacerbate symptoms of low motivation, and anhedonia, and also increase relapse to smoking in those attempting to quit.
The neurobiology relevant for the occurrence of comorbid mood and substance abuse disorders has not been elucidated as most studies exclude comorbid patients. Tobacco smokers are 2 to 5 times more likely to have depressive symptoms than non-smokers (Fergusson et al., 2003; Glassman et al., 1990). To the extent that overlapping disorders might reflect overlapping pathophysiology, comorbid patients may provide important information on the neurobiology of both disorders.
A large body of literature implicates disturbed DA function in substance use disorders (Di Chiara and Imperato, 1988; Volkow et al., 2004), including smoking – both in animals and humans (Brody et al., 2004; Marenco et al., 2004; Tsukada et al., 2005). The addiction process is initiated in part by direct or indirect DA release in the mesocorticolimbic system induced by the acute administration of drugs of abuse. However, although acute drug intake increases DA neurotransmission, chronic drug consumption results in decreased DA activity (Volkow et al., 1997; Volkow et al., 1996; Wang et al., 1997).
Our data supports our hypothesis that chronic cigarette smoking, presumably via the effects of nicotine (and perhaps MAO), produces differences in raclopride displacement as a result of the d-amphetamine challenge between smokers and non-smokers and this difference may be due to decreased dopamine release and/or altered receptor adaptation or sensitivity.
The lesser decrease in [11C]-raclopride displacement after d-amphetamine challenge in smokers found in this study suggests lower DA activity in this population, thus chronic nicotine use may produce a significant alteration in the release of DA in the synaptic cleft when challenged with amphetamine. Several animal studies have already linked acute nicotine administration with DA release in the nucleus accumbens (Brody et al., 2004; Marenco et al., 2004; Tsukada et al., 2005).
Intravenous nicotine has been shown to reduce the BP of [11C]-raclopride in animals, but human studies have not been reported (Brody et al., 2004; Marenco et al., 2004). One study has shown that tobacco-dependent subjects who smoked during an interruption in scanning had greater reductions in [11C]-raclopride BP than those who did not smoke, showing acute smoking-induced DA release (Brody et al., 2004). However, another study (Barrett et al., 2004) did not replicate these findings in nicotine-deprived chronic smokers. This may be due to the different PET methodologies used in each study. In our study, smoking times were standardized across subjects whereby they refrained from smoking for 30 minutes prior to entering the scanner. Following positioning in the scanner, setting up the IV line and conducting the pre-scan procedures, this resulted in an approximate 1 hour abstinence period prior to scanning. This should be an adequate amount of time to equilibrate any effect of smoking on dopamine release and binding. Therefore, smoking prior to scanning most likely was not the reason that smoker subjects showed lower [11C]-raclopride BP at baseline than non-smokers.
While the smaller changes in [11C]-raclopride BP observed with d-amphetamine in non-depressed smokers in this study may be the result of altered dopaminergic activity due to chronic smoking, it can also be that the absence of a significant difference is a power issue and perhaps a larger sample size would have detected it. Although unlikely, smoking one cigarette before the scan may have altered the raclopride BP, but scanning patients in nicotine withdrawal was not a feasible alternative. Further research is needed to clarify and reproduce these findings before advancing any definite conclusions. These subjects may have higher baseline levels of DA in the brain due to a smoking-induced decrease in MAO A and B activity (Fowler et al., 2003; Fowler et al., 2005), allowing for a smaller displacement of raclopride by d-amphetamine (Berlin and Anthenelli, 2001).
Furthermore, a recent PET paper (Meyer et al., 2006) has provided convincing evidence that MAO A density is highly elevated (34%) during an MDD episode in non-smokers and thus provides support for the self-medication hypothesis of comorbidity since tobacco inhibits both forms of MAO in brain. This also may be one explanation for the reduced baseline BP of [11C]-raclopride seen in this study.
The relationship between DA and depression has been obscured by the attention given to serotonin and norepinephrine. Deficits in energy and ability to experience pleasure and motivation are all key diagnostic features of MDD and these deficits are now known to be regulated in part by dopamine systems. Other sources of evidence suggest a role for a diminished dopaminergic transmission in depression (Nestler and Carlezon, 2006; Nutt, 2006). Some neuroimaging studies support the hypothesis that depression is a state of reduced dopaminergic transmission (D’Haenen and Bossuyt, 1994; Meyer et al., 2001). Others, however, are consistent with the results of this study and found no differences between MDD patients and controls (Hirvonen et al., 2008; Montgomery et al., 2007b).
We found no differences in d-amphetamine-mediated [11C]-raclopride displacement in depressed patients compared to controls, showing that MDD per se does not alter DA release after the d-amphetamine challenge. However, positive subjective effects of d-amphetamine were significantly higher (p < 0.05) in depressed subjects (smokers and non-smokers) compared to controls. The enhanced positive subjective response to d-amphetamine in MDD patients replicates previous findings showing an altered response to d-amphetamine in depressed subjects (Cardenas, 2003; Cardenas et al., 2002; Tremblay et al., 2002). Since we did not observe a correlation between positive effects and change in raclopride displacement, the anhedonia observed in depression may not be the result of decreased DA release in these patients but rather an altered state in the sensitivity of DA receptors (Ko et al., 2006; Tremblay et al., 2002).
Perhaps the most interesting finding of this study is that MDD smokers presented significantly lower [11C]-raclopride BP at baseline (p < 0.04) than non-smoking MDD subjects or non-smoking subjects without MDD. This suggests that the differential response in raclopride BP in the comorbid patient are not only present after d-amphetamine challenge, but altered dopaminergic tone may be present prior to the administration of d-amphetamine. The between group differences seen in the post-drug scan may be due to a floor effect in this subgroup whereby no further decrease in [11C]-raclopride BP could be seen following amphetamine challenge because the BP was already so low. However, this finding should be interpreted with caution since the number of subjects studied is limited.
Several limitations of our study must be acknowledged. Our sample size was relatively small, but no different than many other published PET studies. We could not perform separate analysis in ventral striatum relative to caudate/putamen. Despite great discrepancies related to sample size, imaging protocols and image analysis, several studies using PET and functional magnetic resonance imaging (fMRI) have shown differential neural responses in MDD with altered ventrolateral prefrontal cortex, amygdala/ventral striatal activity and caudate putamen (Drevets, 2000; Drevets, 2003; Goldapple et al., 2004). Thus, the results for MDD may reflect the absence of subregion analysis. It would have been helpful to have striatum subregions, however this would have required a higher resolution camera.
There was no correction for possible hormonal differences in the subjects (White et al., 2002) and the study was not age- and gender-matched. Although there were no gender differences by group, there was a negative correlation between baseline BP and age. We did not take advantage of MRI co-registration techniques and finally, we did not explore other brain circuits that are important for depression, namely serotonin and norepinephrine.
In summary, chronic cigarette smoking produces a differential response to d-amphetamine between smokers and non-smokers, and baseline [11C]-raclopride displacement was lower in smokers than in controls, supporting the role of chronic smoking altering dopaminergic pathways and consistent with decreased availability of DA receptors, after chronic use of other psychostimulants (Volkow et al., 2006). Taken together, the likely interpretation of these results is that chronic use of abuse substances including nicotine decreases the availability of D2 receptors – further confirming abnormalities in the reward process of these subjects. Whether this is the result of chronic exposure to drugs or a pre-existing condition that predisposes one to drug use is not known. The study also showed altered dopaminergic pathways in comorbid patients at baseline. The DA system of patients with comorbid tobacco dependence and MDD is more dysfunctional than those of patients that present with one disorder alone. These findings may have implications for smoking cessation treatments in MDD as it may result in more individualized treatment strategies, whereby treatments affecting dopaminergic function may be used in conjunction with nicotine replacement therapy.
Acknowledgments
This work was funded by NIDA grant DA-RO1–13630. The Principal Investigator (Dr. Busto) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We wish to acknowledge the invaluable assistance of Dr. Marco Leyton for his thoughtful review of this manuscript.
References
- Anderson P. Global use of alcohol, drugs and tobacco. Drug Alcohol Rev. 2006;25(6):489–502. doi: 10.1080/09595230600944446. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54(2):65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4(1):33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson E. Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health. 2000;90(7):1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63(7):808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Cardenas L. Dopaminergic Activity in Major Depression and Nicotine Dependence. Toronto: University of Toronto; 2003. [Google Scholar]
- Cardenas L, Houle S, Kapur S, Busto UE. Oral D-amphetamine causes prolonged displacement of [11C]raclopride as measured by PET. Synapse. 2004;51(1):27–31. doi: 10.1002/syn.10282. [DOI] [PubMed] [Google Scholar]
- Cardenas L, Tremblay L, Naranjo C, Herrmann N, Zack M, Busto U. Brain reward system activity in major depression and comorbid nicotine dependence. Journal of Pharmacology and Experimental Therapeutics. 2002;302(3):1265–1271. doi: 10.1124/jpet.302.3.1265. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tobacco use among adults - United States, 2005. Morbidity and Mortality Weekly Report. 2006;55(42):1145–1151. [PubMed] [Google Scholar]
- Krogh C, editor. CPS. Compendium of Pharmaceuticals and Specialties. Ottawa, Ontario, Canada: Canadian Pharmaceutical Association; 1998. [Google Scholar]
- D’Haenen H, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biological Psychiatry. 1994;35:128–132. doi: 10.1016/0006-3223(94)91202-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol Med. 2003;33(8):1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. British Journal of Addiction. 1991;86(12):1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Pappas N, Ferrieri R, Shea C, Garza V, Xu Y, Schlyer D, Gatley SJ, Ding YS, Alexoff D, Warner D, Netusil N, Carter P, Jayne M, King P, Vaska P. Low monoamine oxidase B in peripheral organs in smokers. Proc Natl Acad Sci U S A. 2003;100(20):11600–11605. doi: 10.1073/pnas.1833106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W, Franceschi D, Shea C, Garza V, Xu Y, Ding YS, Alexoff D, Warner D, Netusil N, Carter P, Jayne M, King P, Vaska P. Comparison of monoamine oxidase a in peripheral organs in nonsmokers and smokers. J Nucl Med. 2005;46(9):1414–1420. [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. Jama. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. Canadian Tobacco Use Monitoring Survey (CTUMS) 2006 2007 [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Markkula J, Rasi-Hakala H, Nagren K, Salminen JK, Hietala J. Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology (Berl) 2008;197(4):581–590. doi: 10.1007/s00213-008-1088-9. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity--a population-based study including smoking cessation after three years. Drug Alcohol Depend. 2004;76(3):287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol. 1996;64(4):791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Ko F, Tallerico T, Seeman P. Antipsychotic pathway genes with expression altered in opposite direction by antipsychotics and amphetamine. Synapse. 2006;60(2):141–151. doi: 10.1002/syn.20287. [DOI] [PubMed] [Google Scholar]
- Lammertsma A, Hume S. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27(6):1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine-induced dopamine release in primates measured with [11C]raclopride PET. Neuropsychopharmacology. 2004;29(2):259–268. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63(11):1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12(18):4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: A [11C]raclopride PET study. Synapse. 2007a;61(8):637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Stokes P, Kitamura Y, Grasby PM. Extrastriatal D2 and striatal D2 receptors in depressive illness: pilot PET studies using [11C]FLB 457 and [11C]raclopride. J Affect Disord. 2007b;101(1–3):113–122. doi: 10.1016/j.jad.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl 6):3–8. [PubMed] [Google Scholar]
- Parsey R, Oquendo M, Zea-Ponce Y, Rodenhiser J, Kegeles L, Pratap M, Cooper T, Van Heertum R, Mann J, Laruelle M. Dopamine D2 receptor availability and amphetamine-induced dopamine release in unipolar depression. Biological Psychiatry. 2001;50(5):313–322. doi: 10.1016/s0006-3223(01)01089-7. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res. 2005;7(1):91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK. Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology. 2007;32(2):450–457. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Fujimura Y, Hayashi M, Takano H, Kato M, Okubo Y, Kanno I, Ito H, Suhara T. Enhanced dopamine release by nicotine in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. Int J Neuropsychopharmacol. 2008;11(3):413–417. doi: 10.1017/S1461145707008103. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry. 2002;59(5):409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Harada N, Nishiyama S, Fukumoto D, Kakiuchi T. Nicotine modulates dopamine synthesis rate as determined by L-[beta-11C]DOPA: PET studies compared with [11C]raclopride binding in the conscious monkey brain. Synapse. 2005;57(2):120–122. doi: 10.1002/syn.20157. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20(9):1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Maguire RP, Leenders KL, Mathys K, Angst J. Effects of high amphetamine dose on mood and cerebral glucose metabolism in normal volunteers using positron emission tomography (PET) Psychiatry Res. 1998;83(3):149–162. doi: 10.1016/s0925-4927(98)00033-x. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16(2):174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Levy AV, Fowler JS, Logan J, Alexoff D, Hitzemann RJ, Schyler DJ. MR-PET image coregistration for quantitation of striatal dopamine D2 receptors. J Comput Assist Tomogr. 1996;20(3):423–428. doi: 10.1097/00004728-199605000-00020. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]


