Abstract
This randomized, placebo-controlled, blinded study investigated the hypoalgesic effects of high-frequency transcutaneous electrical nerve stimulation (TENS) delivered via a glove electrode compared with standard self-adhesive electrodes. Fifty-six TENS-naïve, healthy individuals (18 to 50 years old; 28 men, 28 women) were randomly allocated to 1 of 4 groups (n = 14 per group): glove electrode; placebo TENS using a glove electrode; standard electrode; and no treatment control. Active TENS (continuous stimulus, 100 Hz, strong but comfortable intensity) was applied to the dominant forearm/hand for 30 minutes. Placebo TENS was applied using a burst stimulus, 100-Hz frequency, 5-second cycle time for 42 seconds, after which the current amplitude was automatically reset to 0 mA. Pressure pain thresholds (PPTs) were recorded from 3 points on the dominant and nondominant upper limbs before and after TENS. Statistical analyses of dominant PPT data using between-within groups ANOVA showed significant differences between groups at all 3 recording points (P = .01). Post hoc Scheffe tests indicated no significant difference between the standard electrode and glove electrode groups. There was a significant hypoalgesic effect in the standard electrode group compared with the control group and between the glove electrode group and both the control and placebo TENS groups. There was no significant interactive effect between time and group at any of the recording points (P > .05).
Perspective
This study presents a comparison of the hypoalgesic effects of 2 different types of TENS electrode, a novel glove electrode and standard self-adhesive rectangular electrodes. The glove electrode provides a larger contact area with the skin, thereby stimulating a greater number of nerve fibers. The results show that both electrodes have similar hypoalgesic effects and therefore give the clinician another choice in electrode.
Keywords: Transcutaneous electrical nerve stimulation, electrodes, pressure pain threshold
Transcutaneous electrical nerve stimulation (TENS) is a noninvasive modality commonly used for both acute and chronic pain conditions.29 The successful application of TENS involves a period of trial and error in the selection of a number of stimulation parameters, for example, frequency and amplitude of the current and the type and size and location of the electrodes used to deliver the current.19 Several meta-analyses and Cochrane systematic reviews have highlighted the range of TENS treatment regimes used, including various electrode shapes and sizes.3–5,18,20 Although a number of animal and human studies have examined the effects of different current parameters, including frequency, pulse amplitude, pulse duration, and waveform,1,13,14,21,32 few studies have investigated the effects of different types of electrodes and their placement.15,16,30 Manufacturers currently offer a variety of electrode shapes for applications to different body regions, including a novel glove electrode design that is marketed for use in conditions that affect the hand, including arthritis and Reynaud’s syndrome. Only 1 study has previously investigated the glove electrode for pain relief; Renklitepe et al33 reported a significant pain reduction in patients with degenerative arthritis of the hand. However, this study did not compare the effect against a placebo, making interpretation of this finding difficult.
Adequate blinding is an inherent difficulty in both experimental and clinical TENS studies. Several aspects of a study design can promote the effectiveness of blinding, such as using TENS-naïve participants, sham TENS units that are visually identical to real units, and providing identical instructions to all participants.12 Typical TENS placebos utilize a sham device that does not deliver a stimulus.7,23 However, the application of a low-intensity stimulus would appear to be a more credible placebo due to the associated sensations.27 Recently, Rakel and colleagues28 compared the effectiveness of participant and clinician blinding associated with delivering an inactive (no stimulus) TENS placebo versus a TENS placebo that delivered a perceptible stimulus for a short period of time (termed transient placebo, Rakel et al28). The transient placebo and the inactive placebo were similarly effective at blinding the participants; approximately 50% to 60% of the participants identified the inactive placebo and the transient correctly. Importantly, the clinician applying TENS was unable to differentiate between the active unit and the transient placebo but correctly identified 100% of the inactive placebo applications. These preliminary data suggest the ability to perform a clinician-blinded study with TENS and provide justification for further testing of this type of TENS placebo.
Experimental pain models have been used to test various stimulation parameters of TENS, including frequency, pulse amplitude, and site of application.6,10,31 Pressure pain was selected for the current study because its reliability has been demonstrated and it has been used by the authors previously.1,2,9,25,28 The objective of the current study was to compare the hypoalgesic effects of high-frequency TENS delivered via a glove electrode and 1 standard self-adhesive electrode versus 2 standard self-adhesive electrodes on upper limb pressure pain threshold (PPT) in healthy volunteers. A randomized, placebo-controlled, assessor- and participant-blinded design was used that incorporated a new TENS placebo.
Materials and Methods
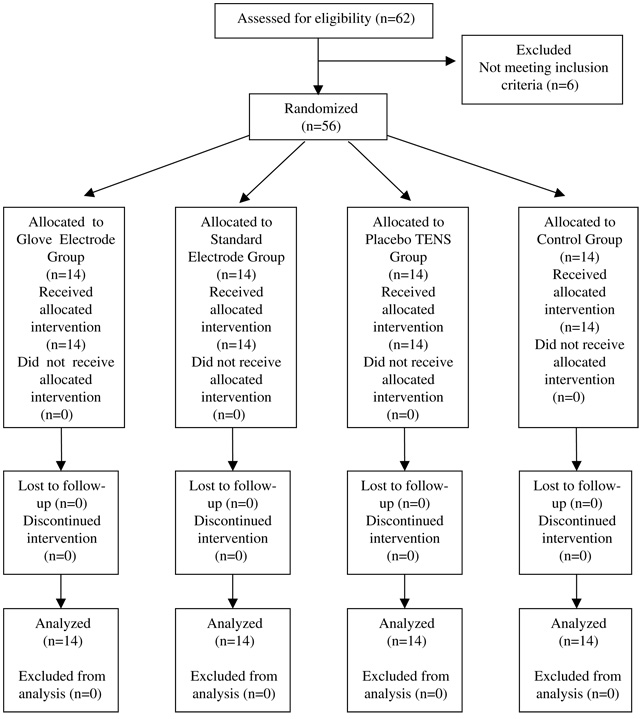
Participants
After obtaining approval from the University of Ulster’s Research Ethics Committee, 56 TENS-naïve, healthy volunteers were recruited from the staff and students of the University (28 men, 28 women; mean age, 28 years; range, 18 to 50 years; 53 right-handed, 3 left-handed). Participants were screened for relevant contraindications including injury or nerve damage to the upper limbs, current pain, pregnancy, chronic illness, cardiac pacemaker, epilepsy, allergies to the TENS electrodes, currently taking pain medication, skin conditions, or deficient skin sensation in the areas of electrode placement. After giving informed written consent, participants were randomly allocated to 1 of 4 groups (n = 14 per group): glove electrode; placebo TENS; standard electrode; and no treatment control (see Fig 1). Randomization was performed using a computer-generated, random-number list compiled by a statistician (E.McC-G.) not involved in data collection; randomization was stratified for gender, thus ensuring equal numbers of men and women in each group. Group allocation was concealed by writing this onto cards and sealing them in consecutively numbered opaque envelopes; envelopes were locked in a secure cabinet with restricted access and opened after the participant provided consent.
Figure 1.
Study design and flow of participants.
Sample size was calculated using a mean difference of 10.1 N and standard deviation of 6.98 N, obtained from previous data on PPT and TENS.24 At a significance level of .05 and power of 80%, it was calculated that 12 participants were required in each group (version 15; Minitab, State College, PA). Allowing for attrition, 14 participants were therefore recruited per group.
Intervention Groups
An investigator (S.C.) who was aware of group allocation prepared participants and applied the intervention. TENS was applied to the dominant upper limb using Select TENS units (Empi, St. Paul, MN) as described below:
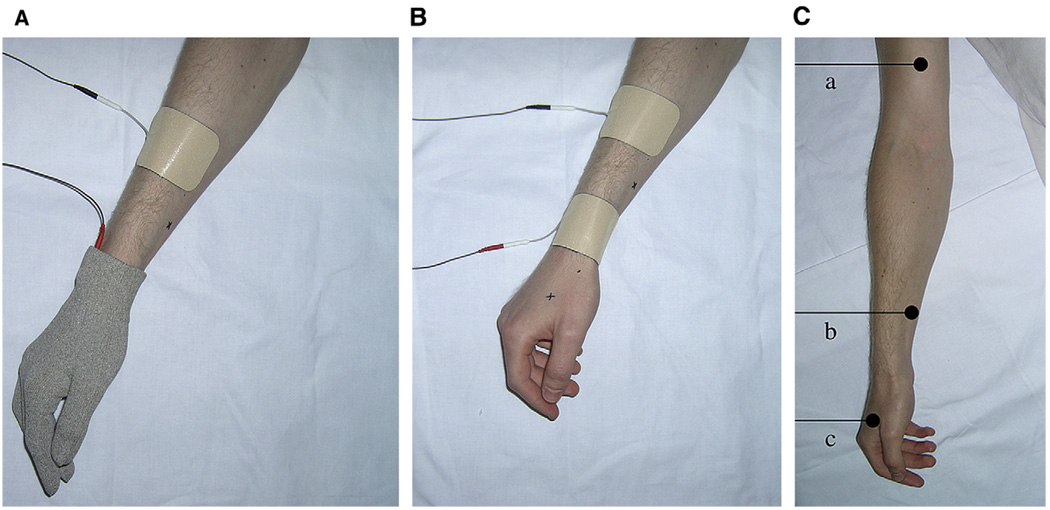
Glove electrode group. One glove electrode and 1 standard 50 × 90-mm self-adhesive electrode (TensCare, Surrey, UK) were applied (see Fig 2A). The standard electrode was positioned on the lateral aspect of the forearm, 10 cm proximal to the distal wrist crease. In the glove electrode, the electrical current is delivered to the lead attachment point close to the wrist and spreads throughout the entire glove, which is made of conductive material. The material in the glove electrode differs from a standard self-adhesive electrode in that it can be washed and therefore the glove electrode itself can be reused (TensCare).
Placebo TENS group. Electrodes were applied as in (1), above. The placebo unit was a Select TENS unit (Empi) modified to deliver a stimulus for a total of 42 seconds (burst stimulus with 100-Hz frequency and a 5-second cycle time), after which time the current amplitude reset to 0 mA. These parameters were chosen because they produced a comfortable stimulus sensation that lasted long enough for the participant to be aware of the sensation but was too brief to have any definite physiological effect.
Standard electrode group. Two 50 × 90-mm standard self-adhesive electrodes (TensCare) were positioned on the lateral aspect of the forearm at the level of the distal wrist crease and the lateral aspect Cowan et al 695 of the forearm 10 cm proximal to the distal wrist crease (see Fig 2B). This electrode position projected paraesthesia into the hand.
Control group. Electrodes were applied as in (1), above, but participants were made aware that the TENS device was not switched on and that no electrical current was delivered.
Figure 2.
A, Electrode position in the glove electrode group. B, Electrode position in the standard electrode group. C, Location of the 3 PPT recording points on the arm (a), forearm (b), and hand (c).
The TENS units were calibrated using an oscilloscope (Model 1602; Gould Electronics, Essex, UK) before the study commencing. In the glove electrode and standard electrode groups, TENS was applied using a frequency of 100 Hz at a “strong but comfortable” intensity for 30 minutes (Select TENS unit, Empi) while the participant lay supine on a treatment plinth. This intensity level was achieved using a standard procedure, during which the pulse amplitude was slowly adjusted to produce a sensation described as strong but comfortable by the participant. Participants were asked every 3 minutes if the intensity of TENS they were feeling had decreased, and if so, the investigator increased the pulse amplitude until the participants reported a “strong but comfortable” intensity level again. The mA value of the arbitrary units displayed on the Select TENS unit was calibrated for each participant. The pulse amplitude was calculated by measuring the peak to peak voltage of the waveform across a 1-kΩ resistor on an oscilloscope (Model 1602, Gould Electronics). The mean maximum pulse amplitude used to produce a strong but comfortable intensity in the 2 active TENS groups was: glove electrode group, 31.4 ± 1.8 mA; standard electrode group, 32.9 ± 1.4 mA (mean ± SEM). The pulse duration of the Select TENS unit increased (up to a maximum of 400 μs) as the pulse amplitude increased; for the mean maximum pulse amplitude values for the 2 active groups described above, the pulse duration was measured as 52 to 55 µs, using a 1-kΩ resistor on the oscilloscope.
In the placebo TENS group, stimulation was delivered at sensory threshold for 42 seconds, after which the TENS amplitude reset to 0 mA; participants in this group were asked every 3 minutes if they were comfortable, but there were no other prompts in relation to TENS sensation. Any questions about the lack of TENS sensation were dealt with by the investigator saying that some types of TENS treatments are barely perceptible. We attempted to blind group allocation for participants in the placebo TENS and active TENS groups as follows: the sham TENS device looked similar to the active TENS device; participants were told that they may or may not feel a tingling sensation; and, all participants were TENS-naïve. In the control group, participants were told that the TENS device would not be switched on and that they would not receive any electrical stimulation.
At the end of data collection, the effectiveness of blinding was assessed by asking the participant and outcome assessor which TENS intervention they thought was delivered, with 3 options for the answer: “real,” “placebo,” or “don’t know.”
Participant Preparation
Participants’ upper limbs were cleaned before marking electrode placement and PPT recording sites using a permanent marker. The electrode placement sites were marked out as described above. Three PPT recording sites (see Fig 2C) were marked out on both the dominant and nondominant upper limbs as follows: (1) 3 cm distal to the distal end of the anatomical snuff box in the midline of the belly of the first dorsal interosseous muscle; (2) on the anterior aspect of the forearm, 7.5 cm proximal to the distal wrist crease; and, (3) on the anterior aspect of the biceps muscle, 10 cm proximal to the elbow flexor crease in the midline of the biceps muscle. These sites were chosen to examine the effects of TENS both within (hand and forearm) and outside (arm) the area of stimulation.
Pressure Pain Threshold Measurements
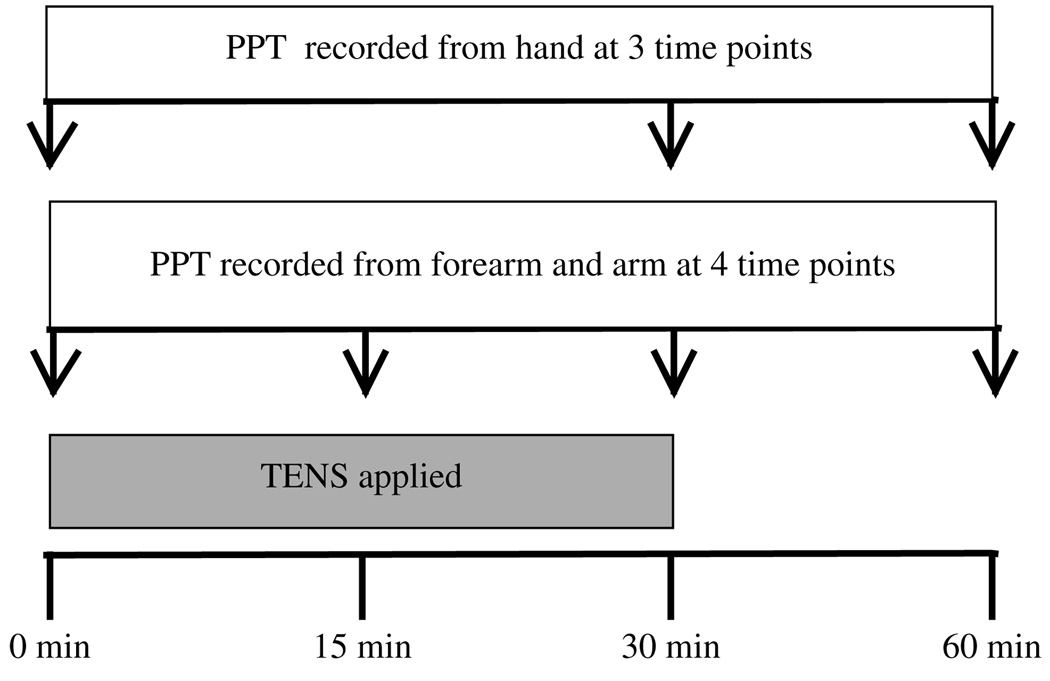
PPT was recorded by an outcome assessor (J.McK.) who was blind to group allocation using a Somedic Type II pressure algometer (Somedic Inc, Hörby, Sweden). The algometer was calibrated daily according to the manufacturers’ instructions. A preliminary reliability study [McKenna et al, 2007, unpublished data] was conducted by this outcome assessor by recording PPT from the 3 recording points described above from 10 healthy volunteers on 2 occasions, 48 hours apart. This reliability study demonstrated excellent overall between-session intrarater reliability for PPT measurements (0.99). In the current study, the outcome assessor was kept blind to the intervention allocated by the investigator (S.C.), masking participants’ dominant hands and distal forearms to conceal if a standard or glove electrode was applied. During recording, the circular probe of the algometer (1 cm2 area) was placed perpendicular to the skin and applied at rate of 50 kPa/s. Participants were asked to say “stop” when the sensation they were feeling turned to pain (distinct from pressure or discomfort); the algometer was immediately removed and the measurement recorded. Two measurements (in kPa) were taken from each recording site at each time point and the average used for data analyses. The TENS application was not interrupted to take PPT recordings. At the forearm and arm recording sites, PPT was recorded at 0 minutes, 15 minutes, 30 minutes, and 60 minutes (see Fig 3). At the hand recording site, PPT was recorded at 0 minutes, 30 minutes, and 60 minutes; there was no 15- minute recording as the glove electrode was in place to deliver TENS.
Figure 3.
Overview of procedure.
During the study, PPT readings from the 3 recording sites were taken in a random order. Randomization was performed using a computer-generated, random-number list compiled by a statistician (E.McC-G.) not involved in data collection. This random order was concealed by writing this onto cards and sealing them in the same opaque envelopes used for group allocation. These cards were given to the outcome assessor only after informed consent was obtained from each participant. All participants were given 2 practice demonstrations of PPT on the deltoid muscle of their nondominant arm to ensure that they understood the concept of PPT recording before the study began. These demonstrations took place immediately before the participant lay supine on the treatment plinth and were given a demonstration of the allocated intervention on the nondominant upper limb.
Data Analysis
Data were analyzed using SPSS (version 15.0; SPSS Inc, Chicago, IL) by the outcome assessor (J.M.), who was blind to group allocation: an average of the 2 PPT scores recorded at each time point was used for analysis. Shapiro-Wilk tests showed that data were normally distributed, therefore parametric tests were used to analyze the data. Difference scores (variation from baseline values) were analyzed using between-within groups ANOVA and post hoc Scheffe tests; significance was set at P < .05.
Results
PPT Data
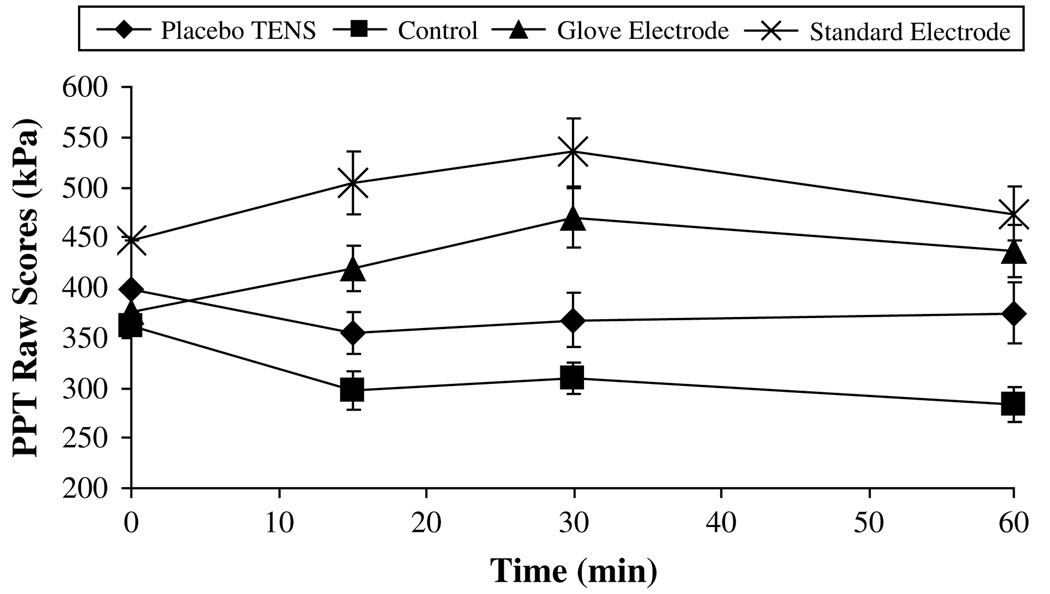
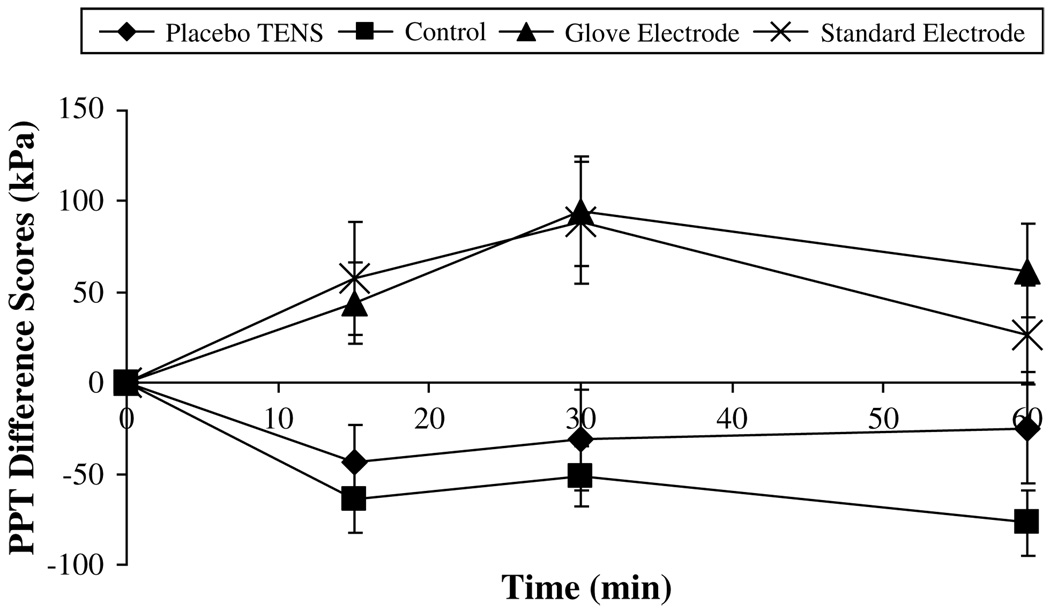
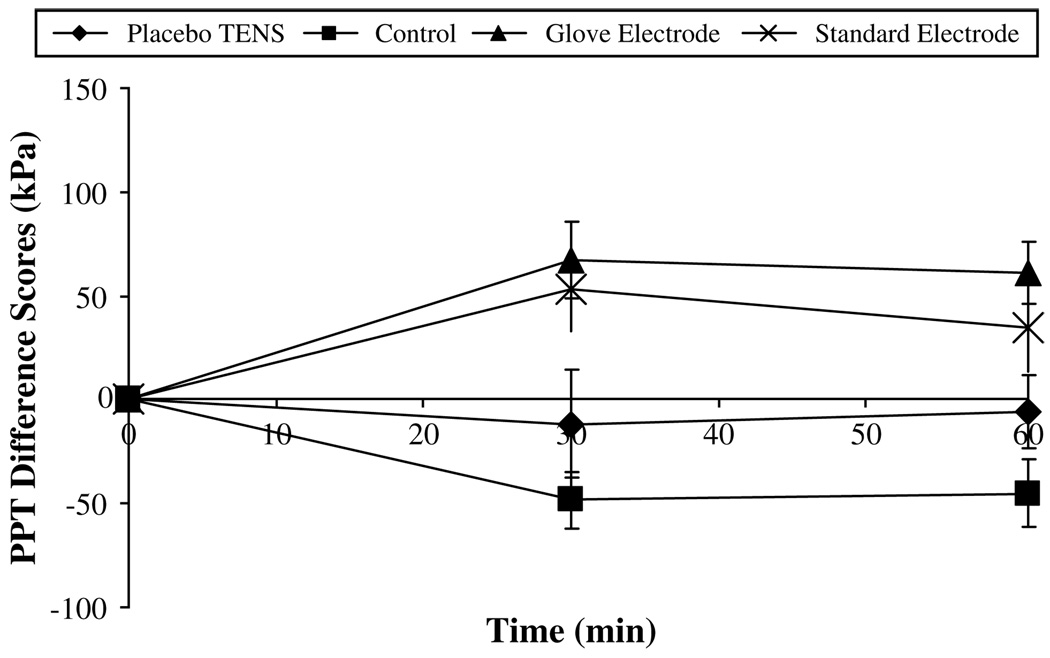
The greatest change in PPT was observed on the dominant side and in particular on the forearm. Fig 4 summarizes the raw PPT data recorded over the 60 minutes for the dominant forearm. Fig 5 displays the PPT difference score data for the dominant forearm. Both active TENS groups show the greatest increase in PPT from baseline, which peaked at 30 minutes (change from baseline: glove electrode, 94.5 ± 30 kPa; standard electrode, 88.4 ± 33.5 kPa, mean ± SEM). At the 60-minute point, PPT values in both of these groups remained at a level above baseline (change from baseline: glove electrode, 61.8 ± 25.7 kPa; standard electrode, 26.7 ± 27 kPa, mean ± SEM). During the entire 60 minutes, the control and placebo TENS groups showed a decrease in PPT indicating a hyperalgesic effect. Fig 6 shows that similar effects were observed at the dominant hand recording point with the 2 active TENS groups, again displaying the greatest increase in PPT from baseline at 30 minutes (change from baseline: glove electrode, 67.3 ± 18.7 kPa; standard electrode, 52.9 ± 19.9 kPa, mean ± SEM); values remained above baseline at the 60-minute point (change from baseline: glove electrode, 60.9 ± 14.9 kPa; standard electrode, 34.7 ± 21.2 kPa, mean ± SEM).
Figure 4.
Raw pressure pain threshold (PPT) data for the dominant forearm (kPa, mean ± SEM).
Figure 5.
Pressure pain threshold (PPT) difference scores for the dominant forearm (kPa, mean ± SEM).
Figure 6.
Pressure pain threshold (PPT) difference scores for the dominant hand (kPa, mean ± SEM).
Statistical analyses of the dominant side difference score PPT data using between-within groups ANOVA showed significant differences: over time at the forearm and arm recording points (P = .004, P = .005); and between groups at the hand, forearm and arm recording points (P < .0001, P < .0001; P = .01). There was no significant interactive effect between time and group at any of the recording points (P > .05). Effect sizes between groups were large ranging from 0.33 for the hand, 0.33 for the forearm and 0.19 for the arm.11 Post hoc Scheffe tests indicated a significant hypoalgesic effect in the standard electrode group compared with the control group at all 3 points; and between the glove electrode group and both the control group and placebo TENS groups at the hand and forearm points. There was no significant difference between the standard electrode and the glove electrode groups at any recording point.
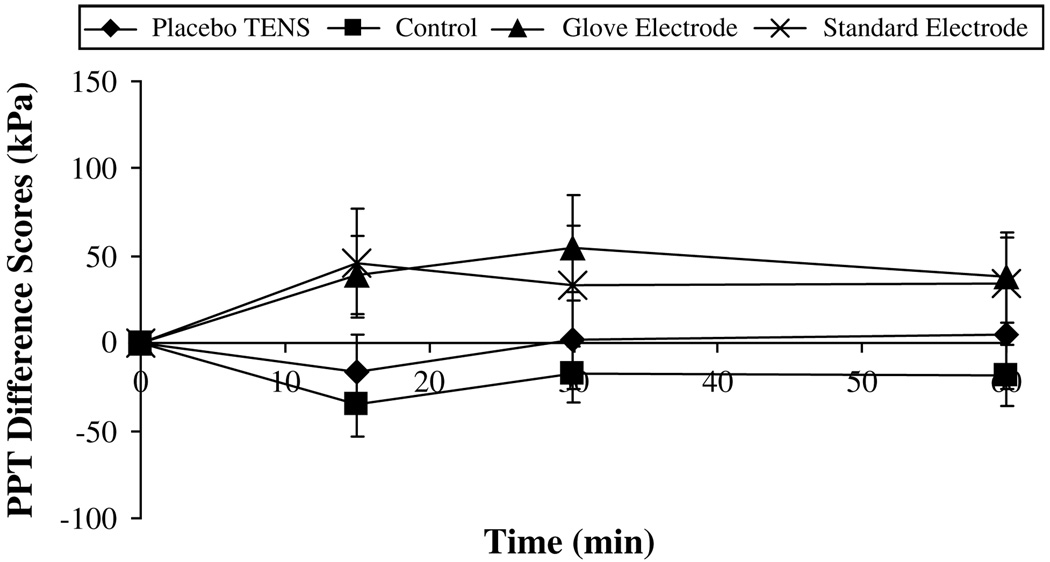
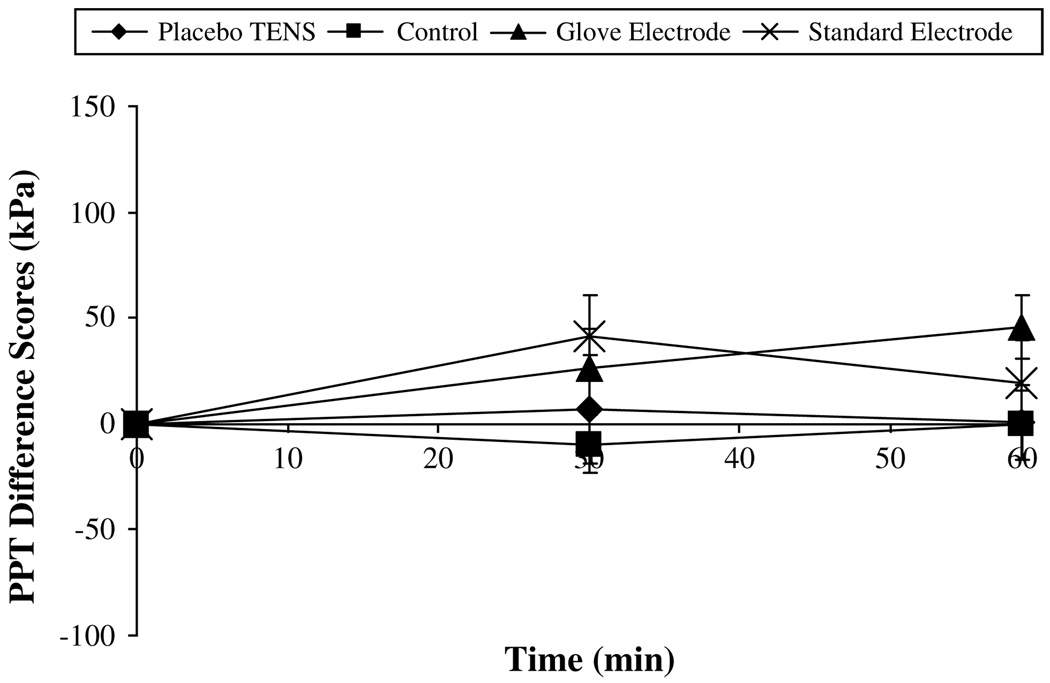
On the nondominant side, analysis of data showed a significant difference between groups at the forearm and arm recording points (P = .015, P = .005). Post hoc Scheffe tests indicated a significant hypoalgesic effect in both the standard electrode group and the glove electrode group compared with the control group at the arm point only. However, there was no significant interactive effect between time and group at any of the recording points or any significant effect over time (P > .05, Fig 7 and Fig 8).
Figure 7.
Pressure pain threshold (PPT) difference scores for the nondominant forearm (kPa, mean ± SEM).
Figure 8.
Pressure pain threshold (PPT) difference scores for the nondominant hand (kPa, mean ± SEM).
Assessment of Blinding
After the 60-minute PPT data were recorded, blinding was assessed by asking both the participants and outcome assessor which TENS intervention they thought was delivered, with 3 options for the answer: “real,” “placebo,” or “don’t know.” In the glove electrode group, 8 of 14 participants correctly identified that they received real TENS, with the remaining 6 participants indicating that they did not know. In the standard electrode group, 7 participants responded that the intervention was real and the other 7 did not know whether the intervention was real or placebo. However, in the placebo TENS group, 6 participants did not know whether the intervention was real or placebo, 4 correctly identified they had received placebo, and 4 thought they received real TENS. The fact that only 29% of placebo participants correctly identified that they received a placebo is a good indicator of successful participant blinding in this study (χ2 test, P = .01). The outcome assessor responded that she did not know group allocation for 100% of participants in the 2 active TENS and the placebo groups.
Discussion
This laboratory study examined the effects of delivering TENS via 2 different electrodes on PPT in healthy volunteers. Results showed no statistically significant differences between TENS delivered via a novel glove electrode or standard electrodes that projected TENS paraesthesia into the hand. Fig 5 illustrates that both types of electrode produced a superior (and statistically significant) increase in PPT compared with the control group. These findings therefore suggest that the glove electrode is as effective as the standard electrodes. This is the first experimental pain study to compare active TENS and placebo TENS delivered through a novel glove electrode. The results demonstrated that the glove electrode produced a significantly greater increase in PPT compared with the placebo TENS at the hand and fore-arm recording points (Fig 5 and Fig 6). However, there was no significant difference between standard electrode and placebo TENS groups.
A limited number of clinical studies have investigated novel shapes of electrode. Oyibo et al26 showed no superior effect of electrical stimulation delivered through stocking electrodes for painful diabetic neuropathy compared with identical control stockings that delivered an “insignificant” current. In contrast, Renklitepe et al33 showed significant pain relief for degenerative joint disease of the hand with TENS delivered via a glove electrode compared with a standard carbon silicon electrode. Unlike the current study, the Renklitepe et al study design did not incorporate a comparison placebo TENS glove electrode group, making interpretation of results difficult.
Ishimaru et al16 examined the influence of electrode type on pain thresholds in various deep tissues (skin, fascia, muscle, and periosteum). Three different types of surface-stimulating electrodes were compared using similar parameters of TENS administered to the femoral region in human volunteers: (1) cone-shaped metal electrodes, (2) rubber electrodes, and (3) large, soft gelatinous electrodes. Electrodes were placed within 30 mm of the site of measurement of pain thresholds, using a pulse algometer. The results showed that TENS applied with surface electrodes significantly increased pain thresholds of skin and fascia but not those of muscle or periosteum. Pre-post TENS pain threshold measurements showed that each of the 3 types of electrode produced a significant post-TENS increase in pain threshold in the skin; only the cone-shaped metal electrodes and the rubber electrodes produced a significant post-TENS increase in pain threshold in fascia. The authors concluded that the shape, material, and size of the surface electrodes produced similar degrees of analgesic effect, except for the large gelatinous electrodes, which had an inferior effect in elevating pain threshold in fascia.
Laboratory studies have demonstrated conflicting findings regarding segmental versus extrasegmental TENS electrode position.8,10,22 In our study, TENS was applied to stimulate the peripheral sensory nerves with both glove and standard electrodes generating paraesthesia in the hand. Clinical trials have also examined the analgesic effect of different electrode placement sites. Previous work by 1 of the authors showed a superior effect for spinal nerve versus painful area electrode placement sites for the treatment of low back pain using a portable interferential unit.15
The effect of electrode size on cold pain threshold has also been investigated by 1 of the authors17 in a sample of healthy volunteers. In this study, continuous TENS was delivered by 2 electrodes placed over the median nerve at the wrist; the hypoalgesic effects of TENS administered through small electrodes (2 cm2) and double-size electrodes (4 cm2) was compared. Both electrode groups increased in cold pain threshold when compared with a no-TENS control, although there were no differences between the groups. The small electrode group but not the double-size electrode group showed a significant increase in cold pain tolerance when compared with no-TENS control. One reason for this difference in hypoalgesia by 2 electrode sizes may be that the higher current density produced by the smaller electrodes was more effective in stimulating the target structure, in this case the median nerve. In the current study, the glove electrode covered a greater surface area and therefore could activate a larger number of afferent fibers directly. The standard electrodes, although smaller in size, were positioned so they could also produce paraesthesia into the hand.
An important aspect of the current study’s design was the use of a different type of placebo TENS. The sham device looked identical to the active TENS unit: the screen displayed arbitrary pulse amplitude values as the current was altered, similar to the active TENS unit. The sham device was modified to deliver a burst stimulus for 42 seconds and then the current output reset to zero. Participants in the active and placebo TENS groups were all TENS-naïve and were told they may or may not feel a tingling sensation. The percentages of participants who correctly identified whether the intervention was real or placebo were 57% in the glove electrode group, 50% in the standard electrode group, and 29% in the placebo TENS group. These data are consistent with the initial study that tested this type of placebo28 and together indicate that it is an acceptable form of placebo TENS. This approach may improve the blinding of clinicians applying TENS, as well as the participants, and should be considered in future clinical trials.
In terms of the clinical application of the 2 different types of electrode used in this study, similar levels of pulse amplitude were required to deliver a strong but comfortable sensation. In addition, no adverse effects were reported for either type of electrode. The experimental pain data indicate that although both electrodes displayed similar magnitudes of hypoalgesia, only the glove electrode produced a statistically significant increase in PPT compared with the placebo TENS, which was also delivered by a glove electrode. It is acknowledged that experimental models of pain have limitations regarding extrapolation of findings to both acute and chronic clinical pain conditions. However, they can be very useful in testing methodological issues encountered in randomized clinical trials, for example, use of an appropriate placebo TENS. Further testing of the type of placebo TENS used in this study is recommended in future laboratory and clinical studies on TENS.
Acknowledgments
The TENS units and electrodes were donated by Empi and TensCare. Ms Ruth Hunter (University of Ulster) is gratefully acknowledged for her assistance in PPT training and recruitment of participants.
Supported by the Research and Development Office, Belfast, Northern Ireland (UK) and Strategic Priority Grant, Department for Employment and Learning, Belfast, Northern Ireland (UK).
References
- 1.Aarskog R, Johnson MI, Demmink JH, Lofthus A, Iversen V, Lopes-Martins R, Joensen J, Bjordal JM. Is mechanical pain threshold after transcutaneous electrical nerve stimulation (TENS) increased locally and unilaterally? A randomized placebo-controlled trial in healthy subjects. Physiother Res Int. 2007;12:251–263. doi: 10.1002/pri.384. [DOI] [PubMed] [Google Scholar]
- 2.Alves-Guerreiro J, Noble JG, Lowe AS, Walsh DM. The effect of three electrotherapeutic modalities upon peripheral nerve conduction and mechanical pain threshold. Clin Physiol. 2001;21:704–711. doi: 10.1046/j.1365-2281.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 3.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption: A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 4.Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain: A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskel Disord. 2007;8:51. doi: 10.1186/1471-2474-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosseau L, Yonge KA, Robinson V, Marchand S, Judd M, Wells G, Tugwell P. Transcutaneous electrical nerve stimulation (TENS) for the treatment of rheumatoid arthritis in the hand. Cochrane Database Syst Rev. 2003;2 doi: 10.1002/14651858.CD004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown L, Tabasam G, Bjordal JM, Johnson MI. An investigation into the effect of electrode placement of transcutaneous electrical nerve stimulation (TENS) on experimentally induced ischemic pain in healthy human participants. Clin J Pain. 2007;23:735–743. doi: 10.1097/AJP.0b013e31814b86a9. [DOI] [PubMed] [Google Scholar]
- 7.Cheing GL, Luk ML. Transcutaneous electrical nerve stimulation for neuropathic pain. J Hand Surg [Br] 2005;30:50–55. doi: 10.1016/j.jhsb.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): Effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain. 2002;99:253–262. doi: 10.1016/s0304-3959(02)00118-5. [DOI] [PubMed] [Google Scholar]
- 9.Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23:760–766. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 10.Claydon LS, Chesterton LS, Barlas P, Sim J. Effects of simultaneous dual-site TENS stimulation on experimental pain. Eur J Pain. 2008;12:696–704. doi: 10.1016/j.ejpain.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JW. Statistical Power Analysis for the Behavioural Sciences. 2nd edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 12.Deyo RA, Walsh NE, Schoenfeld LS, Ramamurthy S. Can trials of physical treatments be blinded? The example of transcutaneous electrical nerve stimulation for chronic pain. Am J Phys Med Rehabil. 1990;69:6–10. doi: 10.1097/00002060-199002000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity and pulse duration of TENS on primary hyperaglesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 14.Hingne PM, Sluka KA. Differences in waveform characteristics have no effect on the anti-hyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) in rats with joint inflammation. J Pain. 2007;8:251–255. doi: 10.1016/j.jpain.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Hurley DA, Minder PM, McDonough SM, Walsh DM, Moore AP, Baxter DG. Interferential therapy electrode placement technique in acute low back pain: A preliminary investigation. Arch Phys Med Rehabil. 2001;82:485–493. doi: 10.1053/apmr.2001.21934. [DOI] [PubMed] [Google Scholar]
- 16.Ishimaru K, Kawakita K, Sakita M. Analgesic effects induced by TENS and electroacupuncture with different types of stimulating electrodes on deep tissues in human subjects. Pain. 1995;63:181–187. doi: 10.1016/0304-3959(95)00030-V. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MI, Ashton CH, Bousfield DR, Thompson JW. Analgesic effects of different pulse patterns of transcutaneous electrical nerve stimulation on cold-induced pain in normal subjects. J Psychosom Res. 1991;35:313–321. doi: 10.1016/0022-3999(91)90086-4. [DOI] [PubMed] [Google Scholar]
- 18.Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta-analysis of randomized controlled trials. Pain. 2007;130:157–165. doi: 10.1016/j.pain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MI. Transcutaneous electrical nerve stimulation (TENS) In: Watson T, editor. Electrotherapy- Evidence Based Practice. 12th edition. Edinburgh, UK: Elsevier; 2008. pp. 253–289. [Google Scholar]
- 20.Khadilkar A, Milne S, Brosseau L, Robinson V, Saginur M, Shea B, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain. Cochrane Database Syst Rev. 2005;3 doi: 10.1002/14651858.CD003008.pub2. [DOI] [PubMed] [Google Scholar]
- 21.King EW, Sluka KA. The effect of varying frequency and intensity of transcutaneous electrical nerve stimulation on secondary mechanical hyperalgesia in an animal model of inflammation. J Pain. 2001;2:128–133. doi: 10.1054/jpai.2001.19963. [DOI] [PubMed] [Google Scholar]
- 22.Kröling P, Gottschild S. TENS increases pressure pain threshold dependent on electrical and topical parameters. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin. 1999;9:48–55. [Google Scholar]
- 23.Limoges MF, Rickabaugh B. Evaluation of TENS during screening flexible sigmoidoscopy. Gastroenterol Nurs. 2004;27:61–68. doi: 10.1097/00001610-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 24.McDowell BC. PhD thesis. University of Ulster; 1995. An investigation of the analgesic and neurophysiological effects of H-wave therapy. [Google Scholar]
- 25.Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Phys Ther. 1998;78:160–169. doi: 10.1093/ptj/78.2.160. [DOI] [PubMed] [Google Scholar]
- 26.Oyibo SO, Breislin K, Boulton AJM. Electrical stimulation therapy through stocking electrodes for painful diabetic neuropathy: A double blind, controlled crossover study. Diabetic Med. 2004;21:940–944. doi: 10.1111/j.1464-5491.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 27.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4:455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 28.Rakel BA, Adams HJ, Cooper NA, Dannen DR, Granquist MJ, Messer BR, Miller CA, Mitschelen AC, Ruggle RC, Vance CGT, Frey Law LA, Walsh DM, Sluka KA. Increasing TENS intensity produces greater analgesia when compared to a new placebo that allows true investigator blinding. Proceedings, International Association for the Study of Pain, 12th World Congress on Pain; Glasgow, UK. 2008. [Google Scholar]
- 29.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: Basic science mechanisms and clinical effectiveness. J Pain. 2003;4:109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 30.Somers DL, Clemente FR. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: The effects of frequency and electrode position on prevention allodynia in a rat model of complex regional pain syndrome type II. Phys Ther. 2006;86:698–709. [PubMed] [Google Scholar]
- 31.Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: Does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil. 2007;88:1344–1349. doi: 10.1016/j.apmr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Vance CG, Radhakrishnan R, Skyba DA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87:44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- 33.Renklitepe N, Dogan N, Kayhan O, Ozaras N. Effects of different TENS electrode types in degenerative joint disease of the hands. Fizik Tedavi Rehabilitasyon Dergisi. 1995;19:204–208. [Google Scholar]