Abstract
In our previous communication we reported the enzymatic recognition of unnatural imidazopyridopyrimidine:naphthyridine (Im:Na) base pairs, i.e. ImON:NaNO and ImNO:NaON, using the Klenow fragment exo− [KF (exo−)]. We describe herein the successful results of (i) improved enzymatic recognition for ImNO:NaON base pairs and (ii) further primer extension reactions after the Im:Na base pairs by Deep Vent DNA polymerase exo− [Deep Vent (exo−)]. Since KF (exo−) did not catalyze primer extension reactions after the Im:Na base pair, we carried out a screening of DNA polymerases to promote the primer extension reaction as well as to improve the selectivity of base pair recognition. As a result, a family B DNA polymerase, especially Deep Vent (exo−), seemed most promising for this purpose. In the ImON:NaNO base pair, incorporation of NaNOTP against ImON in the template was preferable to that of the natural dNTPs, while incorporation of dATP as well as dGTP competed with that of ImONTP when NaNO was placed in the template. Thus, the selectivity of base pair recognition by Deep Vent (exo−) was less than that by KF (exo−) in the case of the ImON:NaNO base pair. On the other hand, incorporation of NaONTP against ImNO in the template and that of ImNOTP against NaON were both quite selective. Thus, the selectivity of base pair recognition was improved by Deep Vent (exo−) in the ImNO:NaON base pair. Moreover, this enzyme catalyzed further primer extension reactions after the ImNO:NaON base pair to afford a faithful replicate, which was confirmed by MALDI-TOF mass spectrometry as well as the kinetics data for extension fidelity next to the ImNO:NaON base pair. The results presented in this paper revealed that the ImNO:NaON base pair might be a third base pair beyond the Watson–Crick base pairs.
INTRODUCTION
The formation of Watson–Crick (WC) base pairs consisting of adenine:thymine (A:T) and guanine:cytosine (G:C) in the DNA duplex is the most fundamental and critical biological event in cells of all living matter. Both pairs are composed of a purine and a pyrimidine nucleobase combination (pu:py), and interact through complementary hydrogen bonds (H-bonds) (two H-bonds in the A:T pair and three H-bonds in the G:C pair). Following these rules, DNA forms a beautiful helical structure, and has functions to store and transmit its own genetic information. The faithful recognition of the WC base pairs is also required for the biotechnology represented by the polymerase chain reaction (PCR). Since the early 1990s, chemists have expanded their efforts to develop artificial extra base pairs beyond the WC base pairs. If these alternative base pairs in the DNA duplex showed specificity in formation of the helical structure and enzymatic replication, they could potentially allow one to expand the genetic code, to explore synthetic biology and to create new genetic systems. Thus far, a number of alternative base pairs have been developed, and successful applications have also been reported (1–6).
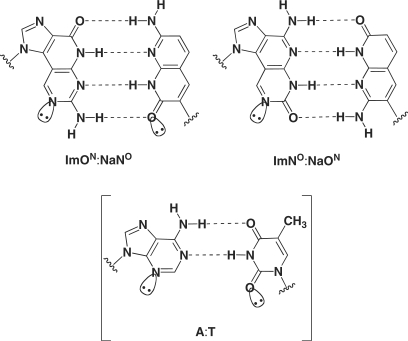
We have undertaken a series of studies aimed at the design of new base pairs consisting of noncanonical four H-bonds to investigate how additional H-bonds in the base pair affect the DNA duplex stability (7,8). Accordingly, we revealed that imidazopyridopyrimidine:naphthyridine (Im:Na) base pairs, i.e. ImON:NaNO and ImNO:NaON (Figure 1) thermally stabilized the duplex structure significantly. In addition, these pairs were specific to each other in the duplex. This thermally stabilizing effect and specificity were considered to arise from (i) the complementary four H-bonds, (ii) the stacking ability of the expanded aromatic surface and (iii) the shape complementarity of the Im:Na base pairs similar to the purine:pyrimidine (pu:py) base pair. With these successful results in hand, we used the Im:Na base pairs to develop a thermally stabilized decoy molecule (9). Furthermore, we recently reported the enzymatic incorporation of dYTP (ImONTP, NaNOTP, ImNOTP or NaONTP) against the complementary base (NaNO, ImON, NaON or ImNO) in the template by the Klenow fragment exo− [KF (exo−)] (10). To the best of our knowledge, this is the first example of enzymatic recognition of base pairs possessing four H-bonds, and thus, we were prompted to expand out investigation of the enzymatic recognition of the Im:Na base pairs.
Figure 1.
Structures of Im:Na base pairs consisting of four H-bonds.
In this article, we describe the results of (i) improvement of the selectivity of base pair recognition of the Im:Na base pairs and (ii) further primer extension reactions after the Im:Na base pair by Deep Vent DNA polymerase (exo−) [Deep Vent (exo−)].
MATERIALS AND METHODS
General methods
The syntheses of chemically modified dNTPs and templates used in this study were described in our previous paper (10). Natural dNTPs were purchased from GE Healthcare, Japan. The FITC-labeled primers and templates consisting of natural nucleotide units were purchased from SIGMA-ALDRICH, Japan. KF (exo−) and Tth DNA polymerases were purchased from Promega. Taq DNA polymerase was purchased from TaKaRa. Vent (exo−) and Deep Vent (exo−) DNA polymerases were purchased from New England Biolabs. KOD Dash DNA polymerase was purchased from TOYOBO. The structure of the 30-mer sequence shown in Figure 6 was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) on an ultraflex TOF/TOF (Bruker Daltonics).
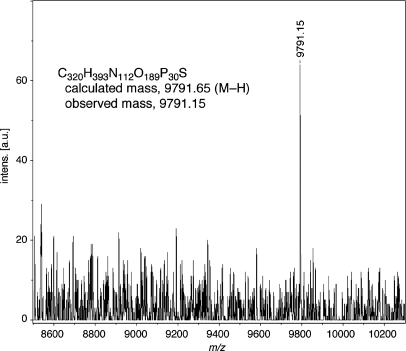
Figure 6.
MALDI-TOF mass spectrum of the full-length 30mer. The sequence of the sample is 5′-FITC-GTTCTGGATGGTCAGCGCACImNOCTTGCCCAC-3′. The exact structure of the 30mer and original spectrum were given in the Supplementary Data (Figure S2).
Primer extension reactions by KF (exo−) (experiment shown in Figure 2)
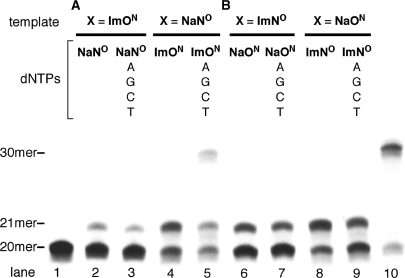
Figure 2.
Primer extension reactions by KF (exo−). (A) The reaction for ImON:NaNO base pair. (B) The reaction for ImNO:NaON base pair. Lanes 2–9 used the primer-template combination 5′-FITC-GTTCTGGATGGTCAGCGCAC-3′ (20mer) and 3′-CAAGACCTACCAGTCGCGTGXGAACGGGTG-5′ (30mer, X = ImON, NaNO, ImNO or NaON). Lane 1 indicates the 20-mer primer, and lane 10 indicates the control 30mer prepared by primer extension reaction (X = T).
A mixture of 0.8 μM duplex consisting of 5′-FITC-labeled primer and template (see legend of Figure 2), 0.2 U of KF (exo−) (19.4 nM) and dNTPs (160 μM) in a buffer (total volume: 10 μl) recommended by the manufacturer was incubated at 37°C for 15 min. The reactions were quenched by adding a loading solution (10 μl) containing 8 M urea, 178 mM Tris–borate, 4 mM Na2EDTA, 0.05% bromophenol blue and 0.05% xylene cyanol, and the mixtures were immediately heated at 100°C for 5 min. The reaction mixtures were resolved by electrophoreses on a 20% polyacrylamide gel containing 8 M urea, and the gels were visualized with FLA-2000 (FUJIFILM).
Screening of DNA polymerases for single nucleotide insertion (experiment shown in Figure 3)
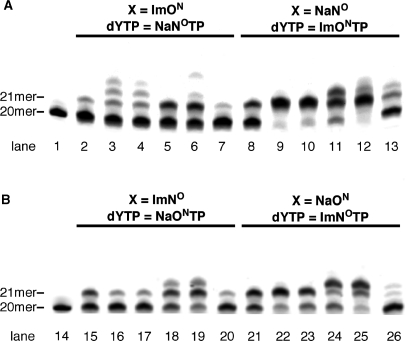
Figure 3.
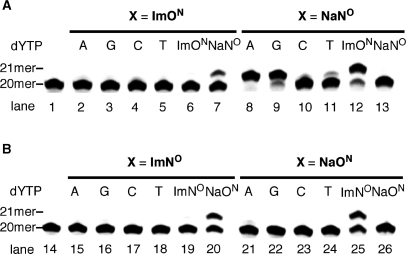
Screening of DNA polymerases for single-nucleotide insertion. (A) The reaction for ImON:NaNO base pair. (B) The reaction for ImNO:NaON base pair. All reactions used the primer-template combination 5′-FITC-GTTCTGGATGGTCAGCGCAC-3′ (20mer) and 3′-CAAGACCTACCAGTCGCGTGXGAACGGGTG-5′ (30mer, X = ImON, NaNO, ImNO or NaON). Lanes 1 and 14 indicate the 20-mer primer; lanes 2, 8, 15 and 21 indicate the results of KF (exo−); lanes 3, 9 16, and 22 indicate those of Taq DNA polymerase; lanes 4, 10, 17 and 23 indicate those of Tth DNA polymerase; lanes 5, 11, 18 and 24 indicate those of Vent (exo−) DNA polymerase; lanes 6, 12, 19 and 25 indicate those of Deep Vent (exo−) DNA polymerase; and lanes 7, 13, 20 and 26 indicate those of KOD Dash DNA polymerase.
A mixture of 0.8 μM duplex consisting of 5′-FITC-labeled primer and template (see legend of Figure 3), DNA polymerase [0.2 U of KF (exo−) (19.4 nM), 2 U of Taq DNA polymerase (236.6 nM), 2 U of Tth DNA polymerase (189.4 nM), 0.75 U of Vent (exo−) DNA polymerase (20.2 nM), 0.75 U of Deep Vent (exo−) DNA polymerase (29.0 nM) or 0.1 U of KOD Dash DNA polymerase (8.9 nM)] and dNTPs (160 μM) in a buffer (total volume: 10 μl) recommended by the manufacturer was incubated at 37°C [KF (exo−)] or 74°C (other polymerase) for 15 min. The reactions were quenched by adding a loading solution (10 μl) containing 8 M urea, 178 mM Tris–borate and 4 mM Na2EDTA, and the mixtures were immediately heated at 100°C for 5 min. The reaction mixtures were resolved by electrophoreses on a 20% polyacrylamide gel containing 8 M urea, and the gels were visualized with FLA-2000.
Single nucleotide insertion by Deep Vent (exo−) (experiment shown in Figure 4)
Figure 4.
Single-nucleotide insertion by Deep Vent (exo−) (selectivity toward natural dNTPs). (A) The reaction for ImON:NaNO base pair. (B) The reaction for ImNO:NaON base pair. All reactions used the primer-template combination 5′-FITC-GTTCTGGATGGTCAGCGCAC-3′ (20mer) and 3′-CAAGACCTACCAGTCGCGTGXGAACGGGTG-5′ (30mer, X = ImON, NaNO, ImNO or NaON). Lanes 1 and 14 indicate the 20-mer primer.
A mixture of 0.8 μM duplex consisting of 5′-FITC-labeled primer and template (see legend of Figure 4), 0.25 U of Deep Vent (exo−) (9.6 nM) and dNTPs (30 μM each when X = ImON, 80 μM each when X = NaNO or 100 μM each when X = ImNO and NaON) in a buffer (total volume: 10 μl) recommended by the manufacture was incubated at 74°C for 2 min. The reactions were quenched by adding a loading solution (10 μl) containing 8 M urea, 178 mM Tris–borate, 4 mM Na2EDTA, 0.05% bromophenol blue and 0.05% xylene cyanol, and the mixtures were immediately heated at 100°C for 5 min. The reaction mixtures were resolved by electrophoreses on a 20% polyacrylamide gel containing 8 M urea, and the gels were visualized with FLA-2000.
Measurement of steady-state kinetics of single nucleotide insertion by Deep Vent (exo−) (experiment shown in Table 1)
Table 1.
Steady-state kinetics data of the single nucleotide insertion by Deep Vent (exo−) DNA Polymerase
| X | dYTP | Km (μM) | Vmax (%ċmin−1) | Vmax/Km (%ċmin−1ċM−1) | Fidelity |
|---|---|---|---|---|---|
| ImON | dATP | 47 ± 30 | 1.8 ± 0.37 | 3.8 × 104 | 4.1 × 10−4 |
| dGTP | 60 ± 14 | 0.98 ± 0.093 | 1.6 × 104 | 1.7 × 10−4 | |
| dCTP | 41 ± 16 | 1.3 ± 0.095 | 3.2 × 104 | 3.4 × 10−4 | |
| dTTP | 54 ± 20 | 1.1 ± 0.13 | 2.0 × 104 | 2.2 × 10−4 | |
| ImONTP | 36 ± 18 | 1.1 ± 0.12 | 3.1 × 104 | 3.3 × 10−4 | |
| NaNOTP | 9.0 ± 6.3 | 4.9 ± 1.6 | 5.4 × 105 | 5.8 × 10−3 | |
| NaNO | dATP | 0.27 ± 0.14 | 15 ± 6.0 | 5.6 × 107 | 6.6 × 10−1 |
| dGTP | 1.7 ± 0.92 | 7.7 ± 2.0 | 4.5 × 106 | 5.2 × 10−2 | |
| dCTP | 33 ± 14 | 3.2 ± 0.22 | 9.7 × 104 | 1.1 × 10−3 | |
| dTTP | 50 ± 35 | 5.1 ± 2.0 | 1.0 × 105 | 1.2 × 10−3 | |
| ImONTP | 2.3±0.18 | 13 ± 2.1 | 5.7 × 106 | 6.7 × 10−2 | |
| NaNOTP | – | – | n.d.a | – | |
| ImNO | dATP | 33 ± 24 | 1.3 ± 0.090 | 3.9 × 104 | 4.2 × 10−4 |
| dGTP | 43 ± 11 | 1.2 ± 0.062 | 2.8 × 104 | 3.0 × 10−4 | |
| dCTP | 16±7.8 | 1.1 ± 0.090 | 7.0 × 104 | 7.5 × 10−4 | |
| dTTP | 53 ± 7.5 | 0.47 ± 0.038 | 9.0 × 103 | 9.7 × 10−5 | |
| ImNOTP | 40 ± 17 | 3.1 ± 0.63 | 7.8 × 104 | 8.4 × 10−4 | |
| NaONTP | 18± 12 | 28 ± 12 | 1.6 × 106 | 1.7 × 10−2 | |
| NaON | dATP | 69 ± 8.1 | 6.8 ± 1.3 | 9.9 × 104 | 1.2 × 10−3 |
| dGTP | 42 ± 26 | 0.69 ± 0.047 | 1.6 × 104 | 1.9 × 10−4 | |
| dCTP | 15 ± 3.7 | 0.72 ± 0.0050 | 4.8 × 104 | 5.6 × 10−4 | |
| dTTP | 53 ± 13 | 0.50 ± 0.065 | 9.5 × 103 | 1.1 × 10−4 | |
| ImNOTP | 14 ± 5.0 | 26 ± 1.4 | 1.9 × 106 | 2.2 × 10−2 | |
| NaONTP | – | – | n.d.a | – | |
| T | dATP | 0.20 ± 0.062 | 17 ± 2.5 | 8.5 × 107 | 1 |
| A | dTTP | 0.55 ± 0.35 | 51 ± 27 | 9.3 × 107 | 1 |
aThe reaction was too insufficient to determine the kinetic parameters.
A mixture of 0.8 μM duplex consisting of 5′-FITC-labeled primer and template (see legend of Figure 4), Deep Vent (exo−) and dNTP in a buffer (total volume: 10 μl) recommended by the manufacturer was incubated at 74°C. The amount of Deep Vent (exo−) used (0.25–0.5 U, 9.6–19.2 nM), the reaction time (2–20 min), and the gradient concentration of dYTP (0.1–200 μM) were adjusted to give reaction extents of 25% or less. The reactions were quenched by adding a loading solution (10 μl) containing 8 M urea, 178 mM Tris–borate, 4 mM Na2EDTA, 0.05% bromophenol blue and 0.05% xylene cyanol, and the mixture were immediately heated at 100°C for 5 min. The reaction mixtures were resolved by electrophoreses on a 20% polyacrylamide gel containing 8 M urea, and the reaction extents were measured with FLA-2000. Relative velocities (υ0) were calculated as the extents of the reaction divided by the reaction time, and were normalized to the enzyme concentration (9.6 nM) for the various enzyme concentrations used. The kinetic parameters (Km and Vmax) were obtained from Hanes–Woolf plots. The data presented are averages of three independent assays. The fidelity was calculated based on the data of dATP incorporation against T in the template, when Na nucleotides were located in the template. In contrast, the fidelity was calculated based on the data of dTTP incorporation against A in the template, when Im nucleotides were located in the template.
Primer extension reactions by family B DNA polymerases (experiment shown in Figure 5)
Figure 5.
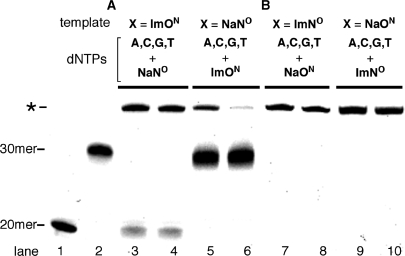
Primer extension reactions by family B DNA polymerases. (A) The reaction for ImON:NaNO base pair. (B) The reaction for ImNO:NaON base pair. All reactions used the primer-template combination 5′-FITC-GTTCTGGATGGTCAGCGCAC-3′ (20mer) and 3′-CAAGACCTACCAGTCGCGTGXGAACGGGTG-5′ (30mer, X = ImON, NaNO, ImNO or NaON). The reaction was carried out in the presence of dYTP (NaNOTP, ImONTP, NaONTP or ImNOTP) and natural dNTPs. Lane 1 indicates the 20-mer primer; lane 2 indicates the control 30mer prepared by primer extension reaction (X = T); lanes 3, 5, 7 and 9 indicate the results of Vent (exo−); and lanes 4, 6, 8 and 10 indicate those of Deep Vent (exo−) DNA polymerase.
A mixture of 0.8 μM duplex consisting of 5′-FITC-labeled primer and template (see legend of Figure 5), DNA polymerase [1 U of Vent (exo−) DNA polymerase (26.9 nM) or 1 U of Deep Vent (exo−) DNA polymerase (38.7 nM)] and dNTPs (160 μM) in a buffer (total volume: 10 μl) recommended by the manufacturer was incubated at 74°C for 15 min. The reactions were quenched by adding a loading solution (10 μl) containing 8 M urea, 178 mM Tris–borate, 4 mM Na2EDTA, 0.05% bromophenol blue and 0.05% xylene cyanol, and the mixtures were immediately heated at 100°C for 5 min. The reaction mixtures were resolved by electrophoreses on a 20% polyacrylamide gel containing 8 M urea, and the gels were visualized with FLA-2000.
Preparation of full-length 30mer for MALDI-TOF mass measurement (experiment shown in Figure 6)
A mixture of 0.8 μM duplex consisting of 5′-FITC-labeled primer and template (X = NaON), 50 U of Deep Vent (exo−) (38.7 nM) and ImNOTP and dNTPs (160 μM each) in a buffer (total volume: 500 μl) recommended by the manufacturer was incubated at 74°C for 60 min. The reaction was quenched by adding a stop solution (500 μl) containing 7 M urea, 89 mM Tris–borate and 2 mM Na2EDTA. This enzymatic reaction was carried out in eight tubes, and then the reaction mixtures were combined. The mixtures were desalted and dried in SpeedVac. The residue was dissolved in 500 μl of loading solution (7 M urea, 89 mM Tris–borate, 2 mM Na2EDTA, 0.05% bromophenol blue and 0.05% xylene cyanol). The resulting full-length 30mer was purified on 20% polyacrylamide gel containing 8 M urea, and desalted and dried in SpeedVac. Then, the sample was analyzed by MALDI-TOF MS.
RESULTS
In our previous communication (10), we reported the results of one nucleotide incorporation for ImON:NaNO and ImNO:NaON base pairs by the KF (exo−). For the ImON:NaNO base pair, NaNOTP was incorporated selectively against ImON in the template, while incorporation of dATP competed with that of ImONTP against NaNO. This result was explained by the fact that the NaNO in the template was recognized as a thymine (T) analog by KF (exo−). On the other hand, enzymatic recognition of the ImNO:NaON base pair was rather selective. In order to develop these base pairs as an alternative and versatile base pair beyond the WC base pair, however, further improvement of the base pair selectivity for 1-nt incorporation would be required as would primer extension reactions after the incorporation of the Im:Na base pair. Therefore, we first examined whether the desired primer extension reactions after the incorporation of ImON:NaNO and ImNO:NaON base pairs occurred with KF (exo−). As shown in Figure 2, enzymatic reactions by KF (exo−) were carried out using a 30-mer template (X = ImON, NaNO, ImNO or NaON) and a 5′-FITC-labeled 20-mer primer in the presence of (i) dYTP alone (dYTP = NaNOTP, ImONTP, NaONTP or ImNOTP) and (ii) dYTP together with natural dNTPs. As a result, NaNOTP, for example, was incorporated against ImON in the template (lane 2); however, no further primer extension was observed in spite of the presence of natural dNTPs (lane 3). The same results were obtained in other combinations with one exception. As shown in lane 5, a band corresponding to the full-length 30-mer was observed along with the 21-mer band in PAGE analysis (a control 30mer is shown in lane 10). Although this band may be a desired full-length product containing ImON at its 21st position, it is nevertheless possible that it came from the misincorporation of natural dATP against NaNO in the template (as described above, the NaNO in the template behaved as a T analog), followed by the primer extension. In order to determine which of the two possibilities was responsible for the effect, the primer extension reaction using a 5′-FITC-labeled 21-mer primer (5′-GTTCTGGATGGTCAGCGCACImON-3′) and a 30-mer template (X = NaNO) in the presence of natural dNTPs was conducted. No primer extension was observed under these conditions (the result of PAGE is given in the Supplementary Data; Figure S1), indicating that the band corresponding to the 30mer observed in lane 5 of Figure 2 comes from the misincorporation of dATP against NaNO in the template, followed by the primer extension. From these results, it was shown that KF (exo−) did not catalyze the primer extension reaction after the Im:Na base pair at the 21st position.
In order to improve the selectivity of base pair recognition for the Im:Na base pairs, and to promote the primer extension reaction after the Im:Na base pair, we carried out a screening of DNA polymerases, including family A polymerases [KF (exo−), Taq and Tth] and family B polymerases [Vent (exo−), Deep Vent (exo−), and KOD Dash]. First, 1-nt incorporation for the ImON:NaNO and ImNO:NaON base pairs was examined using the aforementioned DNA polymerases. As shown in Figure 3, all enzymes afforded the 21-mer fragment, and in some cases, fragments longer than a 21mer were observed. This may indicate the possibility of the primer extension reaction, which of course comes from an undesired misincorporation. This phenomenon was not observed at all in the reactions using KF (exo−). As in the case of KF (exo−) (lane 2 versus lane 8 and lane 15 versus lane 21) (10), incorporation of ImONTP (and ImNOTP) against NaNO (and NaON) in the template appeared to occur rather than that of NaNOTP (and NaONTP) against ImNO (and ImON). Among the DNA polymerases examined, family B polymerases, especially Deep Vent (exo−), seemed most promising for the 1-nt incorporation (lanes 6, 12, 19 and 25).
Accordingly, we next examined the 1-nt incorporation using Deep Vent (exo−) by examining the base pair selectivity against other dNTPs. For the ImON:NaNO base pair (Figure 4A), NaNOTP was incorporated selectively against ImON in the template to afford a 21-mer sequence (lane 7). When NaNO was placed in the template (lanes 8, 9 and 12), not only was ImONTP incorporated but also dATP and dGTP. The incorporation of dATP competed with that of ImONTP when KF (exo−) was used (10), a tendency we observed in our previous study. However, in this case, the incorporation of dATP appeared to be more effective compared with other dNTPs based on the results of PAGE analysis (lane 8). Unlike the ImON:NaNO base pair, recognition of the ImNO:NaON base pair by Deep Vent (exo−) was selective (Figure 4B), and the base pair seemed much higher than in our previous study using KF (exo−). Therefore, we determined the kinetic parameters (Km = the Michaelis constant, Vmax = the maximum rate of the enzyme reaction and Vmax/Km = the elongation efficiency) of every 5′-triphosphate at various concentrations (Table 1) [the kinetic parameters obtained by KF (exo−) are listed in the Supplementary Data for comparison; Table S1]. Quantitative analyses revealed that Deep Vent (exo−) incorporated NaNOTP preferentially against ImON in the template, and the efficiency was 14–34-fold higher than for the other dNTPs (Vmax/Km; 5.4 × 105 versus 1.6 × 104–3.8 × 104). When NaNO was placed in the template, dATP was the best substrate as the complementary 5′-triphosphate. Incorporation of ImONTP against NaNO in the template was 10-fold less relative to dATP (Vmax/Km; 5.7 × 106 versus 5.6 × 107) and was the same as that of dGTP (Vmax/Km; 5.7 × 106 versus 4.5 × 106). These quantitative data are in good agreement with the results of PAGE analysis (Figure 4A). From these results, it can be concluded that the selectivity of base pair recognition in the ImON:NaNO base pair by Deep Vent (exo−) was lower than that by KF (exo−) as discussed in our previous communication (10). On the other hand, recognition of the ImNO:NaON base pair by Deep Vent (exo−) was improved relative to that by KF (exo−) in terms of efficiency and selectivity. Thus, incorporation of NaONTP against ImNO in the template showed Vmax/Km = 1.6 × 106, which was higher than that by KF (exo−). In addition, the efficiency was 20–177-fold higher than other dNTPs (Vmax/Km; 1.6 × 106 versus 7.8 × 104–9.0 × 103). When NaON was placed in the template, incorporation of ImNOTP showed similar efficiency and selectivity (Vmax/Km; 1.9 × 106 versus 9.9 × 104–9.5 × 103).
As shown in Figure 2, KF (exo−) did not promote the primer extension reaction after the Im:Na base pair. Since the Im:Na base pairs, especially the ImNO:NaON base pair, were recognized selectively by Deep Vent (exo−) as well as by KF (exo−), we then examined whether Deep Vent (exo−) would promote the further extension reaction after the Im:Na base pair. All combinations, i.e. ImON:NaNO and ImNO:NaON base pairs, and Vent (exo−) as well as Deep Vent (exo−), which belong to the family B polymerases, were tried (Figure 5). In all attempts, the band corresponding to the 20-mer primer was nearly consumed and no band corresponding to a 21mer was observed. These results indicated that both polymerases promoted primer extension reactions different from those by KF (exo−). However, all reactions afforded a band (marked with an asterisk) that appeared longer than the expected 30mer (lane 2). When NaNO was placed in the template, a band possessing the same gel mobility as the control 30mer (lanes 5 and 6) was observed as the major product. However, the band marked with an asterisk was observed partially in both cases. In our previous communication (10), we revealed that the oligodeoxynucleotides (ODNs) containing Im and Na nucleotide units had reduced gel mobilities than the usual ODNs consisting of natural nucleotide units in spite of their identical length. This being the case, it can be inferred that the bands marked with an asterisk would be the desired full-length 30mer, while the bands possessing the same gel mobilities as the control 30mer observed in lanes 5 and 6 should come from the misincorporation of dATP against NaNO in the template, followed by further primer extension. The kinetic parameters of dATP and ImONTP against NaNO in the template listed in Table 1 may strongly suggest this consideration. However, there is no evidence that the bands marked with an asterisk are the desired full-length 30mer. In the primer extension reaction, it is known that DNA polymerases sometimes add the nontemplated extra nucleotides on the 3′-end of a resulting full-length ODN (11), and this activity might afford a longer sequence than the desired 30mer. In order to confirm the structures of the bands marked with an asterisk, the main band observed in lane 10 was purified and the resulting fragment was analyzed by MALDI-TOF mass spectrometry. As shown in Figure 6, the observed mass (m/z = 9791.15) agreed well with the calculated value of the desired full-length 30mer containing ImNO at its 21st position [C320H393N112O189P30S 9791.65 (M–H)] (the structure of the 30mer and its original MALDI-TOF mass spectrum are given in the Supplementary Data; Figure S2). Thus, it was confirmed that the selective incorporation of ImNOTP against NaON in the template, followed by further primer extension reactions, occurred to afford the 30mer in the reaction shown in lane 10. Likewise, all bands marked with an asterisk would be the desired 30mers, while the major bands observed in lanes 5 and 6 would be the 30mers arising from misincorporation of dATP. As listed in Table 1, Deep Vent (exo−) incorporated dATP 10-fold higher than ImONTP against NaNO in the template, and thus, this consideration appeared to be reasonable. Since there were no bands corresponding to the 30mer arising from such misincorporation of natural dNTPs in lanes 7–10, it can be concluded that the ImNO:NaON base pair was recognized selectively as the complementary base to be incorporated at the 21st position and the further primer extension reactions were promoted to afford the desired full-length 30mer by family B polymerases such as Deep Vent (exo−). (Additional evidence that the band marked with an asterisk corresponded to the desired 30mer containing Im or Na base is given in the Supplementary Data; see Figure S3.) In order to determine the extension fidelity next to the ImNO:NaON base pair, we compared the kinetic parameters of every 5′-triphosphate using a 5′-FITC-labeled 21mer (5′-GTTCTGGATGGTCAGCGCACNaON-3′) and a 30-mer template (3′-CAAGACCTACCAGTCGCGTGImNOGAACGGGTG-5′). As a result, dCTP was precisely incorporated against dG in the template next to the ImNO:NaON base pair (the kinetic parameters are listed in the Supplementary Data; Table S2).
DISCUSSION
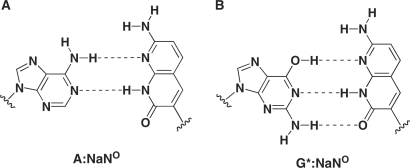
The most striking result in this study was that, unlike KF (exo−), family B polymerases, such as Deep Vent (exo−), catalyzed the further primer extension reaction after the Im:Na base pair to afford full-length DNA. Furthermore, it is also worth noting that Deep Vent (exo−) showed higher selectivity for the ImNO:NaON base pair. This selectivity would arise from: (i) the complementary four H-bonds, (ii) the stacking ability of the expanded aromatic surface and (iii) the shape complementarity of the Im:Na base pairs similar to the pu:py base pair. Contrary to the ImNO:NaON base pair, the selectivity of the ImON:NaNO base pair was low, especially when the NaNO was placed in the template. The preferable incorporation of dATP against NaNO in the template can be explained by the similarity of NaNO with T in terms of its H-bonding pattern (Figure 7A). In this base pair, incorporation of dGTP against NaNO in the template also competed with that of ImONTP, whose efficiency was much less than that of dATP. Since incorporation of dGTP against NaNO in the template was not observed in our previous study using KF (exo−) (10), this phenomenon was an unexpected result. As one explanation for dGTP misincorporation against NaNO, the formation of the base pair between a minor tautomeric form of G (G*) and NaNO through three H-bonds (Figure 7B) can be proposed, as observed in the base pair between isocytidine and isoguanosine (12). The higher selectivity of base pair recognition by the ImNO:NaON base pair relative to the ImON:NaNO base pair presumably would be attributed to the difference in their H-bonding patterns (Figure 1). Thus, it has been suggested that interaction of the N3 of purine bases and the O2 of pyrimidine bases as proton acceptors located in the minor groove with the DNA polymerase is vital for dNTP incorporation (see A:T pair in Figure 1) (13,14). In the case of the ImON:NaNO base pair, a similar interaction with DNA polymerases is expected, which may also cause the misincorporation of natural dNTPs such as dATP and dGTP to result in the lower selectivity by Deep Vent (exo−) as well as KF (exo−). In contrast, the proton acceptor corresponding to the O2 of pyrimidine bases is missing in the ImNO:NaON base pair, and thus, this unusual H-bonding pattern is thought to account for the higher selectivity. The question still remains as to why Deep Vent (exo−), unlike KF (exo−), catalyzed the further primer extension reactions after the Im:Na base pair to afford full-length DNA. Thus far, a number of chemically modified dNTPs have been prepared, and their enzymatic incorporation has been investigated. In these studies, we sought to determine if not only base pair selectivity, but also the fact that the further extension reaction occurs after the incorporation of the modified dNTPs might pose a problem (15–18). In many cases, the incorporation of the modified dNTPs by KF (exo−) without the promotion of further extension reactions has been observed. However, family B polymerases generally tolerate chemical modifications in their substrates, and therefore their capacity may promote further extension reactions. One explanation for this permissive capacity of family B polymerases such as Deep Vent (exo−) may be attributed to the difference in strictness of the minor groove interaction between family A and family B DNA polymerases (19–21). Thus, crystallographic studies have shown a higher ordered interaction in the minor groove of the double helix DNA with family A polymerases compared with family B polymerases (22–25). As depicted in Figure 1, for example, the ImON:NaNO base pair has alternative H-bonding acceptors corresponding to the N3 of purine bases and the O2 of pyrimidine bases in the minor groove; however, the geometry of their acceptors differs somewhat from those of a natural pu:py base pair system. In addition, the Im bases can be considered as ring-expanded analogs of purines toward the minor groove direction, while the Na bases are ring-expanded analogs of pyrimidines toward the major groove direction. In summary, unlike KF (exo−), Deep Vent (exo−) allowed these overall structural differences because of its permissive capacity to afford full-length products.
Figure 7.
Structures of possible base pairs between NaNO and natural bases.
CONCLUSION
In this paper, we have investigated how the thermally stable ImON:NaNO and ImNO:NaON base pairs are recognized by DNA polymerases. The investigations focused on improving the selectivity of the Im:Na base pairs and promoting primer extension reactions after Im:Na base pairs. Accordingly, the ImNO:NaON base pair was selectively recognized as a complementary base pair and the further primer extension reactions occurred when Deep Vent (exo−) was used. The selectivity of the ImNO:NaON base pair was considered to be due to the four H-bonds between the nucleobases, the unusual H-bonding pattern relative to the WC base pairs, and the shape complementarity similar to the WC base pairs. Our results would be a contribution toward developing alternative stable base pairs to expand the genetic code and explore synthetic biology. In the research field developing an alternative new base pair other than the WC base pairs, Benner has proposed base pairs consisting of three H-bonds. According to his theory, 23 = 8 H-bonding patterns are conceivable. Since the Im:Na base pairs consist of four H-bonds, one can in theory imagine 24 = 16 H-bonding patterns (26). Only two pairs are readily accessible now. Among the remaining 14 patterns, more selective and effective base pair systems may exist. Further investigations are underway.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science (No. 18109001). Funding for open access charge: Japan Society of Promotion of Science.
Conflict of interest statement. None declared.
Supplementary Material
Footnotes
Present address: Noriaki Minakawa, Graduate School of Pharmaceutical Sciences, The University of Tokushima, Shomachi 1-78-1, Tokushima, 770-8505, Japan.
REFERENCES
- 1.Henry AA, Romesberg FE. Beyond A, C, G and T: augmenting nature's alphabet. Curr. Opin. Chem. Biol. 2003;7:727–733. doi: 10.1016/j.cbpa.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Seo YJ, Matsuda S, Romesberg FE. Transcription of an expanded genetic alphabet. J. Am. Chem. Soc. 2009;131:5046–5047. doi: 10.1021/ja9006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benner SA, Sismour AM. Synthetic biology. Nat. Rev. Genet. 2005;6:533–543. doi: 10.1038/nrg1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirao I. Unnatural base pair systems for DNA/RNA-based biotechnology. Curr. Opin. Chem. Biol. 2006;10:622–627. doi: 10.1016/j.cbpa.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Hirao I, Mitsui T, Kimoto M, Yokoyama S. An efficient unnatural base pair for PCR amplification. J. Am. Chem. Soc. 2007;129:15549–15555. doi: 10.1021/ja073830m. [DOI] [PubMed] [Google Scholar]
- 6.Krueger AT, Kool ET. Model systems for understanding DNA base pairing. Curr. Opin. Chem. Biol. 2007;11:588–594. doi: 10.1016/j.cbpa.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minakawa N, Kojima N, Hikishima S, Sasaki T, Kiyosue A, Atsumi N, Ueno Y, Matsuda A. New base pairing motifs. The synthesis and thermal stability of oligodeoxynucleotides containing imidazopyridopyrimidine nucleosides with the ability to form four hydrogen bonds. J. Am. Chem. Soc. 2003;125:9970–9982. doi: 10.1021/ja0347686. [DOI] [PubMed] [Google Scholar]
- 8.Hikishima S, Minakawa N, Kuramoto K, Fujisawa Y, Ogawa M, Matsuda A. Synthesis of 1,8-naphthyridine C-nucleosides and their base-pairing properties in oligodeoxynucleotides: thermally stable naphthyridine:imidazopyridopyrimidine base-pairing motifs. Angew. Chem. Int. Ed. 2005;44:596–598. doi: 10.1002/anie.200461857. [DOI] [PubMed] [Google Scholar]
- 9.Hikishima S, Minakawa N, Kuramoto K, Ogata S, Matsuda A. Synthesis and characterization of oligodeoxynucleotides containing naphthyridine:imidazopyridopyrimidine base pairs at their sticky ends. Application as thermally stabilized decoy molecules. ChemBioChem. 2006;7:1970–1975. doi: 10.1002/cbic.200600318. [DOI] [PubMed] [Google Scholar]
- 10.Minakawa N, Ogata S, Takahashi M, Matsuda A. Selective recognition of unnatural imidazopyridopyrimidine:naphthyridine base pairs consisting of four hydrogen bonds by the Klenow fragment. J. Am. Chem. Soc. 2009;131:1644–1645. doi: 10.1021/ja807391g. [DOI] [PubMed] [Google Scholar]
- 11.Hu GX. DNA polymerase-catalyzed addition of nontemplated extra nucleotides to the 3′-end of a DNA fragment. DNA Cell Biol. 1993;12:763–770. doi: 10.1089/dna.1993.12.763. [DOI] [PubMed] [Google Scholar]
- 12.Switzer CY, Moroney SE, Benner SA. Enzymatic recognition of the base pair between isocytidine and isoguanosine. Biochemisty. 1993;32:10489–10496. doi: 10.1021/bi00090a027. [DOI] [PubMed] [Google Scholar]
- 13.Guo M, Hildbrand S, Leumann CJ, McLaughlin LW, Waring MJ. Inhibition of DNA polymerase reactions by pyrimidine nucleotide analogues lacking the 2-keto group. Nucleic Acids Res. 1998;26:1863–1869. doi: 10.1093/nar/26.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spratt TE. Identification of hydrogen bonds between Escherichia coli DNA polymerase I (Klenow fragment) and the minor groove of DNA by amino acid substitution of the polymerase and atomic substitution of the DNA. Biochemisty. 2001;40:2647–2652. doi: 10.1021/bi002641c. [DOI] [PubMed] [Google Scholar]
- 15.Vastmans K, Pochet S, Peys A, Kerremans L, Aerschot AV, Hendrix C, Marlière P, Herdewijn P. Enzymatic incorporation in DNA of 1,5-anhydrohexitol nucleotides. Biochemisty. 2000;39:12757–12765. doi: 10.1021/bi001297g. [DOI] [PubMed] [Google Scholar]
- 16.Jäger S, Rasched G, Kornreich-Leshem H, Engeser M, Thum O, Famulok M. A versatile toolbox for variable DNA functionalization at high density. J. Am. Chem. Soc. 2005;127:15071–15082. doi: 10.1021/ja051725b. [DOI] [PubMed] [Google Scholar]
- 17.Veedu RN, Vester B, Wengel J. Polymerase chain reaction and transcription using locked nucleic acid nucleotide triphosphates. J. Am. Chem. Soc. 2008;130:8124–8125. doi: 10.1021/ja801389n. [DOI] [PubMed] [Google Scholar]
- 18.Hwang GT, Romesberg FE. Unnatural substrate repertoire of A, B, and X family DNA polymerases. J. Am. Chem. Soc. 2008;130:14872–14882. doi: 10.1021/ja803833h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summerer D, Marx A. Differential minor groove interactions between DNA polymerase and sugar backbone of primer and template strands. J. Am. Chem. Soc. 2002;124:910–911. doi: 10.1021/ja017244j. [DOI] [PubMed] [Google Scholar]
- 20.Strerath M, Marx A. Tuning PCR specificity by chemically modified primer probes. Angew. Chem. Int. Ed. 2002;41:4766–4769. doi: 10.1002/anie.200290043. [DOI] [PubMed] [Google Scholar]
- 21.Pasquale FD, Fischer D, Grohmann D, Restle T, Geyer A, Marx A. Opposed steric constraints in human DNA polymerase β and E. coli DNA polymerase I. J. Am. Chem. Soc. 2008;130:10748–10757. doi: 10.1021/ja8028284. [DOI] [PubMed] [Google Scholar]
- 22.Eom SH, Wang J, Steitz TA. Structure of Taq polymerase with DNA at the polymerase active site. Nature. 1996;382:278–281. doi: 10.1038/382278a0. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Korolev S, Waksman G. Crystal structures of open and closed forms of binary and ternary complexes of the large fragment of Thermus aquaticus DNA polymerase I: structural basis for nucleotide incorporation. EMBO J. 1998;17:7514–7525. doi: 10.1093/emboj/17.24.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin MC, Wang JM, Steitz TA. Structure of the replicating complex of a Pol alpha family DNA polymerase. Cell. 2001;105:657–667. doi: 10.1016/s0092-8674(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickson CL, Devine KG, Benner SA. Probing minor groove recognition contacts by DNA polymerases and reverse transcriptases using 3-deaza-2′-deoxyadenosine. Nucleic Acids Res. 2004;32:2241–2250. doi: 10.1093/nar/gkh542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benner SA. Redesigning genetics. Science. 2004;306:625–626. doi: 10.1126/science.1101104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.