Abstract
The accessory subunit of mitochondrial DNA polymerase γ, POLGβ, functions as a processivity factor in vitro. Here we show POLGβ has additional roles in mitochondrial DNA metabolism. Mitochondrial DNA is arranged in nucleoprotein complexes, or nucleoids, which often contain multiple copies of the mitochondrial genome. Gene-silencing of POLGβ increased nucleoid numbers, whereas over-expression of POLGβ reduced the number and increased the size of mitochondrial nucleoids. Both increased and decreased expression of POLGβ altered nucleoid structure and precipitated a marked decrease in 7S DNA molecules, which form short displacement-loops on mitochondrial DNA. Recombinant POLGβ preferentially bound to plasmids with a short displacement-loop, in contrast to POLGα. These findings support the view that the mitochondrial D-loop acts as a protein recruitment centre, and suggest POLGβ is a key factor in the organization of mitochondrial DNA in multigenomic nucleoprotein complexes.
INTRODUCTION
Mitochondria maintain a separate genome from the nucleus, which produces essential components of the oxidative phosphorylation system. Mammalian mitochondrial (mt) DNA is ∼16 kb and most cells contain over a thousand copies of the mitochondrial genome. Because there is no evidence for mitochondrial histones, it was widely believed for many years that mtDNA was largely naked. However, in the past decade a host of proteins has been found to be associated with mtDNA (1–4) and so it is now generally accepted that mtDNA is arranged in nucleoprotein complexes, or nucleoids. Although considerable progress has been made in recent years in cataloguing the protein components of mitochondrial nucleoids much less is known about how they contribute to nucleoid architecture and dynamics.
In mammals, many mtDNA molecules contain a short triple-stranded region or D-loop (5,6) located in the major non-coding region of mtDNA, or NCR. The length of the third strand is somewhat variable (7), but as they sediment at seven svedbergs, they are collectively known as 7S DNA. The D-loop spans ∼650 nt in humans, and is located entirely within the NCR or control region of mtDNA. Synthesis of 7S DNA is believed to depend ultimately on mtRNA polymerase to provide an RNA primer derived from 7S RNA (8), whose 5′ end maps to the light-strand promoter (LSP). The 3′ end of 7S-DNA is located close to a sequence predicted to form a clover-leaf structure, which has been proposed to act as a termination signal for 7S-DNA synthesis, and so it is called the Termination Associated Sequence, or TAS (7). The abundance of 7S DNA varies considerably among tissues and cells, and its synthesis is enhanced by electrical stimulation of muscle (9), as the electrical stimulus also induces mitochondrial biogenesis and an increase in mtDNA mass, it implies that 7S DNA plays a positive role in mtDNA metabolism. In the orthodox model of mtDNA replication 7S DNA is presumed to be a stalled or aborted replication intermediate (7); however, the orthodox model has been challenged in recent years (10,11), thereby calling into question this hypothesis. Moreover, the identification of a candidate nucleoid protein, ATAD3p, with specific D-loop-binding properties suggested that the mitochondrial D-loop might be involved in protein recruitment (2), among other things (12).
DNA polymerase γ (POLγα or POLGα) is the sole recognized DNA polymerase of animal mitochondria (13), and so presumably synthesizes 7S DNA, but beyond this little is known of the regulation of the process. Certainly POLGα is essential for mtDNA maintenance (14), and together with mitochondrial single-stranded (ss) DNA-binding protein (mtSSB) and the DNA helicase Twinkle it can be used to synthesize 16-kb stretches of DNA in vitro (15). POLGα has an accessory subunit POLGβ, which enhances polymerase processivity in vitro (16,17), and is assumed to be a component of mitochondrial replisomes in vivo (18). Analysis of the behaviour of mutants and resolution of the structure of POLGβ indicate that binding to POLGα and binding to DNA involve distinct domains (19). POLGβ binds to double-stranded (ds) DNA in vitro; however, this function is not required for stimulation of DNA synthesis on a ss template (18–21). POLGβ has sequence and structural similarity to type IIa aminoacyl–tRNA synthetases, and to a lesser extent to thioredoxin, which is known to play an accessory role in the priming of DNA replication by T7 DNA polymerase. Hence, it has been proposed that POLGβ may be involved in primer recognition as well as DNA strand elongation (22).
The dsDNA-binding properties of POLGβ suggested to us that it might have a general role in mtDNA maintenance. In the current study, POLGβ expression was modulated to determine the effect on mtDNA stability. Gene-silencing of POLGβ decreased mtDNA multimers, increased the number of mitochondrial nucleoids and altered their structure in cultured cells, whereas elevated expression of POLGβ increased the size of mitochondrial nucleoids. Experiments with recombinant protein indicated that POLGβ is a specific D-loop-binding protein, and so it is concluded that this property enables POLGβ to contribute to the organization of mtDNA in multigenomic complexes, in addition to its previously reported role as a replication processivity factor.
MATERIALS AND METHODS
Cell culture and RNAi
Human cultured cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 110 mg/l pyruvate and 10% fetal bovine serum. For RNAi, 143B human osteosarcoma (HOS) cells were maintained on standard six-well plates. Forty-eight hours before transfection, cells were harvested and diluted such that, on the day of transfection, cells were 25–30% confluent. Then cells were transfected with 3.6 µl of Lipofectamine2000 (Invitrogen) per 0.6 ml of OptiMEM (Invitrogen), pre-incubated for 20 min at room temperature with 5 nM dsRNA (final concentration); after 4 h, 0.6 ml of DMEM/30% FBS was added. Cells were diluted 4-fold after 24 h and transfected a second time, at 72 h, with the cells ∼30% confluent. Cells were diluted 2-fold at 96 h. After 120 or 144 h cells were examined by confocal microscopy, or lysed for total RNA or DNA extraction. The dsRNA, 5′-UGAAGAAGGCCGGAAAGGAAACAAAAG-3′; 3′-UAACUUCUUCCGGCCUUUCCUUUGUUU-5′ (dsRNA-840), purchased from iGene (Tokyo, Japan) corresponded to nt 840–864 of the POLGβ open reading frame.
Quantification of transcripts and mtDNA copy number
POLGβ mRNA abundance was estimated relative to GAPDH. Total RNA was extracted from cells using TRIzol reagent (Invitrogen) and cDNA generated using Ominiscript reverse transcription kit with random hexamer primers and oligo dT (Qiagen) according to manufacturer's instructions. mtDNA copy number of control and siRNA treated osteosarcoma cells was estimated by amplifying a portion of the cytochrome c oxidase II (COX II) gene of mtDNA and comparing it to the nuclear single copy gene, Amyloid Precursor protein, or APP, as previously described (23). Total DNA samples were prepared from cells lysed in 500 μl 75 mM NaCl, 50 mM EDTA, 0.2% SDS pH 8.0. Cell lysates were incubated at 50°C for 2 h with 400 μg/ml Proteinase K. DNA was precipitated by addition of an equal volume of isopropanol, pelleted by centrifugation at 8500 × gmax for 30 min, and dissolved in 100 μl of TE buffer pH 8.0. The DNA concentration was adjusted to 5 ng/µl and 25 ng was used per real-time PCR reaction. Real-time PCR reactions employed Amplitaq Gold DNA polymerase with gold buffer (Applied Biosystems) according to manufacturer's instructions, except that 5% w/v glycerol was added. Primer sequences were POLGβ forward 5′-CAATGTGTCTAAATTACATGGCCGAG-3′, POLGβ reverse 5′-CGGTCTAGGTCCCCATTTACAGA-3′, POLGβ probe 5′-TGGACGAAAAAATGTGGTTCCTTGTGTTC-3′. GAPDH forward 5′-GAAGGTGAAGGTCGGAGTCAAC-3′, GAPDH reverse 5′-CAGAGTTAAAAGCAGCCCTGGT-3′, GAPDH probe 5′-TTTGGTCCGTATTGGGCG-3′. COXII forward 5′-CGTCTGAACTATCCTGCCCG-3′, COXII reverse 5′-TGGTAAGGGAGGGATCGTTG-3′, COXII probe 5′-CGCCCTCCCATCCCTACGCATC-3′. APP forward 5′-TTTTTGTGTGCTCTCCCAGGTCT-3′, APP reverse 5′-TGGTCACTGGTTGGTTGGC-3′, APP probe 5′-CCCTGAACTGCAGATCACCAATGCGGTAG-3′. Attached to all probes were a FAM flurophore at the 5′ end and a TAMRA quencher at the 3′ end (Sigma Genosys). Cycle conditions were the default setting on the ABI sequence detection system 7700 (95°C 10 min, then 40 cycles of 95°C for 15 s and 60°C for 1 min).
DNA analysis
In the case of DNA isolated without the use of proteinase K, siRNA-treated and mock transfected HOS cells were lysed with extraction buffer containing 75 mM NaCl, 50 mM EDTA, 20 mM HEPES–NaOH (pH 7.8) and 0.5% SDS. The lysate was extracted successively with phenol and chloroform/isoamyl alcohol (24 : 1), DNA was precipitated by addition of an equal volume of isopropanol, washed with 70% ethanol and resuspended in extraction buffer, and re-extracted as before. DNA was finally suspended in 10 mM Tris, 1 mM EDTA pH 8. For 2D agarose gel electrophoresis (2D-AGE) of DNA isolated without protease, 10 μg of total cell DNA was digested with AccI and fractionated on a 0.4% TBE agarose gel at 1.7 V/cm for 15 h at room temperature; second dimension electrophoresis conditions were 0.875% agarose, 3 V/cm for 24 h at 4°C. DNA for analysing RIs and other non-linear molecules was derived from HOS cells by lysis in extraction buffer and incubation at 4°C for 2 h with 500 µg/ml Proteinase K and then phenol/chloroform extracted as above. Proteinase K-treated DNA was AccI digested and separated by 2D-AGE using 0.55% agarose and 0.9 V/cm for 20 h in the first dimension, and 1.55% agarose for 7 h at 260 mA at 4°C in the second dimension. Southern blots of 2D gels were hybridized to radiolabelled probes by overnight incubation at 65°C in 7% SDS, 0.25 M sodium phosphate pH 7.2. The human mtDNA probed spanned nt 16 343-151 (24), whereas the mouse mtDNA probes covered nt 15 007–15 805 and nt 13 874–14 525 (25). Post-hybridization washes were 1× SSC followed by 1× SSC, 0.1% SDS, twice for 30 min at 65°C. Filters were exposed to X-ray film and developed after 3–7 days, or exposed to phosphor imaging plates for quantification of spots of RIs and unit length fragments (1n) with a Typhoon™ phosphorimager (GE Healthcare).
Immunoblotting
Aliquots of 30 μg of total cell lysate were separated on 4–12% SDS–polyacrylamide gels (Invitrogen) at 100–150 V for 1.5 h, before transfer to Protran nitrocellulose membrane (Whatman), using a Trans-Blot Semy-Dry transfer cell (Bio-Rad), in a buffer comprising 48 mM Tris pH 8.5, 390 mM Glycine, 0.37% SDS, 20% methanol at 150 mA/per gel for 1 h at room temperature. Membranes were blocked with 5% milk in 1× PBS, 0.1% Tween20 for 1 h at room temperature with shaking. Proteins were detected by specific antibodies: goat anti-PolGα 1 : 500, goat anti-GAPDH 1 : 500 (SantaCruz) and donkey anti-goat 1 : 4000 (Promega); rabbit anti-POLGβ 1 : 1000, goat anti-rabbit 1 : 5000.
Inducible expression of POLG2 in human embryonic kidney cells
POLGβ with a haemagglutinin (HA) tag was cloned into pcDNA5 and transfected into human embryonic kidney (HEK) flp-in T-Rex cells (Invitrogen), where expression of the transgene is under the control of a tetracycline/doxycyclin inducible promoter. Whole cell lysates derived from HEK cells incubated with and without 10 ng/ml doxycyclin were analysed by immunoblotting using 1 in 2000 anti-HA peroxidase antibody (3F10) from Roche.
Confocal microscopy
For live-stains, cells were washed with 1 ml PBS and incubated with 1 ml DMEM containing 3 µl PicoGreen (Invitrogen, P11495) and 200 nM of Mitotracker Orange (Invitrogen) for 30 min at 37°C. The cells were washed three times in 2 ml PBS before mounting on slides. A LSM 510 Meta confocal microscope (Zeiss) was used for cell imaging in conjunction with LSM 510 software, images were edited using Photoshop Element (Adobe). Images were acquired with a Zeiss 63×/1.40 oil immersion objective, set at zoom 2.4 at 22–24°C in Zeiss Immersol®. The excitation/emission wavelengths for PicoGreen and Mitotracker Orange were 502/523 nm and 554/576 nm, respectively. MitoTracker orange signal was pseudo-coloured red to improve contrast.
POLGβ.HA overexpressing U2 osteosarcoma (U2OS) cells were made by transient transfection using TransIT®-LT1 (Mirus) and the manufacturer's protocol with a sequence verified POLGβ.HA expression plasmid (pcDNA3.1(+)Hygro). Two days after transfection cells were fixed with 4% paraformaladehyde in cell culture medium at RT for 30 min and lysed in PBS/10% FCS/0.5% TritonX-100 for 15 min at RT. mtDNA (Figure 4B and C) was detected using a 1 : 100 dilution of an anti-DNA antibody (1 µg/ml PROGEN). HA tagged POLGβ was detected using a 1 : 400 dilution of a polyclonal HA antibody (Covance). Secondary antibodies (from Invitrogen) used were anti-mouse IgM Alexa Fluor® 488 (DNA), and anti-rabbit Alexa Fluor® 568 (HA polyclonal) both diluted at 1:1000. All antibodies were diluted in PBS/10% FCS. Slides were mounted using ProLong® Gold antifade with DAPI (Invitrogen). Samples were examined by Confocal Laser Microscopy using a Perkin Elmer/Wallac UltraView LCI system equipped with appropriate excitation and emission filters and with an Andor iXon DV885 EMCCD camera and Andor iQ software. Nucleoid size used full-width half- maximum measurements (FWHM) of the anti-DNA antibody stain, which was measured for each nucleoid at the plain of maximum intensity and focus. Nucleoid numbers were counted by generating maximum projection images of z-stacks (Andor iQ), which were exported to ImageJ software (http://rsb.info.nih.gov/ij/) for foci counting. Nucleoid numbers and size determination was done on POLGβ. HA overexpressing and non-expressing cells from the same transfection sample as judged by the anti-HA immunofluorescence.
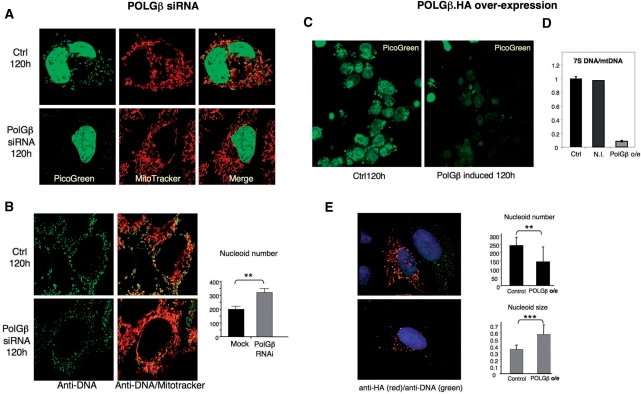
Figure 4.
Modulating the expression of POLGβ affects mitochondrial nucleoid structure, number and size. HOS cells displayed a substantial decrease in PicoGreen staining of mitochondrial nucleoids after POLGβ siRNA (A and Supplementary Figure 1). Cells were twice mock-transfected (control), or transfected with dsRNA-840-targeting POLGβ (POLGβ RNAi). Transfections were at t = 0 and t = 72 h; live cell imaging was performed at t = 120 h. DNA was stained with Picogreen and mitochondria with Mitotracker™ (Invitrogen); both reagents depend on a membrane potential for mitochondrial uptake. (B) Mock and POLGβ siRNA treated HOS cells were immunolabelled using anti-DNA antibody, visual inspection of cells suggested that nucleoids were more numerous after POLGβ siRNA and this was confirmed by analysis of 29 cells of each type from three independent experiments with Axiovision software (Zeiss): P = 0.004 based on the unpaired two-tailed student's t-test (**P < 0.01). Control and dsRNA-840 treated cells with the most intense signal were selected for nucleoid counting from several different fields of cells, as such cells were invariably well spread and in good condition. Induction of POLGβ transgene in HEK cells for 120 h led to a loss of Picogreen signal (C) and a marked decrease in 7S DNA level after 72 h treatment with 10 ng/ml doxycyclin, compared to the same cells not induced (N.I.) with the drug and control (Ctrl) HEK cells lacking the POLGβ transgene (D). (E) Over-expression of HA-tagged POLGβ in human U2OS osteosarcoma cells resulted in the formation of enlarged nucleoids (stained with anti-DNA antibodies) (**P < 0.01), which were fewer in number than in untransfected cells (***P < 0.001) (see ‘Results’ section and Supplementary Figure 3). U2OS cells were preferred to HEK cells for confocal analysis because they spread well and therefore yielded better quality images.
Production of recombinant POLG and EMSAs
POLGβ (his)10 was cloned into vector pRun (a gift of Drs Runswick and Walker) and expressed in E. coli BL21 cells. Cells were disrupted by sonication in a buffer comprising 0.5 M NaCl, 20 mM Phosphate buffer pH 7.4, 20 mM Imidazole, 0.2% Triton X-100 and 0.4 mM PMSF and after centrifugation at 48 000 × gmax for 30 min at 4°C, the supernatant was loaded on a nickel Sepharose column. The bulk of non-specifically bound protein was removed by washing with 100 mM Imidazole, and his-tagged POLGβ was eluted by applying a gradient of 100–500 mM Imidazole to the column. After collection protein containing fractions were dialysed overnight at 4°C, against 300 mM NaCl, 20 mM Tris pH 7.4, 20% glycerol, 2 mM MgCl2 and 5 mM DTT, concentrated on a VIVASPIN 2 ml column (Vivascience Sartorius Group) and stored in glycerol at −20°C. In some cases, POLGβ was purified further by gel filtration chromatography in buffer comprising: 300 mM NaCl, 20 mM Tris pH 7.4, 20% Glycerol, 2 mM MgCl2, 2 mM DTT, using a Superose12 column (GE Healthcare). The two major peaks contained POLGβ and the second of these contained no appreciable contamination based on commassie staining after SDS–PAGE (Supplementary Figure 1A and B).
PolGα was cloned into pFastBac HT (Invitrogen) and baculovirus produced in Sf9 cells according to manufacturer's instructions. PolGα was expressed and purified from High Five cells infected with the baculovirus. Cells were grown in suspension and harvested three days after infection and lysed by sonication in 100 ml of buffer A (50 mM Tris–HCl pH 8, 300 mM NaCl, 10 mM imidazole, 0.1% 2-mercaptoethanol) supplemented with DNase, RNase and protease inhibitor. After centrifugation, supernatant was loaded onto HisTrap HP column (GE Healthcare) pre-equilibrated with buffer A. After washing, protein was eluted with 0.01–1 M imidazole. The His-tag was cleaved with TEV protease overnight. Released protein was loaded onto a HiTrap Heparin HP column (GE Healthcare) and eluted with 0–1 M NaCl. Fractions containing PolGα were pooled, concentrated and loaded onto a HiLoad 26/60 Superdex 200 column (GE Healthcare).
Synthetic D-loop was made by incubating 32P end-labelled oligonucleotide C (5′-ACCGCTATGTATTTCGTACATTACTGCCAGCCACCATGAATATTGTACGGTACCATAAATACTTG ACCACCTGTAGTACATAAAAACCCAATCCACATCAAAACCCCCTCCCCATGCTTA-3′) with pNCR (Bluescript plasmid with an insert containing nt 15 839–668 of human mtDNA, which includes the entire NCR) in the presence of RecA, as previously described (2). POLGβ (his10) or POLGα (untagged) protein was incubated with gel-extracted D-loop C in binding buffer: 130 mM NaCl, 25 mM Tris–Cl pH 8.0, 10% glycerol, 5 mg/ml BSA, 1 mM EDTA, 2 mM DTT, for 20 min at 37°C and 10 min on ice. The mixtures were separated by native 1D-AGE: 70 V for 6 h at 4°C, in a 0.8% agarose gel in 1× Tris–borate EDTA buffer, pH 8.0. In the case of oligonucleotides, protein and DNA (oligo C, a 33-mer 5′-CCGGTCGTACGGAAAACCTGTATTTTCAGGGCT-3′ or a 37-mer ds oligonucleotide 5′-CCGGTCGTACGGAAAACCTGTATTTTCAGGGCTCCGG-3′) were incubated for 20 min at 30°C in binding buffer (130 mM NaCl, 25 mM Tris–HCl pH 8.0, 10% glycerol, 0.5 mg/ml BSA, 1 mM EDTA, 1 mM DTT) and separation conditions were 8% PAGE at 200 V for 5 h in 1× TBE at 4°C.
Resolution of mitochondrial nucleoprotein complexes on iodixanol gradients
Mitochondria (10 mg/ml) were suspended in 20 mM HEPES pH 7.6, 2.5 mM EDTA, 25 mM NaCl, 1 mM DTT with 1/50 Roche protease inhibitor™ and lysed with 0.4% DDM and centrifuged for 10 min at 1000 × gmax; the supernatent was loaded on a 20–45% iodixanol gradient (20 mM HEPES pH 7.6, 1 mM EDTA, 25 mM NaCl, 1 mM DTT, 0.1% DDM with protease inhibitor) and centrifuged at 100 000 × gmax for 12 h. Nucleic acid was extracted from a portion of each fraction of the gradient and after Southern blotting hybridized to a radiolabelled probe to estimate the level of mtDNA, the remainder of each fraction was analysed by immunoblotting after SDS–PAGE. POLGβ transgene product was detected using an anti HA-Per conjugate at a dilution of 1 : 2000. PolGα detection utilized goat anti-PolGα (1 : 500) and donkey anti-goat (1 : 4000). Tfam: Rabbit anti-Tfam (1 : 5000) and goat anti-rabbit (1 : 5000). mtSSB: Rabbit anti-mtSSB (1 : 500) and goat anti-rabbit (1:5000).
RESULTS
POLGβ gene-silencing depletes mitochondrial D-loops
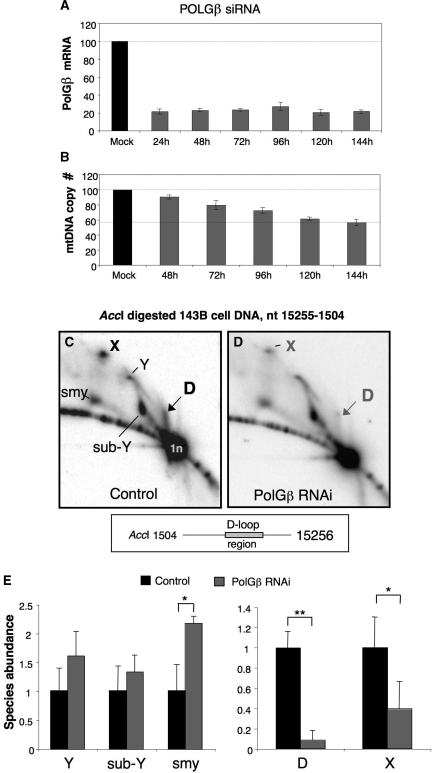
To investigate the role of POLGβ in mtDNA metabolism, POLGβ mRNA levels were repressed in human 143B osteosarcoma (HOS) cells by transfection with dsRNA targeting POLGβ. Transcript levels were ∼20% of control levels 24 h after transfection and gene-silencing could be extended to 144 h by re-transfecting cells with dsRNA, 72 h after the first transfection (Figure 1A). RNA interference led to a slow but inexorable decline in mtDNA copy number, such that by Day 6 the amount of mtDNA per cell had almost halved compared to mock transfected cells (Figure 1B). By comparison, gene-silencing of the mtDNA helicase Twinkle was more dramatic, reducing mtDNA copy number to half the control value, 72 h after transfection (2,23). This suggested that mtDNA replication was inhibited in POLGβ silenced cells, but not halted entirely, and so mitochondrial replication intermediates were screened from POLGβ siRNA treated cells. Total cellular DNA was harvested 5 or 6 days after the first transfection and AccI digested fragments of DNA were separated by neutral 2D-AGE and hybridized to a probe detecting the 2.8-kb fragment of human mtDNA spanning nt 15 255–1504, which includes the NCR (Figure 1C and D). Based on previous analysis (11,26), several of the non-linear species correspond to replication intermediates. These were the molecules resolving near the apex of a standard replication fork (Y) arc, partially degraded replication forks migrating below the standard Y arc (sub-Y), and replication forks joined to the adjacent fragment (nt 8157–15 254), creating a compressed slow-moving Y-like arc (smy). In addition there was an uncharacterized molecular species, D and molecules resolving close to the apex of a so-called X arc. X-arcs comprise DNA molecules with a four-way junction (27), which may be termination or recombination intermediates. Quantification of the various species indicated a modest 1.4- to 2-fold increase in the abundance of mitochondrial replication intermediates, i.e. the species comprising the Y, sub-Y and smy arcs, relative to non-replicating linear mtDNA (1n) (Figure 1E). In contrast, there were marked decreases in the abundance of species’ D and X (Figure 1C), which were significant (P < 0.01 and P < 0.05, respectively) (Figure 1E). In an earlier study, we noted that mtDNA fragments including the major non-coding region where the D-loop forms were associated with a prominent spot or smear on the Y or initiation arc, close to the linear fragment (26). Further analysis of fragments of mouse mtDNA confirmed that species D was exclusive to fragments containing the D-loop region (Figures 2A–D). Extraction of species D from 2D gels and its subsequent separation after heat denaturation demonstrated that such species contain 7S DNA (Figure 2E), and so species D represents fragments of mtDNA with a D-loop.
Figure 1.
POLGβ siRNA causes a 45% decrease in mtDNA copy number and a 90% decrease in a novel species, D, resolved by 2D-AGE. (A) RNA was isolated at daily intervals from mock transfected (mk) HOS cells and cells transfected with 5 nM dsRNA targeting POLGβ. In the case of time points greater than 72 h the cells were transfected a second time with the same dsRNA. POLGβ transcript levels were determined by qPCR with reference to GAPDH. The results represent the outcome of between two and six independent experiments for each time point. (B) DNA samples harvested from cells transfected as above were assayed with primers and probes detecting the COXII gene of mtDNA and the nuclear APP gene, to derive a relative mtDNA copy number. In one experiment, there was no appreciable effect on mtDNA number 24 h after POLGβ-dsRNA treatment (data not shown). AccI digested DNA from untreated HOS cells (control) or cells exposed to 5 nM dsRNA-840 for 120 h (POLGβ siRNA) was separated by 2D-AGE and hybridized to a probe spanning nt 16 343–151, which detects a 2.8-kb fragment of mtDNA, nt 15 255–1504 (C and D, respectively). The DNA in these experiments was isolated using Proteinase K. (E) In order to quantify changes to mtRIs and other species, the abundance of the various molecular species relative to the unit length fragment (1n), of non-replicating mtDNA was determined using a Typhoon phosphorimager (GE Healthcare) *P < 0.05, **P < 0.01.
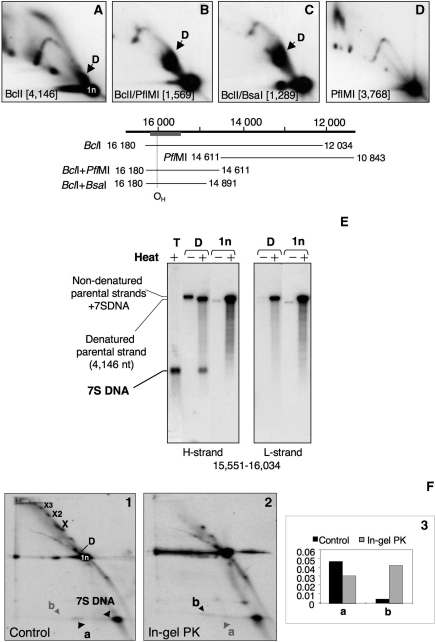
Figure 2.
Both molecular species depleted by POLGβ siRNA contain 7S DNA. Restriction fragments of mouse mtDNA containing the D-loop region were visualized by hybridization with a radiolabelled probe after separation by 2D-AGE (A–C); all had a product equivalent to species D of human mtDNA (Figure 1C), and this was absent from fragments lacking the D-loop region (D). The co-ordinates of the restriction fragments analysed in (A–D) are illustrated schematically immediately below the 2D gel images. (E) Extraction of species D after 2D-AGE of BclI digested mouse liver mtDNA (A) and subsequent heat denaturation released 7S DNA, which was resolved and detected by 1D-AGE and Southern hybridization. 7S DNA was present in undigested mouse mtDNA samples (T), but was not associated with the unit length BclI fragment (1n) spanning nt 12 034–16 180. (F) A substantial amount of 7S DNA is released following restriction enzyme digestion of HOS cell mtDNA (gel image 1). Species D is presumed to be present (based on previous analysis (26), but was not clearly separated from the unit length fragment (1n) under the conditions used here. Species ‘a’ has the same mobility as 7S DNA in the second dimension but not the first; therefore it is inferred to be 7S DNA that is released between the first and second dimension electrophoresis steps. Another species with the same mass as 7S DNA (b) is released from molecules resolving close to the apex of the X arc when the same material is treated with proteinase K between the first and second dimension gel electrophoresis steps (gel image 2). (Image 3) phosphorimager quantification of species a and b. Note that the species at the apex of the X-arc may contain 7S DNA, but if so, its stability is not dependent on the presence of protein.
When mtDNA is extracted without the use of protease, restriction fragments of mtDNA encompassing the D-loop region are held together by protein, producing a series of X-like species upon 2D-AGE (2). To determine whether or not the protease sensitive X-like species contained 7S DNA, whole cellular DNA extracted without protease treatment was digested with AccI and separated by 2D-AGE. After first dimension electrophoresis, the gel slice was treated with or without proteinase K, separated in the second dimension, blotted and hybridized to a probe that detects the nt 15 255–1504 mtDNA fragment containing the NCR (Figure 2F). A spot at the apex of the simple X arc persisted after in-gel protease treatment, but the adjacent less mobile species collapsed, yielding linear molecules and a much smaller molecular species (b), which, based on its mobility in the second dimension and the probe applied (nt 16 343–151), is 7S DNA. Therefore, the protein-dependent X-like species, which declined in response to POLGβ siRNA appears to contain 7S DNA.
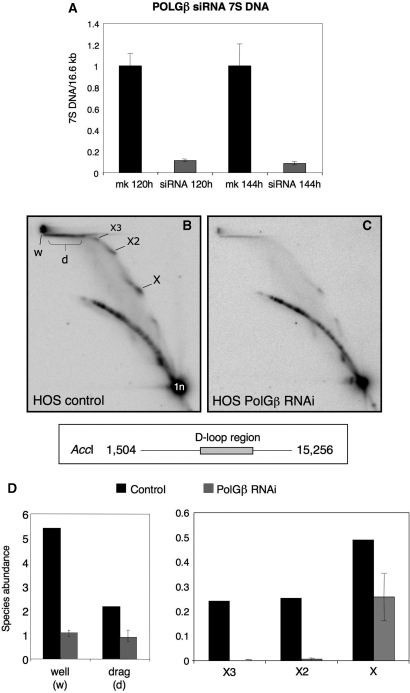
In light of the decrease in the abundance of the two species containing 7S DNA (D and X), the level of total 7S DNA in POLGβ siRNA cells was determined. POLGβ siRNA treatment produced a 90% decrease in 7S DNA level compared to controls, based on the ratio of 7S DNA to genome-length molecules of mtDNA (16.6 kb), analysed by Southern blotting (Figure 3A).
Figure 3.
POLGβ siRNA results in a 90% decrease in total cellular 7S DNA and the loss of mtDNA multimers held together by protein. Whole cell nucleic acid was isolated from cells treated twice with dsRNA-840 for 120 or 144 h. Aliquots of 3 μg of DNA were digested with PvuII, which cuts human mtDNA once to produce a linear fragment of 16.6 kb, 7S DNA was released from mitochondrial D-loops by incubation at 70°C for 10 min and separated by 1D-AGE. Southern blots were hybridized with a radiolabelled fragment of mtDNA corresponding to part of the D-loop region (∼nt 16 111–191). The amount of 7S DNA was expressed as a fraction of linear mtDNA (7S DNA/16.6 kb) (A). AccI digested DNA extracted from HOS cells, without the use of proteinase K, after transfection without (B) or with dsRNA targeting POLGβ (C) separated by 2D-AGE and hybridized with probe detecting the 2.8-kb fragment of human mtDNA nt 15 255–1504. W—mtDNA trapped in the well of the first dimension gel, D—mtDNA separated by 1D-AGE but unable to penetrate the second dimension gel (drag). X—molecules resolving near the apex of a standard X arc (2). (D) The abundance of the various species relative to the unit length fragment (1n) based on phosphorimager analysis.
ATAD3p binds to synthetic D-loops in vitro and ATAD3 gene-silencing leads to the loss of mtDNA multimers from DNA samples extracted from cultured human cells without a protease treatment (2). We concluded that ATAD3p might be recruited to mtDNA via the D-loop; if this is true, a shortage of mitochondrial D-loops should also lead to a loss of mtDNA multimers. Therefore, DNAs from POLGβ gene-silenced and control cells were isolated without a protease step and after digestion with AccI separated by 2D-AGE. Hybridization to a probe detecting the NCR-containing fragment revealed a series of X-like spots resolving well above the linear duplex arc in controls (Figure 3B), whereas such species were greatly reduced after POLGβ siRNA treatment (Figure 3C and D), as was the case with ATAD3 gene-silencing (2).
POLGβ expression affects nucleoid size, number and structure
To determine if the level of expression of POLGβ affected the organization of mitochondrial nucleoids, human cultured cells were examined by confocal microscopy after inducing or repressing POLGβ gene expression. Staining of mitochondrial nucleoids with the DNA intercalating dye, Picogreen, was markedly reduced after POLGβ gene-silencing (Figure 4A and Supplementary Figure 2). There was no such marked decrease in mitochondrial nucleoid signal based on anti-DNA antibody labelling (Figure 4B). We have shown previously that a 50% decrease in mtDNA copy number is difficult to detect using anti-DNA antibodies, and that Picogreen signal is reduced when DNA is supercoiled (2); hence the marked reduction in Picogreen signal is attributed to changes in the structure or topology of mitochondrial nucleoids and not merely to the 40% decrease in mtDNA copy number associated with 120 h of POLGβ siRNA (Figure 1B). On the other hand, anti-DNA labelling revealed a significant increase in mitochondrial nucleoid number (P < 0.01), after POLGβ siRNA (Figure 4B). The maximum number of nucleoids in one airy plane (z-section) was 198 ± 21 in mock transfected cells, whereas POLGβ dsRNA treated cells contained 318 ± 34 nucleoids. In theory, the increase in nucleoid number (decreased mtDNA number per nucleoid) could be a general response to a decrease in mtDNA copy number, rather than a specific effect of limiting POLGβ. However, inhibiting mtDNA replication with dideoxycytidine to decrease mtDNA copy number to the same extent as POLGβ siRNA had no effect on mitochondrial nucleoid number (Supplementary Figure 4A), nor was there any suggestion of increased nucleoid numbers in Twinkle siRNA treated HOS cells, where the copy number was 50% of controls (Supplementary Figure 4B). The effects on mitochondrial nucleoids of modulating POLGβ expression could have been indirect, as the protein forms a heterotrimer with POLGα; i.e. a shortage of POLGβ might destabilize POLGα. However, POLGα levels were unaffected by POLGβ siRNA (Supplementary Figure 5A), indicating that the changes in mtDNA documented above were not related to the abundance of POLGα.
Over-expression of POLGβ was investigated in HEK and U2OS cells. Doxycyclin induced expression of HA tagged POLGβ in HEK cells caused a loss of Picogreen signal, indistinguishable from POLGβ siRNA treatment (Figure 4C) and moreover this was accompanied by a substantial decrease in the steady-state level of 7S DNA (Figure 4D). To test whether these results reflected aggregation of the transgene product and endogenous POLGβ, i.e. a situation where over-expression was tantamount to gene-silencing, mitochondrial lysates were fractionated on iodixanol gradients. HA-tagged POLGβ was found to co-fractionate with mtDNA (Supplementary Figure 6), indicating that the transgene product was binding to mtDNA. Furthermore, immunocytochemistry of U2OS cells expressing of POLGβ.HA revealed good co-localization of the protein and mtDNA (Figure 4E and Supplementary Figure 5). Based on visual inspection, the mitochondrial nucleoids appeared larger and less numerous in U2OS cells over-expressing POLGβ and this impression was confirmed by quantitative analysis: the maximum number of foci (nucleoids) labelled with anti-DNA antibody in one airy plane, was 244 ± 47 per cell in controls, with a mean nucleoid size of 0.350 ± 0.056 µm (full width half maximum), whereas the same cell type overexpressing POLGβ had 145 ± 87 nucleoids per cell, sized 0.569 ± 0.133 µm. The differences in nucleoid number and size were significant, P < 0.007 and P < 0.0001, respectively, based on the unpaired Student's t-test (Figure 4E). The increase in POLGβ expression was not accompanied by a concomitant increase in POLGα expression (Supplementary Figure 5B) and nor could the increase in nucleoid size be explained by enhanced replication, as the mtDNA copy number was unchanged after induction of POLGβ, based on quantitative PCR analysis (Supplementary Figure 5C).
POLGβ preferentially binds to plasmids bearing a D-loop
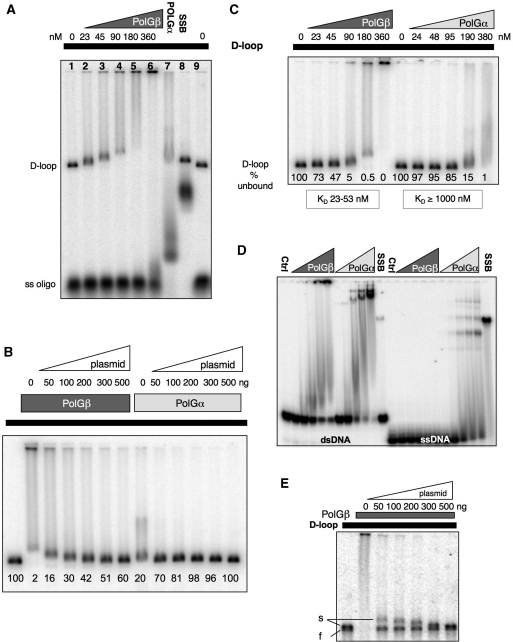
The similar effects on mtDNA of POLGβ (Figures 3B and C; 4A) and ATAD3 siRNA (2) was consistent with the proteins contributing to two steps in a pathway: POLGβ being required for D-loop synthesis and the D-loops serving to recruit ATAD3p to mtDNA. Accordingly, the decrease in mtDNA multimers observed after POLGβ siRNA (Figure 3C) could be explained by a lack of ATAD3p in mitochondrial nucleoids. Alternatively the D-loop could act as a recruitment centre for both POLGβ and ATAD3p, in which case both proteins would be capable of interacting directly with D-loops. To test the latter hypothesis, recombinant POLGβ tagged with 10 histidine residues at the carboxy terminus was produced and incubated with synthetic D-loops and the products resolved on native agarose gels. POLGβ bound to all the available plasmid with a D-loop at concentrations of protein that had no effect on free oligonucleotide, whereas both POLGα and SSB bound to free oligonucleotide as well as to D-loop bearing molecules (Figure 5A). This bias did not merely reflect an ability to bind dsDNA and not ssDNA as the protein preferred D-loops to plasmid DNA when cold competitor was present in considerable excess (Figure 5B). Thus, POLGβ bound to the vast majority of D-loop bearing molecules when synthetic D-loops were mixed with a ∼250-fold excess (50 ng) of ‘cold’ plasmid, prior to incubation with the protein (Figure 5B). In contrast, the same amount of competitor plasmid was much more effective at reducing the binding of POLGα to D-loops (Figure 5B).
Figure 5.
POLGβ preferentially binds plasmid bearing a D-loop. (A) Increasing amounts of recombinant POLGβ (0, 22.5, 45, 90, 180 and 360 nM) were incubated with synthetic D-loop C in binding buffer and the mixtures separated by native 1D-AGE (see ‘Materials and Methods’ section). The concentrations of POLGα and SSB were 190 and 300 nM, respectively. The free radiolabelled oligonucleotide (ss oligo) at the foot of the gel is the result of D-loop dissociation during extraction. (B) Approximately 0.2 ng radiolabelled D-loop C was mixed on ice with 0–500 ng of cold competitor, supercoiled plasmid (Bluescript or pUC19) and then incubated with 180 nM POLGβ or 190 nM POLGα for 20 min at 37°C. Based on other gels run for shorter periods, there was no detectable increase in free oligonucleotide with increasing amounts of protein, or cold competitor DNA. (C) Labelled D-loop C was co-eluted with unlabelled plasmid and incubated with increasing concentrations of POLGβ (0, 22.5, 45, 90, 180 and 360 nM) or POLGα (0, 24, 47.5, 95, 190 and 380 nM) and separated by non-denaturing AGE. The proportion (%) of unbound D-loop is indicated at the foot of each lane in (B and C). (D) A short piece of dsDNA (37 nt pairs) or a 33-mer ss oligonucleotide were titrated against 0, 24, 47.5, 95, 190 and 380 nM of POLG1 or 0, 22.5, 45, 90, 180 and 360 nM of POLGβ and separated by 6% non-denaturing PAGE. (E) In some experiments the D-loop resolved as two bands: a slow (s) and a fast (f) migrating form (lane 1). Both forms of synthetic D-loop showed a mobility shift when incubated with 190 mM POLGβ (lane 2). Unlabelled plasmid, mixed with the synthetic D-loops prior to incubation with POLGβ (lanes 3–7), was a considerably more effective competitor of fast migrating D-loops than slow migrating D-loops. POLGβ bound equally well to linear and supercoiled DNA without a D-loop (Supplementary Figure 8).
There was some cold plasmid in every D-loop EMSA, as some of the D-loops dissociated to plasmid and free oligonucleotide during isolation. Presumably this was less of a problem in the experiment shown in Figure 5A, as it gave the lowest KD for POLGβ binding to D-loops (1.5 nM), although even here free oligonucleotide (ss oligo) was clearly detectable at the bottom of the gel. Moreover plasmid with and without a D-loop had similar mobility and so a variable amount of contaminating ‘cold’ plasmid was extracted together with the synthetic D-loops. Therefore it was critical to compare POLGβ and POLGα with the same D-loop preparation in order to determine the relative affinity of binding. In a series of experiments, the KD of binding to radio-labelled D-loops was of the order of 50-fold higher for POLGα than POLGβ (Figure 5C), despite the fact that our preparation of POLGα had greater affinity for dsDNA than POLGβ, and POLGα but not POLGβ bound to ssDNA (Figure 5D). The KD for POLGβ binding to ssDNA was incalculably high, whilst that of POLGα was 92 nM; and with a KD of 124 nM, POLGα's affinity for dsDNA was 4-fold greater than POLGβ (490 nM). Taken together these results suggest that POLGβ is a specific D-loop-binding protein.
Synthetic D-loops were of two types: slow (s) and fast (f) migrating forms; and when POLGβ was incubated with synthetic D-loops, in the presence of increasing amounts of cold competitor plasmid, it preferentially bound to the slow migrating species’ (Figure 5E), whereas ATAD3B preferentially bound to the faster migrating D-loops [(2) and Supplementary Figure 7A]. Tightly supercoiled plasmids with a D-loop are refractory to restriction digestion (28); here XhoI proved capable of cleaving the slow-migrating D-loop, but not the fast migrating form (Supplementary Figure 7B), and so the fast migrating D-loop is inferred to be the more tightly supercoiled of the two.
DISCUSSION
POLGβ has been recognized as a processivity factor and accessory subunit of mtDNA polymerase γ for several years (16,17); the new data reported here indicate that this is not the protein's only role. Human POLGβ is now implicated in mtDNA organization. The concept of bifunctional mitochondrial nucleoid proteins has several precedents. There is evidence that the mammalian mitochondrial protein Tfam has roles in DNA maintenance and transcription (29–31), and in yeast bifunctional mitochondrial nucleoid proteins are common currency: they include Ilv5, α-ketoglutarate dehydrogenase 2, Aconitase and Hsp60 (1).
POLGβ and nucleoid maintenance
POLGβ's DNA-binding properties are superfluous to its function as a processivity factor (18,20), and most processivity factors do not bind to DNA. Therefore the dsDNA-binding properties of POLGβ fully support the idea of one or more additional functions for POLGβ. The effects of modulating POLGβ expression suggest that POLGβ is a key determinant of mtDNA copy number per nucleoid. Several cultured human cell lines, including HOS cells have been estimated to contain on average five mtDNA molecules per nucleoid (32–34). Therefore, this is predicted to rise to eight mtDNAs per nucleoid in cells overexpressing POLGβ and to fall to between one and two in cells where POLGβ expression has been repressed by RNA interference, when the decrease in mtDNA copy number is taken into consideration. The long-term effect of altering the mtDNA copy number of nucleoids is not known, although it would be expected to affect the mathematics of mtDNA segregation and therefore the maintenance of heteroplasmy (35), as found in mitochondrial diseases (36).
Accounting for the changes in 7S DNA levels is less straightforward, given that both increased and decreased expression of POLGβ caused a precipitous fall in the steady-state level of 7S DNA. However, a protein in excess having the converse effect to normal has many precedents; Tfam, for example, is essential for maintaining mtDNA (14) and can in moderation increase mtDNA copy number (30), yet a large excess of Tfam causes mtDNA depletion (37). Thus, POLGβ's propensity for binding D-loop structures (Figure 5) suggests it is ordinarily recruited to mtDNA via the mitochondrial D-loop, whereas in great excess, the dsDNA-binding properties of POLGβ may be sufficient to ensure its recruitment to mtDNA in the absence of D-loop structures. Moreover, the fact that 7S DNA (D-loop) levels fell when POLGβ was over-expressed (Figure 4D), implies that cells cannot sustain normal levels of D-loops and abundant POLGβ, perhaps because this would inhibit nucleoid division to such an extent that they would grow ever larger and the cell would be unable to partition mtDNA at cell division. Thus, the decrease in 7S DNA may be designed to avoid such an eventuality; it might reflect an active process involving another nucleoid protein, such as Twinkle DNA helicase, alternatively POLGβ loading onto mtDNA may be self-limiting, i.e. high occupancy of mtDNA by POLGβ may occlude D-loop synthesis.
POLGβ is not a universal mitochondrial nucleoid protein
The role of POLGβ in mitochondrial nucleoid maintenance may well be restricted to mammals and vertebrates, as Drosophila POLGβ is shorter than its mammalian homologue (38), and the section missing from insect POLGβ has been shown to be important for dimerization and DNA binding of mouse POLGβ (19,39). Based on the sequence differences between mouse and fly POLGβ, it was suggested that mammalian POLGβ would have an additional function not found in the insect protein (38); in light of the new findings we conclude that mtDNA organization is one such function. If this is true, Drosophila POLGβ will not display the same affinity for D-loops as mammalian POLGβ, although this has yet to be tested.
POLGβ's DNA-binding properties distinguish it from both SSB and POLGα
Given that both POLGβ and ATAD3p readily bind to D-loops [(2) and this report], it was conceivable that we had identified a general property of many nucleic-acid-binding proteins. However the fact that neither SSB nor POLGα behave similarly suggests otherwise. SSB bound to synthetic D-loops and ssDNA equally well (Figure 5A, lane 8), whereas POLGβ, at best, binds to ssDNA only weakly (Figure 5A and D). Indeed, the binding of POLGβ to ssDNA may be entirely dependent on secondary structure. Unlike POLGβ, POLGα bound to ssDNA and dsDNA equally well, to a first approximation (Figure 5D). The affinity of binding of recombinant POLGα to dsDNA and ssDNA (Figure 5D) was similar to that reported for a 21/45 nt primer–template (21). POLGβ's affinity for dsDNA was greater than we observed in one report (20), but similar to another study (17). Although POLGα did bind to synthetic D-loops, its preference for D-loops was considerably less than POLGβ (Figures 5B and C). Thus, there were marked differences between POLGβ, POLGα and SSB in respect of their preferences for binding to different forms of DNA. Alone among the three proteins tested here, POLGβ bound to D-loop structures in preference to dsDNA or ssDNA, and so we infer that the mitochondrial D-loop is likely to be a physiologically relevant substrate of POLGβ, especially as altered expression of POLGβ had a pronounced effect on mitochondrial D-loop stability. Ultimately it will be necessary to generate cells with mutant forms of POLGβ that are incapable of binding DNA, or D-loops, in order to determine the precise contribution of these features of the protein to mtDNA organization.
Notwithstanding the remarks about SSB above, mtSSB may also be recruited to mtDNA via the D-loop. Historically, SSB was thought to be plentiful in animal mitochondria because it was believed to coat long stretches of ssDNA that formed during replication (7); however, recent work has suggested that there is no greater requirement for ssDNA in mtDNA replication than nuclear DNA replication (11). Certainly mtSSB is abundant in mature Xenopus oocytes (40), where mtDNA replication is dormant, supporting the idea that it is, like POLGβ, a nucleoid maintenance factor, as well as being essential for DNA replication.
POLGβ's and ATAD3p's contribution to mitochondrial nucleoid status
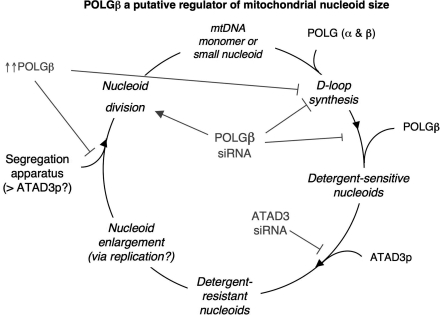
ATAD3p's assignment as a mitochondrial nucleoid protein that directly binds to mtDNA (2) has been questioned (41). However, the striking parallels between the behaviour of ATAD3p (2) and POLGβ (this report) both in vitro and in cultured cells lend additional support to the idea that ATAD3p is a bona fide mitochondrial nucleoid protein. POLGβ and ATAD3p share a marked preference for DNA molecules with a D-loop; and silencing of either gene destabilizes detergent-resistant mtDNA multimers and alters the structure of mitochondrial nucleoids. The findings to date can be reconciled with a ‘nucleoid cycle’ (Figure 6). Namely, in conjunction with POLGα, POLGβ is needed for D-loop synthesis, but it also stabilizes D-loop structures, unless it is present in excess. Both ATAD3p and POLGβ are required to form a detergent-resistant nucleoid, and nucleoid size will naturally increase with time owing to DNA replication. Although nucleoid expansion could in theory be augmented by nucleoid fusion, this appears to be a rare event based on a recent study in human cultured cells (32). Nucleoid division is imperative for transmission of mtDNA and so the apparatus for partitioning mitochondrial nucleoids must be recruited at some stage. This could involve additional copies of ATAD3p, as previously suggested (12), or as yet unknown factors. If the recruitment of the mtDNA segregation apparatus is D-loop dependent, then the D-loop is needed for different purposes at different stages of the nucleoid cycle, and this could explain how the loss of 7S DNA was associated with both increasing and decreasing nucleoid size.
Figure 6.
A model for POLGβ and ATAD3p involvement in mitochondrial nucleoid formation, enlargement and division. The changes in mitochondrial nucleoids effected by modulating the expression of POLGβ (this report) and ATAD3p (2) suggest that each contributes to nucleoid homeostasis. The synthesis of a short triple-stranded region creates a D-loop, which is mediated by mtDNA polymerase γ holoenzyme (POLGα and β). The D-loop structures are the preferred substrate of ATAD3p [a putative nucleoid protein, see ref. (2) and POLGβ (this report)], hence D-loop synthesis is followed by POLGβ and ATAD3p recruitment. Additional factors may be required for nucleoid division, which are inhibited by an excess of POLGβ (see text for further details).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
UK Medical Research Council, the Academy of Finland [Grants 110689, 103213 and CoE funding]; Juselius Foundation; Tampere University Hospital Medical Research Fund; University of Tampere; European Union FP6 Mitocombat Integrated Programme (to I.J.H., M.D.R., A.R., S.G. and J.N.S.); UK Muscular Dystrophy Campaign (to T.Y.); Academy of Finland grants 110463 and 108380 (to P.M.); European Union FP6 Proteomage (to A.R.F. and T.S.W.). Funding for open access charge: Medical Research Council.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
Tfam and mtSSB antibodies were kind gifts of Drs Rudi Weisner and Massimo Zeviani, respectively.
REFERENCES
- 1.Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- 2.He J, Mao CC, Reyes A, Sembongi H, Di Re M, Granycome C, Clippingdale AB, Fearnley IM, Harbour M, Robinson AJ, et al. The AAA+ protein ATAD3 has displacement loop binding properties and is involved in mitochondrial nucleoid organization. J. Cell Biol. 2007;176:141–146. doi: 10.1083/jcb.200609158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman BA, Newman SM, Hallberg RL, Slaughter CA, Perlman PS, Butow RA. In organello formaldehyde crosslinking of proteins to mtDNA: identification of bifunctional proteins. Proc. Natl Acad. Sci. USA. 2000;97:7772–7777. doi: 10.1073/pnas.140063197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Bogenhagen DF. Human MTDNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J. Biol. Chem. 2006;281:25791–25802. doi: 10.1074/jbc.M604501200. [DOI] [PubMed] [Google Scholar]
- 5.Arnberg A, van Bruggen EF, Borst P. The presence of DNA molecules with a displacement loop in standard mitochondrial DNA preparations. Biochim. Biophys. Acta. 1971;246:353–357. doi: 10.1016/0005-2787(71)90147-x. [DOI] [PubMed] [Google Scholar]
- 6.Kasamatsu H, Robberson DL, Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc. Natl Acad. Sci. USA. 1971;68:2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton DA. Replication of animal mitochondrial DNA. Cell. 1982;28:693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- 8.Ojala D, Crews S, Montoya J, Gelfand R, Attardi G. A small polyadenylated RNA (7 S RNA), containing a putative ribosome attachment site, maps near the origin of human mitochondrial DNA replication. J. Mol. Biol. 1981;150:303–314. doi: 10.1016/0022-2836(81)90454-x. [DOI] [PubMed] [Google Scholar]
- 9.Annex BH, Williams RS. Mitochondrial DNA structure and expression in specialized subtypes of mammalian striated muscle. Mol. Cell Biol. 1990;10:5671–5678. doi: 10.1128/mcb.10.11.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowmaker M, Yang MY, Yasukawa T, Reyes A, Jacobs HT, Huberman JA, Holt IJ. Mammalian mitochondrial DNA replicates bidirectionally from an initiation zone. J. Biol. Chem. 2003;278:50961–50969. doi: 10.1074/jbc.M308028200. [DOI] [PubMed] [Google Scholar]
- 11.Yasukawa T, Reyes A, Cluett TJ, Yang MY, Bowmaker M, Jacobs HT, Holt IJ. Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. EMBO J. 2006;25:5358–5371. doi: 10.1038/sj.emboj.7601392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt IJ, He J, Mao CC, Boyd-Kirkup JD, Martinsson P, Sembongi H, Reyes A, Spelbrink JN. Mammalian mitochondrial nucleoids: organizing an independently minded genome. Mitochondrion. 2007;7:311–321. doi: 10.1016/j.mito.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Ropp PA, Copeland WC. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics. 1996;36:449–458. doi: 10.1006/geno.1996.0490. [DOI] [PubMed] [Google Scholar]
- 14.Hance N, Ekstrand MI, Trifunovic A. Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum. Mol. Genet. 2005;14:1775–1783. doi: 10.1093/hmg/ddi184. [DOI] [PubMed] [Google Scholar]
- 15.Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23:2423–2429. doi: 10.1038/sj.emboj.7600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrodeguas JA, Kobayashi R, Lim SE, Copeland WC, Bogenhagen DF. The accessory subunit of Xenopus laevis mitochondrial DNA polymerase gamma increases processivity of the catalytic subunit of human DNA polymerase gamma and is related to class II aminoacyl-tRNA synthetases. Mol. Cell Biol. 1999;19:4039–4046. doi: 10.1128/mcb.19.6.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim SE, Longley MJ, Copeland WC. The mitochondrial p55 accessory subunit of human DNA polymerase gamma enhances DNA binding, promotes processive DNA synthesis, and confers N-ethylmaleimide resistance. J. Biol. Chem. 1999;274:38197–38203. doi: 10.1074/jbc.274.53.38197. [DOI] [PubMed] [Google Scholar]
- 18.Farge G, Pham XH, Holmlund T, Khorostov I, Falkenberg M. The accessory subunit B of DNA polymerase gamma is required for mitochondrial replisome function. Nucleic Acids Res. 2007;35:902–911. doi: 10.1093/nar/gkl1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrodeguas JA, Theis K, Bogenhagen DF, Kisker C. Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase gamma, Pol gamma B, functions as a homodimer. Mol. Cell. 2001;7:43–54. doi: 10.1016/s1097-2765(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 20.Carrodeguas JA, Pinz KG, Bogenhagen DF. DNA binding properties of human pol gammaB. J. Biol. Chem. 2002;277:50008–50014. doi: 10.1074/jbc.M207030200. [DOI] [PubMed] [Google Scholar]
- 21.Fan L, Kim S, Farr CL, Schaefer KT, Randolph KM, Tainer JA, Kaguni LS. A novel processive mechanism for DNA synthesis revealed by structure, modeling and mutagenesis of the accessory subunit of human mitochondrial DNA polymerase. J. Mol. Biol. 2006;358:1229–1243. doi: 10.1016/j.jmb.2006.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan L, Sanschagrin PC, Kaguni LS, Kuhn LA. The accessory subunit of mtDNA polymerase shares structural homology with aminoacyl-tRNA synthetases: implications for a dual role as a primer recognition factor and processivity clamp. Proc. Natl Acad. Sci. USA. 1999;96:9527–9532. doi: 10.1073/pnas.96.17.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyynismaa H, Sembongi H, Bokori-Brown M, Granycome C, Ashley N, Poulton J, Jalanko A, Spelbrink JN, Holt IJ, Suomalainen A. Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum. Mol. Genet. 2004;13:3219–3227. doi: 10.1093/hmg/ddh342. [DOI] [PubMed] [Google Scholar]
- 24.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 25.Bayona-Bafaluy MP, Acin-Perez R, Mullikin JC, Park JS, Moreno-Loshuertos R, Hu P, Perez-Martos A, Fernandez-Silva P, Bai Y, Enriquez JA. Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res. 2003;31:5349–5355. doi: 10.1093/nar/gkg739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasukawa T, Yang MY, Jacobs HT, Holt IJ. A bidirectional origin of replication maps to the major non-coding region of human mitochondrial DNA. Mol. Cell. 2005;18:651–662. doi: 10.1016/j.molcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Friedman KL, Brewer BJ. Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 1995;262:613–627. doi: 10.1016/0076-6879(95)62048-6. [DOI] [PubMed] [Google Scholar]
- 28.Nimonkar AV, Boehmer PE. The herpes simplex virus type-1 single-strand DNA-binding protein (ICP8) promotes strand invasion. J. Biol. Chem. 2003;278:9678–9682. doi: 10.1074/jbc.m212555200. [DOI] [PubMed] [Google Scholar]
- 29.Alam TI, Kanki T, Muta T, Ukaji K, Abe Y, Nakayama H, Takio K, Hamasaki N, Kang D. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003;31:1640–1645. doi: 10.1093/nar/gkg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 31.Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991;252:965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- 32.Gilkerson RW, Schon EA, Hernandez E, Davidson MM. Mitochondrial nucleoids maintain genetic autonomy but allow for functional complementation. J. Cell Biol. 2008;181:1117–1128. doi: 10.1083/jcb.200712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iborra FJ, Kimura H, Cook PR. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2004;2:9. doi: 10.1186/1741-7007-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legros F, Malka F, Frachon P, Lombes A, Rojo M. Organization and dynamics of human mitochondrial DNA. J. Cell Sci. 2004;117:2653–2662. doi: 10.1242/jcs.01134. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs HT, Lehtinen SK, Spelbrink JN. No sex please, we're mitochondria: a hypothesis on the somatic unit of inheritance of mammalian mtDNA. Bioessays. 2000;22:564–572. doi: 10.1002/(SICI)1521-1878(200006)22:6<564::AID-BIES9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 37.Pohjoismaki JL, Wanrooij S, Hyvarinen AK, Goffart S, Holt IJ, Spelbrink JN, Jacobs HT. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 2006;34:5815–5828. doi: 10.1093/nar/gkl703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaguni LS. DNA polymerase gamma, the mitochondrial replicase. Annual Rev. Biochem. 2004;73:293–320. doi: 10.1146/annurev.biochem.72.121801.161455. [DOI] [PubMed] [Google Scholar]
- 39.Yakubovskaya E, Chen Z, Carrodeguas JA, Kisker C, Bogenhagen DF. Functional human mitochondrial DNA polymerase gamma forms a heterotrimer. J. Biol. Chem. 2006;281:374–382. doi: 10.1074/jbc.M509730200. [DOI] [PubMed] [Google Scholar]
- 40.Bogenhagen DF, Wang Y, Shen EL, Kobayashi R. Protein components of mitochondrial DNA nucleoids in higher eukaryotes. Mol. Cell Proteomics. 2003;2:1205–1216. doi: 10.1074/mcp.M300035-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Bogenhagen DF, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 2008;283:3665–3675. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.