Abstract
Emotionally arousing events are typically well remembered, but there is a large interindividual variability for this phenomenon. We have recently shown that a functional deletion variant of ADRA2B, the gene encoding the α2b-adrenergic receptor, is related to enhanced emotional memory in healthy humans and enhanced traumatic memory in war victims. Here, we investigated the neural mechanisms of this effect in healthy participants by using fMRI. Carriers of the ADRA2B deletion variant exhibited increased activation of the amygdala during encoding of photographs with negative emotional valence compared with noncarriers of the deletion. Additionally, functional connectivity between amygdala and insula was significantly stronger in deletion carriers. The present findings indicate that the ADRA2B deletion variant is related to increased responsivity and connectivity of brain regions implicated in emotional memory.
Keywords: ADRA2B, fMRI, polymorphism, imaging genetics
Enhanced memory for emotionally arousing events is a highly adaptive phenomenon, which helps us to remember both dangerous and favorable situations (1). Under certain conditions, however, this mechanism can also lead to intrusive and persistent traumatic memories of an extremely aversive event, thereby contributing to the development and symptoms of posttraumatic stress disorder (PTSD) (2, 3). Elucidating the mechanisms of emotional memory formation thus represents an important aim of modern neuroscience not only in terms of better understanding basic mnemonic processes, but also with regard to the clinical relevance (4, 5).
Studies in rodents have indicated that the amygdala is critically involved in mediating the memory-enhancing effect of emotional arousal (6–8). Specifically, emotional arousal leads to a release of norepinephrine (NE) within the amygdala (9), and it has been shown that such amygdala activation strengthens the storage of many different kinds of information via its widespread network of efferent projections to other brain regions (10). Several human studies support this notion by showing that a pharmacologically induced increase of central noradrenergic transmission enhances memory performance (11–13), whereas a pharmacological blockade of noradrenergic transmission abolishes the enhancement of memory for emotional information and prevents enhanced amygdala activation in response to emotional stimuli (14, 15).
Recently, we reported that carriers of a functionally relevant deletion variant of ADRA2B, the gene encoding the α2b-adrenoreceptor, exhibit enhanced memory for emotional vs. neutral pictures compared with noncarriers (16). In that study, 435 participants were presented with neutral and emotional pictures taken from the international affective picture system (IAPS) (17). Delayed free recall was tested after 10 min, during which participants performed on a working memory task. The finding of increased emotional memory in deletion carriers of ADRA2B was followed up in 202 survivors of the Rwandan civil war who had experienced highly aversive emotional situations. In that sample, carriers of the deletion variant had a significantly higher score for reexperiencing traumatic memories than noncarriers, indicating the clinical relevance of this genotypic difference in noradrenergic neurotransmission. However, the neural substrates of the differences in emotional memory formation with regard to this genetic variation within the noradrenergic system remain unknown.
Because of the important role of the amygdala and the noradrenergic transmission within this region in mediating the memory-enhancing effect of emotional arousal, we hypothesized differential amygdala activity during encoding of emotional memories in dependence of the ADRA2B genotype. To test this hypothesis, we used fMRI in 57 young healthy participants who viewed emotional and neutral IAPS pictures in the MR scanner and freely recalled them 10 min later. During the 10-min period, participants performed on a working memory task (n-back). Procedure and design were adopted from our previous study to maximize comparability (16). Because the statistical power to detect an effect of similar size (d = 0.4) as in the previous study (n = 435 subjects) is 32% with 57 subjects (18), we did not expect statistically significant genotype-dependent differences in emotional memory on the behavioral level. However, we expected statistically significant genotype-dependent differences in brain activity because estimation of genetic effects on the level of neural systems has been shown repeatedly to be more sensitive than on the behavioral level (19–22). Based on the findings of our previous study (16), we hypothesized that carriers of the ADRA2B deletion variant exhibit higher activation of the amygdala during encoding of emotional events compared with noncarriers of the deletion. In addition, we hypothesized that the genotype-dependent increase in amygdala activity is accompanied by stronger coupling with brain regions related to storage of episodic memories enhanced by emotional arousal, such as the medial temporal lobe or neocortical brain regions (23).
Results
Genotyping and Behavioral Results.
In our study sample of 57 subjects, 22 subjects (18 students, three males, age 24.8 ± 0.9 years) were heterozygous and eight subjects (all students, two males, age 22.3 ± 1.3 years) homozygous carriers of the deletion variant of ADRA2B. A total of 27 subjects (23 students, 11 males, age 24.0 ± 0.9 years) did not carry the deletion variant (for genotyping procedure, see SI Methods). Genotype was not significantly associated with level of education, sex, or age (all P > 0.10). Genotype frequencies were in Hardy–Weinberg equilibrium (P > 0.50) and corresponded to the frequencies typically observed in Caucasians (24). As in our previous study (16), homozygote and heterozygote carriers of the deletion variant of ADRA2B (n = 30) were statistically treated as one group (deletion group) because of the small number of homozygous carriers.
Participants showed a high degree of interindividual variability in memory enhancement for emotional pictures. On average, subjects recalled 94% ± 17% more negative (range, −46% to 750%) and 106% ± 14% more positive (range, −22% to 650%) pictures relative to neutral pictures (both P < 0.0001). The emotional enhancement of memory was comparable for negative and positive pictures (P > 0.25). Consistent with our previous study (16), carriers of the ADRA2B deletion variant exhibited increased emotional memory for negative pictures (113% ± 18%) compared with noncarriers of the deletion (73% ± 19%). The size of the genotype effect on emotional memory for negative pictures was identical to the effect observed in the previous large behavioral study (both d = 0.4) but, as expected, did not reach statistical significance in the current study because of low statistical power (P = 0.14, two-tailed). Emotional memory for positive pictures did not differ significantly between groups (114% ± 15% vs. 97% ± 16%; P = 0.42; d = 0.2). Recall performance of neutral pictures was identical for both genotype groups (7.4 ± 0.6 pictures vs. 7.4 ± 0.7 pictures; P > 0.90). Subjective arousal ratings (three-point scale) were highest for negative (2.4 ± 0.4), intermediate for positive (1.9 ± 0.4), and lowest for neutral (1.4 ± 0.3; all P < 0.001, for pairwise comparisons) pictures. Genotype groups did not differ in emotional arousal ratings of the pictures (all P > 0.40) and working memory performance in the n-back task (all P > 0.50).
Genotype-Independent Brain Activation.
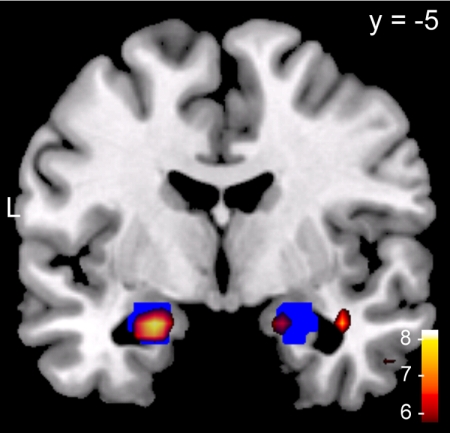
We first tested whether encoding of emotional pictures activated the amygdala. We performed a genotype-independent analysis comparing brain activity during viewing of emotional and neutral pictures. As expected, we observed significantly higher activity in the left and right amygdala for negative vs. neutral pictures. The activation in the amygdala was highly robust, surviving even the very conservative familywise error (FWE) correction for the whole brain [left: (−22, −6, −20), t(56) = 7.79; right: (19, 0, −20), t(56) = 6.85; both P(FWE) < 0.001, FWE corrected for the whole brain; see Fig. 1). Consistent with findings from similar studies (for metaanalyses, see refs. 25 and 26), we observed additional increased activations in response to negative pictures in the visual cortex, medial prefrontal cortex, and insula (Table S1). No brain region showed higher activity for neutral compared with negative pictures. For positive pictures, similar but smaller effects were observed. Activity during encoding of positive vs. neutral pictures was higher in the left amygdala [(−19, −3, −16), t(56) = 4.00; P(SVC) < 0.006] and right amygdala [(19, −3, −20), t(56) = 3.64; P(SVC) < 0.02; small volume-corrected (SVC) for the bilateral amygdala]. Again, additional activation was observed in the visual cortex, cingulate gyrus, and inferior frontal gyrus/insula (Table S2). Directly comparing negative and positive pictures revealed a significantly stronger bilateral amygdala activation during encoding of negative compared with positive pictures [left: (−22, −6, −20), t(56) = 5.11; right: (22, 0, −20), t(56) = 4.40; both P(SVC) < 0.001). This difference may be due to the significantly higher arousal ratings of negative compared with positive pictures.
Fig. 1.
Genotype-independent amygdala activation during encoding of negative vs. neutral pictures. The t values from the one-sample t test (red-white) are overlaid on a T1 template and displayed at a threshold of P(FWE) < 0.05, FWE-corrected for the whole brain. The anatomical defined bilateral amygdala (Talairach atlas) is indicated in blue. Left corresponds to the left side of the brain.
Genotype-Dependent Analysis.
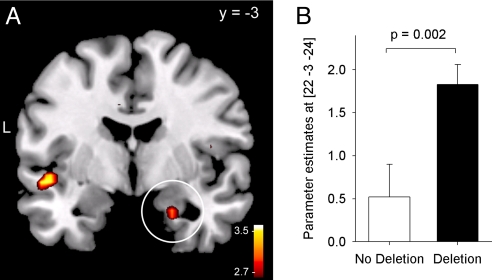
In the next step, we compared activation levels in the amygdala between ADRA2B deletion carriers and noncarriers. In accordance with our hypothesis, deletion carriers activated the amygdala to a significantly higher degree than noncarriers of the deletion in response to negative vs. neutral pictures. Particularly in the right amygdala, the genotype-dependent group difference was robust, surviving SVC for the bilateral amygdala search volume [(22, −3, −24), t(55) = 3.10, P = 0.002; P(SVC) < 0.05; Fig. 2 and Table 1]. The effect did not depend on sex (interaction effect, F1,53 = 0.1; P > 0.94). Activity in the left amygdala was also significantly higher in deletion carriers compared with noncarriers, although the effect was not large enough to survive the correction for multiple comparisons in the bilateral search volume [(−22, 0, −20), t(55) = 2.55; P = 0.007; P(SVC) = 0.14]. Also, during encoding of positive pictures, the left amygdala exhibited increased but nonsignificant activation in deletion carriers vs. noncarriers [(−25, 3, −20), t(55) = 2.04; P < 0.03; P(SVC) = 0.35). According to our statistical criterion (i.e., SVC), we focused on genotype-dependent differences in the right amygdala during encoding of negative pictures in our further analysis.
Fig. 2.
Genotype-dependent brain activity in the amygdala. (A) Carriers of the ADRA2B deletion variant activated the right amygdala in response to negative vs. neutral images to a greater extent compared with noncarriers [P(SVC) < 0.05, displayed at an uncorrected threshold of P = 0.005]. The white circle indicates the activation in the right amygdala. (B) Parameter estimates at the peak voxel in the right amygdala for noncarriers and carriers of the deletion.
Table 1.
ADRA2B genotype-dependent differences in brain activity during encoding of negative vs. neutral pictures
| Region | BA | No. of voxels | L/R | MNI coordinates |
t(55) | Z | P | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Amygdala | 9 | R | 22 | −3 | −24 | 3.10 | 2.96 | 0.002* | |
| 3 | L | −22 | 0 | −20 | 2.55 | 2.47 | 0.007 | ||
| Inferior parietal lobule | 40 | 6 | L | −30 | −41 | 48 | 3.69 | 3.47 | 0.000 |
| Superior temporal gyrus | 38 | 6 | R | 39 | 17 | −20 | 3.68 | 3.46 | 0.000 |
| Insula | 13 | 3 | L | −47 | −6 | −8 | 3.42 | 3.24 | 0.001 |
Results of the contrast “deletion > no deletion carriers for negative > neutral pictures” are shown. L and R indicate left and right hemisphere, respectively.
*P < 0.05 after SVC for the bilateral amygdala.
In addition to the genotype-dependent differences in amygdala activity, exploratory whole-brain analysis revealed that carriers of the deletion variant of ADRA2B exhibited increased activation in the left insula in response to negative pictures. The same activity pattern was also observed in the inferior parietal lobule as well as the superior temporal gyrus (Table 1). During encoding of positive pictures, noncarriers of the deletion had higher activation in the cingulate gyrus [(−22, −44, 24), t(55) = 3.86; P < 0.001). No other genotype-dependent suprathreshold activation was observed.
Emotional Subsequent Memory Analysis.
Event-related fMRI also allows for investigation of brain regions involved in memory formation by analyzing differential activity during encoding of subsequently remembered vs. subsequently forgotten events (subsequent memory analysis). The emotional subsequent memory (ESM) analysis identifies brain regions in which the subsequent memory effect (“difference due to memory,” or Dm) is larger for emotional than neutral items (Dmemotional > Dmneutral). Independently of genotype, ESM effects for negative pictures occurred in the fusiform gyrus, parietal regions, parahippocampus/hippocampus, and insula, as well as the amygdala in our study (Table S3). ESM effects for positive pictures were only observed in the anterior hippocampus, weakly extending in amygdala regions (Table S3). These results are generally consistent with previous findings (27, 28) and further indicate that the reported brain regions that show genotype-dependent activity differences belong to a network important for emotional memory.
We also compared ESM effects between genotype groups. It is important to note that this analysis may ignore group differences in brain activity related to emotional memory performance because it is based on comparing mean brain activity between the categories “remembered” and “forgotten,” regardless of the number of remembered and forgotten items and corresponding neural correlates. Thus, brain regions contributing to a genotype-dependent behavioral difference may be missed in this analysis. However, this analysis can identify brain regions that are differentially recruited during successful emotional memory encoding, depending on the genotype. In our sample, carriers of the deletion variant of ADRA2B (compared with noncarriers) exhibited a significantly stronger ESM effect in the left insula (Table S4). In contrast, noncarriers had a higher ESM effect for negative pictures in the occipital lobe. No genotype-dependent differences in ESM effects were observed in the amygdala. Separate analyses in each genotype group revealed that ESM effects in the amygdala were of similar dimension in carriers and noncarriers of the deletion variant (Table S3).
Functional Connectivity.
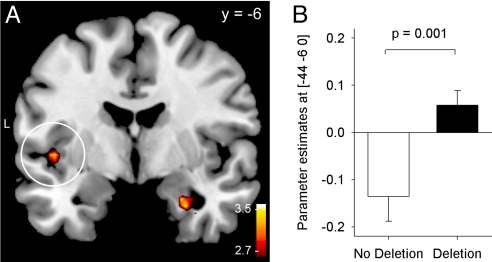
To further investigate the influence of the ADRA2B polymorphisms on neural substrates of emotional memory encoding, we calculated the functional connectivity [psychophysiological interaction (PPI)] between the right amygdala and all other brain areas during encoding of negative vs. neutral pictures in carriers and noncarriers of the deletion. This method allowed us to identify brain regions that differ in their association with the seed region between negative and neutral picture conditions, excluding general associations due to neuroanatomical constraints or simple perceptual processes. We observed that coupling during encoding of negative pictures between the right amygdala and left insula was significantly higher in deletion compared with no-deletion carriers [(−44, −6, 0), t(55) = 3.29; P = 0.001; deletion vs. no-deletion group; Fig. 3). Connectivity with the right amygdala in deletion carriers was also increased in the left inferior frontal gyrus [Brodmann's area (BA) 47; (−33, 14, −16), t(55) = 3.61; P < 0.001] and the left postcentral gyrus [BA 3; (−36, −25, 48); t(55) = 3.63; P < 0.001). No brain regions exhibited significantly decreased connectivity with the right amygdala for carriers vs. noncarriers of the deletion variant of ADRA2B.
Fig. 3.
Genotype-dependent differences in connectivity between the right amygdala and the left insula. (A) In deletion carriers, amygdala and insula activity was positively coupled during encoding of negative pictures, whereas an inverse association was observed in noncarriers. The difference in connectivity estimates between the genotype groups was significant (P = 0.001, displayed at a threshold of P = 0.005). The white circle indicates the increase in connectivity in the left insula. (B) Parameter estimates for the genotype-specific difference in connectivity with the amygdala in the left insula.
Discussion
In accordance with our main hypothesis, carriers of the ADRA2B deletion variant exhibited higher amygdala activation in response to negative vs. neutral pictures compared with noncarriers. This differential responsivity of the amygdala to emotional stimuli is in line with the known role of the noradrenergic system and the amygdala in mediating the memory-enhancing effects of emotional arousal (6).
Evidence for the involvement of the amygdala in the formation of emotional memories is very strong (ref. 4, for a review). As known from numerous studies in rodents, the enhancing effect of emotion on memory critically depends on the functional integrity of the amygdala and the noradrenergic neurotransmission within the amygdala during and shortly after learning (29–31). According to the modulation hypothesis, the amygdala is not the primary storage site for memories strengthened by emotional arousal, but it promotes synaptic plasticity underlying memory formation in other brain regions via increased release of the neurotransmitter noradrenaline (see refs. 5, 10, 32, and 33 for reviews). Several human studies support this notion by indicating impairment of emotional memories in patients with selective amygdala lesions (34–36) and dependence of emotional memory formation on noradrenergic neurotransmission (11–14). Moreover, the noradrenergic system seems to be a promising target for the pharmacological prevention/reduction of traumatic memories in PTSD (37). Imaging studies have consistently shown that activity in the amygdala during encoding is correlated with retrieval success of memories tested after several hours, weeks, or years (38–42). Based on this background and our data, we conclude that the ADRA2B genotype-dependent differences are related to altered amygdala activation in response to emotional events.
In vitro, the deletion of three glutamic acid residues of the α2b-adrenergic receptor studied here results in inhibition of adenylcyclase, but also in decreased agonist-promoted phosphorylation and receptor desensitization (24). Because these data suggest both agonistic and antagonistic effects, the exact molecular mechanism by which the deletion variant influences noradrenergic neurotransmission during emotional memory encoding remains to be determined. However, both the data of our behavioral as well as of the present imaging study strongly suggest that the deletion variant results in increased noradrenergic availability in response to emotional events, leading to increased amygdala activation as well as enhanced emotional memories (16). Because the α2-adrenoceptors act as autoreceptors inhibiting NE release, our data suggest that the deletion variant acts primarily as a loss-of-function polymorphism of the α2b-adrenergic receptor in the regulation of emotional memories.
With regard to the time course, our imaging results speak for a genotype-dependent difference in amygdala activation already during the encoding phase, which explains why the ADRA2B genotype alters emotional memory recall as early as 10 min after learning (16). Because differences in amygdala activity and connectivity during the encoding phase readily predict recall of memories tested after several weeks or years (40, 43), the degree of the initial response of the amygdala to emotional stimuli also appears to be crucial for initializing processes of memory formation for the long term. Noradrenergic transmission is known to modulate both short-term memory processes (44) and long-term memory consolidation (45) of emotionally arousing information. Therefore, it is possible that genotype-dependent differences in α2b-adrenoreceptor function not only affect memory encoding but also affect later phases of emotional memory formation and involve additional brain regions. In fact, enhancing effects of emotion on memory become even more pronounced after longer retention intervals (46). Although the current study focused on encoding and early memory formation processes, future studies will have to examine the effects of genetic variations in the adrenergic system on long-term consolidation of emotional memories and their neural underpinnings.
Independently of genotype group differences in general amygdala responsivity and memory performance, no genotype-dependent difference in the amygdala was observed when comparing mean brain activity for the picture categories “remembered” and “forgotten” in the ESM analysis, because the ESM effects in the amygdala were of similar dimension in both genotype groups. Thus, a similar ESM effect occurs at different general responsivity levels of the amygdala, depending on ADRA2B genotype. Together with the observed genotype-dependent increase in general amygdala responsivity, our results suggest that the amygdala is involved in successfully encoding emotional memories in both genotype groups, but at different levels. In the left insula, however, ESM effects were larger for carriers of the deletion variant of ADRA2B, indicating that in deletion carriers, the insula might play a more pronounced role in relation to successful emotional memory encoding compared with noncarriers. This notion is compatible with the observed increased connectivity between the amygdala and the insula during the encoding of negative pictures in deletion carriers. Such increased connectivity between the amygdala and the insula points to a tightened communication within brain areas known to be involved in affective processing (47, 48). The insular cortex is known to be highly important for the acquisition and long-term consolidation of aversive memories in rodents and acts in close interaction with the amygdala to promote memory formation (32, 33). Posttraining infusions of cAMP in the insula enhance inhibitory avoidance in rats (49), and in vivo tetanic stimulation of the amygdala induces long-term potentiation in the insular cortex (50, 51).
Evidence for an involvement of the insula in emotional memory is also provided by human studies. During aversive conditioning, increases in brain activity associated with the conditioned stimulus are consistently observed in insular regions (52–54). Also, during encoding of negative pictures, higher insula activation was observed in young participants, who exhibited a stronger extent of emotional memory enhancement than older participants (55). Importantly, heightened amygdala and insula responsivities to emotional- or trauma-related stimuli have been observed in PTSD patients (47, 56, 57) as well as in several other affective disorders (48). This hyperactivity is related to symptom severity in PTSD patients (58). Moreover, insula activity correlates directly with the intensity of intrusions (“flashbacks”) of traumatic memories (59). Interestingly, in our previous study we found evidence that the ADRA2B deletion variant is not only related to stronger emotional memories in healthy subjects but also to stronger traumatic memories in war victims (16). Thus, the present finding of an ADRA2B deletion variant-related increase in amygdala activity and amygdala–insula interaction during encoding of emotional events in healthy subjects might also have implications for understanding the mechanisms underlying the development of traumatic memories in PTSD.
Materials and Methods
Participants.
A total of 57 healthy young subjects (mean age, 24.1 ± 0.6 years; range, 18–38 years; 16 males) participated in the study. All subjects were free of any lifetime neurological or psychiatric illness and did not take any medication at the time of the experiment. The experiments were approved by the ethics committee of the University of Zürich. Written informed consent was obtained from all subjects before participation.
Picture Task.
Stimuli consisted of 72 pictures that were selected from the IAPS (17) as well as from in-house standardized picture sets that allowed us to equate the pictures for visual complexity and content (e.g., human presence). On the basis of normative valence scores (from 1 to 9), pictures were assigned to emotionally negative (2.3 ± 0.6), emotionally neutral (5.0 ± 0.3), and emotionally positive (7.6 ± 0.4) conditions, resulting in 24 pictures for each emotional valence. Negative and positive pictures were equated for valence extremity (P > 0.60). Mean normative arousal ratings were 5.9 ± 0.9 (negative), 3.4 ± 0.5 (neutral), and 4.9 ± 0.8 (positive). Picture categories significantly differed in normative arousal ratings (negative > positive > neutral; P < 0.001). Four additional pictures showing neutral objects were used to control for primacy and recency effects in memory. Two of these pictures were presented in the beginning and two at the end of the picture task. They were not included in the analysis. In addition, 24 scramble pictures were used. The background of the scramble pictures contained the color information of all pictures used in the experiment (except primacy and recency pictures), overlayed with a crystal and distortion filter (Adobe Photoshop CS3). In the foreground, a mostly transparent geometrical object (rectangle or ellipse of different sizes and orientations) was shown. Pictures were presented in the scanner by using MR-compatible LCD goggles (Visuastim XGA; Resonance Technology). Eye correction was used when necessary.
Encoding Phase.
The pictures were presented for 2.5 seconds in a quasirandomized order so that at maximum, four pictures of the same category occurred consecutively. A fixation cross appeared on the screen for 500 msec before each picture presentation. Trials were separated by a variable intertrial period of 9–12 sec (jitter) that was equally distributed for each stimulus category. During the intertrial period, participants subjectively rated the picture showing scenes according to valence (negative, neutral, positive) and arousal (large, medium, small) on a three-point scale (Self Assessment Manikin) by pressing a button with a finger of their dominant hand. For scramble pictures, participants rated form (vertical, symmetric, or horizontal) and size (large, medium, small) of the geometrical object in the foreground. The encoding phase of the picture had a total duration of 22 min. Participants were not told that they had to remember the pictures for later recall.
Participants were instructed and trained on the picture task before being positioned in the scanner. Training consisted of presentation and rating of five pictures, including scenes and scrambled pictures, which were not used during scanning.
Free Recall.
At 10 min after picture presentation, memory performance was tested by using a free-recall task, which required participants to write down a short description (a few words) of the previously seen pictures. Remembered primacy and recency pictures as well as training pictures were excluded from the analysis. No time limit was set for this task. Two trained investigators independently rated the descriptions for recall success (interrater reliability, >99%). No details were required for correct scoring because we used only distinct pictures. As in our previous study (16), we used the relative difference between successfully recalled negative (or positive) and neutral pictures as dependent variables, with the number of successfully recalled neutral pictures set to 100%. The absolute number of recalled neutral pictures was used as a covariate in the statistical analysis of emotional memory performance to control for differences in memory capacity.
Procedure.
After receiving general information about the study and giving their informed consent, participants were instructed and then trained on the picture task and the n-back task (described in the SI Methods) they later performed in the scanner. After training, they were positioned in the scanner. The participants received earplugs and headphones to reduce scanner noise. Their head was fixated in the coil by using small cushions, and they were told not to move their heads. Functional MR images were acquired during the performance of the picture task and the n-back task in two separate sessions (total scanning time approximately 30 min). After finishing the tasks, participants left the scanner and were taken to a separate room for free recall of the pictures. Finally, participants filled out questionnaires, gave saliva for genotype analysis, and were debriefed. The total length of the experimental procedure was ≈3 h. Participants received 25 Swiss francs per hour for participation.
fMRI Data Acquisition and Processing.
Measurements were performed on a Philips Intera 3T whole-body MR unit equipped with an eight-channel Philips SENSE head coil. Functional time series were acquired with a sensitivity-encoded (60), single-shot echo-planar sequence (SENSE-sshEPI). We used the following acquisition parameters: echo time = 35 ms, field of view (FOV) = 22 cm, acquisition matrix = 80 × 80, interpolated to 128 × 128, voxel size: 2.75 × 2.75 × 4 mm3, and SENSE acceleration factor R = 2.0. By using a midsagittal scout image, 32 contiguous axial slices were placed along the anterior–posterior commissure plane covering the entire brain with a repetition time = 3,000 msec (θ = 82°). The first two acquisitions were discarded because of T1 saturation effects.
Preprocessing and data analysis was performed by using SPM5 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, U.K.; http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 2008a (Mathworks). Volumes were slice-time-corrected to the first slice, realigned to the first acquired volume, normalized into standard stereotactic space (template provided by the Montreal Neurological Institute, Montreal, Canada), and smoothed by using an 8-mm full-width-at-half-maximum Gaussian kernel. A 128-sec-cutoff high-pass filter was added to the confound partition of the design matrix to account for low-frequency drifts, and a correction for intrinsic autocorrelations was included in the analysis. For each subject, evoked hemodynamic responses to event types were modeled with a delta (stick) function corresponding to stimulus presentation convolved with a canonical hemodynamic response function within the context of a general linear model. The pictures accounting for possible primacy and recency effects as well as button presses during valence and arousal ratings were modeled separately. In addition, six movement parameters from spatial realigning were included as regressors of no interest.
For all genotype-independent analyses, we used a threshold of P < 0.05, corrected for multiple comparisons in the whole brain (FWE t(54) = 5.24), in a minimum of 20 adjacent voxels (k = 20). In the comparisons of genotype groups, an uncorrected threshold of P < 0.001 in a minimum number of three adjacent voxels (k = 3) was used for an exploratory analysis of the whole brain. According to our hypotheses, the left and right amygdala were regions of interest (ROIs) where SVC was applied with a threshold of P < 0.05. The ROIs contained the left and right amygdala as defined by the Talairach atlas (61) implemented in the software WFU PickAtlas v2.4 (62).
Functional Connectivity Analyses.
To further investigate the association of ADRA2B genotype with amygdala BOLD response and network coupling during memory encoding, we performed a PPI analysis as a measure of task-related functional connectivity (63) within SPM5. We used as seed the peak of activation in the right amygdala identified in the comparison between the genotype groups (Montreal Neurological Institute coordinates: x = 22, y = −3, z = −24). We did not include as additional seed the peak in the left amygdala, given that it did not survive correction for multiple comparisons. The time course of the seed was extracted for each subject, mean-centered, high-pass-filtered, and deconvolved. A general linear model was then computed by using three regressors: a physiological regressor (the time course response in the seed region), a psychological regressor (the contrast between negative and neutral pictures), and a PPI term, calculated as the cross-product of the previous two terms. The individual PPI contrasts were entered in a second-level random-effects analysis to investigate differences in connectivity between genotypes (P < 0.001 uncorrected; k = 3).
Supplementary Material
Acknowledgments.
This work was supported by Swiss National Science Foundation Grants PP00P3-123391 and CRSIK0_122691 (to D.J.-F.d.Q.) and PP00P3-114813 and CRSIK0_122691 (to A.P.), the European Science Foundation (EUROStress) (D.J.-F.d.Q. and A.P.), and a grant from the Zürich Center for Integrative Human Physiology (to D.J.-F.d.Q.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 18881.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907425106/DCSupplemental.
References
- 1.McGaugh JL. Memory and Emotion: The Making of Lasting Memories. New York: Columbia Univ Press; 2003. [Google Scholar]
- 2.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 4.Labar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 5.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Cahill L, McGaugh JL. The neurobiology of memory for emotional events: Adrenergic activation and the amygdala. Proc West Pharmacol Soc. 1996;39:81–84. [PubMed] [Google Scholar]
- 9.McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 10.McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: Interaction with other brain systems. Neurobiol Learn Mem. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- 11.O'Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med. 1999;29:1083–1088. doi: 10.1017/s0033291799008703. [DOI] [PubMed] [Google Scholar]
- 12.Southwick SM, et al. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am J Psychiatry. 2002;159:1420–1422. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- 13.Hurlemann R, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. J Neurosci. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 15.Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Quervain DJ, et al. A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- 17.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- 18.Rasch B, Friese M, Hofmann W, Naumann E. Quantitative Methods I. Heidelberg: Springer; 2006. [Google Scholar]
- 19.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 20.Mattay VS, Goldberg TE. Imaging genetic influences in human brain function. Curr Opin Neurobiol. 2004;14:239–247. doi: 10.1016/j.conb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Tan HY, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter M, et al. A functional variant of the tryptophan hydroxylase 2 gene impacts working memory: A genetic imaging study. Biol Psychol. 2008;79:111–117. doi: 10.1016/j.biopsycho.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.McGaugh JL. Memory–a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 24.Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- 25.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 26.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 27.Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learn Mem. 2008;15:172–182. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer T, Glascher J, Moritz S, Buchel C. Emotional enhancement effect of memory: Removing the influence of cognitive factors. Learn Mem. 2008;15:569–573. doi: 10.1101/lm.995108. [DOI] [PubMed] [Google Scholar]
- 29.Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- 30.Ferry B, Roozendaal B, McGaugh JL. Involvement of alpha1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur J Pharmacol. 1999;372:9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- 31.Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bermudez-Rattoni F, Ramirez-Lugo L, Gutierrez R, Miranda MI. Molecular signals into the insular cortex and amygdala during aversive gustatory memory formation. Cell Mol Neurobiol. 2004;24:25–36. doi: 10.1023/B:CEMN.0000012722.45805.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welzl H, D'Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 34.Markowitsch HJ, et al. The amygdala's contribution to memory–a study on two patients with Urbach-Wiethe disease. Neuroreport. 1994;5:1349–1352. [PubMed] [Google Scholar]
- 35.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 36.Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 37.Pitman RK, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 38.Cahill L, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolcos F, Labar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Dolcos F, Labar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 42.Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: An event-related FMRI investigation. Cereb Cortex. 2008;18:2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behav Neurosci. 2004;118:420–428. doi: 10.1037/0735-7044.118.2.420. [DOI] [PubMed] [Google Scholar]
- 45.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 46.Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106:538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur Psychiatry. 2007;22:387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: Involvement of the basolateral amygdala. Learn Mem. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Escobar ML, Chao V, Bermudez-Rattoni F. In vivo long-term potentiation in the insular cortex: NMDA receptor dependence. Brain Res. 1998;779:314–319. doi: 10.1016/s0006-8993(97)01175-x. [DOI] [PubMed] [Google Scholar]
- 51.Jones MW, French PJ, Bliss TV, Rosenblum K. Molecular mechanisms of long-term potentiation in the insular cortex in vivo. J Neurosci. 1999;19:RC36. doi: 10.1523/JNEUROSCI.19-21-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 53.Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St Jacques P, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Psychol Sci. 2008;20:74–84. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rauch SL, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 57.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann NY Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 58.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 59.Osuch EA, et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- 60.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 61.Lancaster JL, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 63.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.