Abstract
Marine microalgae support world fisheries production and influence climate through various mechanisms. They are also responsible for harmful blooms that adversely impact coastal ecosystems and economies. Optimal growth and survival of many bloom-forming microalgae, including climatically important dinoflagellates and coccolithophores, requires the close association of specific bacterial species, but the reasons for these associations are unknown. Here, we report that several clades of Marinobacter ubiquitously found in close association with dinoflagellates and coccolithophores produce an unusual lower-affinity dicitrate siderophore, vibrioferrin (VF). Fe-VF chelates undergo photolysis at rates that are 10–20 times higher than siderophores produced by free-living marine bacteria, and unlike the latter, the VF photoproduct has no measurable affinity for iron. While both an algal-associated bacterium and a representative dinoflagellate partner, Scrippsiella trochoidea, used iron from Fe-VF chelates in the dark, in situ photolysis of the chelates in the presence of attenuated sunlight increased bacterial iron uptake by 70% and algal uptake by >20-fold. These results suggest that the bacteria promote algal assimilation of iron by facilitating photochemical redox cycling of this critical nutrient. Also, binary culture experiments and genomic evidence suggest that the algal cells release organic molecules that are used by the bacteria for growth. Such mutualistic sharing of iron and fixed carbon has important implications toward our understanding of the close beneficial interactions between marine bacteria and phytoplankton, and the effect of these interactions on algal blooms and climate.
Keywords: algal blooms, iron acquisition, vibrioferrin, Marinobacter, photochemistry
The growth and species composition of microalgal communities in the ocean are often regulated by the micronutrient iron (1), which in turn influences climate by controlling biogenic calcification, oceanic sequestration of CO2, and biological release of dimethyl sulfide (2, 3). Iron may also influence blooms of toxic or harmful algae, which have occurred with increasing frequency in recent decades, and have caused substantial ecological and economic damage worldwide (4). Many bloom-forming algal species, including climatically important dinoflagellates and coccolithophores (2, 3), are known to form beneficial or obligatory close associations with certain bacteria, but the reasons for these associations remain obscure (5–8). The bacteria that closely associate with phytoplankton are believed to be involved in a wide range of interactions, including metabolite/nutrient uptake, provision and remineralization (5, 6), cell differentiation (7), as well as algicidal or bacterioprotective effects (8, 9). To our knowledge, however, these interactions are poorly understood, and only a few well-studied examples exist.
Among the most intriguing factors that may involve algal–bacterial interactions is iron acquisition. Iron is an essential element for photosynthesis and respiration, and it limits primary productivity and bacterial growth in much of the ocean because of its poor solubility and resultant exceedingly low concentration (1, 10, 11). To alleviate limitation of this key micronutrient, many marine heterotrophic bacteria and some cyanobacteria produce siderophores, small organic molecules that tightly bind iron and thereby increase its solubility (12). The bacteria then take up the siderophores via outer-membrane transporters that are specific for different groups of siderophores. By contrast, eukaryotic phytoplankton are not known to produce siderophores or to directly take up bacterially derived Fe(III)-siderophore complexes. However, many eukaryotic phytoplankton are able to access iron from siderophores or other chelates via ferrireductases and adjacent Fe(II) transporters on their outer cell membranes, for which genomic evidence exists in diatoms and green algae (13).
Results
We have previously observed that the bacterial community cooccurring with the toxic dinoflagellate Gymnodinium catenatum bears a remarkable similarity to the bacterial communities of other marine dinoflagellates from different algal collections (14). Among the most notable members of these communities were bacteria phylogenetically affiliated with the α-proteobacterial Roseobacter and the γ-proteobacterial Marinobacter clades. Here, examining a larger set of cultures, we find that members of the genus Marinobacter were detected in 83% of dinoflagellate and 87% of the coccolithophorid cultures that we examined, whereas Marinobacter spp. were found less frequently in diatom cultures (Tables S1 and S2). These closely related Marinobacter spp. have also been cultured from dinoflagellates by others, corroborating the idea that several species of Marinobacter are very frequently associated with dinoflagellates and coccolithophores (15, 16).
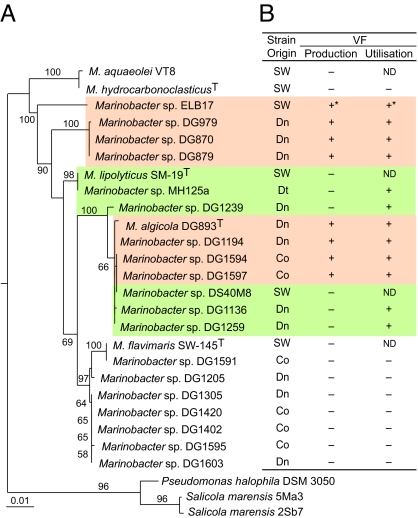
Although these algal-associated species were closely related to other Marinobacter species (e.g., Marinobacter hydrocarbonoclasticus or Marinobacter sp. DS40M8), most of the tested strains did not produce the siderophores commonly produced by free-living members of the Marinobacter genus (12). This observation prompted us to screen many Marinobacter isolates for their siderophore production using a combination of liquid chromatography (LC)-MS, NMR, growth promotion assays, and PCR of selected siderophore biosynthetic genes (Fig. 1). The known molecule vibrioferrin (VF) (Fig. 2A) was the only siderophore detected in two particular algal-associated subclades, suggesting that: (i) siderophore production may be a useful chemotaxonomic marker for algal-associated Marinobacter species, and (ii) there might be some unique functional significance for VF production (17, 18).
Fig. 1.
The 16S rRNA gene phylogeny of the Marinobacter clade and VF production profile. (A) Maximum-likelihood neighbor-joining tree with bootstrap support (≥50%) of Marinobacter 16S rRNA genes. (B) Production and utilization of VF by Marinobacter for iron acquisition, as determined by LC-MS, NMR, PCR screening of VF biosynthetic genes, and siderophore growth-promotion assays. Dn, dinoflagellate; Co, coccolithophore; Dt, diatoms; ND, not determined. Orange shading indicates those strains capable of VF production and uptake; green shading indicates VF uptake only. Bar denotes nucleotide substitutions per site.*For Marinobacter sp. ELB17, production and uptake were presumed based on the presence of close homologs of VF biosynthetic and uptake genes.
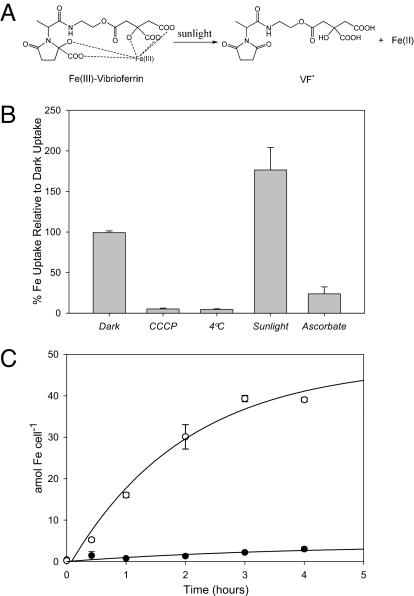
Fig. 2.
VF-mediated iron uptake in Marinobacter sp. DG879 and the dinoflagellate S. trochoidea in the dark and in the presence of sunlight. (A) The photolysis of Fe(III)-VF in sunlight produces a photoproduct (VF*) and Fe(II)′, which is quickly oxidized in SW to Fe(III)′. (B) 55Fe uptake rates by Marinobacter sp. DG879. Uptake was performed using 1 μM Fe(III) and 3 μM total VF either in the dark or in the presence of attenuated natural sunlight (450 μE·m−2·s−1) at 20 °C. The uncoupler of oxidative phosphorylation, CCCP, and incubation of cells at 4 °C were used as metabolic inhibitors; incubations were carried out in the dark. The reductant ascorbate was used to prevent the oxidation of photochemically produced Fe(II) in bacteria exposed to sunlight. (C) 55Fe uptake by axenic S. trochoidea cells. Uptake was performed in trace-metal- and EDTA-free Aquil using 50 nM Fe(III) and 500 nM VF either in the dark (filled circles) or in the presence of attenuated natural sunlight (open circles) as above. Error bars in B and C represent SD of triplicate cultures. Bars were not shown when smaller than the symbol.
VF is a member of the carboxylate class of siderophores containing two α-hydroxy acid groups. It was originally isolated from Vibrio parahaemolyticus, an estuarine enteropathogenic bacterium associated with seafood-borne gastroenteritis (17). We observed several unique features of VF and its iron chemistry that differed substantially from most other siderophores produced by free living marine bacteria. The first was its only moderate affinity for binding iron (conditional stability constant in seawater (SW) relative to Fe′, log Kcond (FeL, Fe′) = 10.93 ± 0.03) owing to the presence of only five iron-binding ligand groups and placing it on par with the weaker class of generic iron binding ligands found in the ocean (19). Most other marine siderophores contain six donor groups, and thus, form much more stable ferric chelates (12). However, the most remarkable feature of VF is the striking sensitivity of its iron complex to light. Siderophores containing α/β-hydroxy acid moieties are known to undergo photochemical reactions involving the oxidative cleavage of a carboxylate group and the concomitant reduction of Fe(III) to Fe(II) (20, 21). The Fe(II) produced can then dissociate from the “degraded” siderophore, and be reoxidized to soluble inorganic ferric hydrolysis species [designated Fe(III)′], which are highly bioavailable to algal cells (22, 23). It has been shown that the photolysis of certain ferric chelates and the resultant production of dissolved Fe(II) and Fe(III)′ species increases the uptake of iron by marine phytoplankton (20, 23). However, the efficiency of this mechanism in increasing the bioavailability of iron is open to question because of the unexpected observation that the photoproducts formed for many photoactive marine siderophores retain the ability to strongly coordinate Fe(III) (20, 24, 25). Indeed, in some cases, the photoproduct is actually a better Fe(III) chelator than the parent siderophore (24, 25).
Because VF contains two α-hydroxy acid moieties, we anticipated that Fe(III)-VF chelates would be highly photolabile (Fig. 2A), and indeed, Fe(III)-VF demonstrated exceptional sensitivity to light. It underwent photolysis at a 10- to 20-fold higher rate than other photoactive siderophores such as petrobactin, marinobactin, and aquachelin (Table 1). Also, unlike any other marine siderophore examined so far, the resulting photoproduct (VF*) (Fig. 2A) has no significant affinity for iron (see Methods), which maximizes net Fe(III)′ production compared with those siderophores whose photoproducts are still efficient ferric chelators. Thus, the photochemistry of Fe(III)-VF is an irreversible process that leads to the destruction of the siderophore. Such a scenario would seem to be extremely disadvantageous for the producing bacterium, prompting us to examine the iron uptake kinetics of photolyzed and unphotolyzed Fe(III)-VF by Marinobacter sp. DG879 and its dinoflagellate host.
Table 1.
Rates of Fe(II) production during the photolysis of the ferric complexes of VF and other marine siderophores
| Siderophore | Initial specific rate, h−1 (2,000 μE·m−2·s−1) | Rate of photolysis, h−1 (80μE·m−2·s−1) | Ref. |
|---|---|---|---|
| Vibrioferrin | 12.9 (0.017) | 0.031 | This study |
| Aquachelin C | 0.6 (0.015) | — | 20 |
| Petrobactin | — | 0.003 | This study |
| Marinobactin | — | <0.001 | This study |
Initial specific rates were determined by monitoring LMCT of Fe2+(BPDS)3 at 535 nm as described in Methods. BPDS, bathophenanthrolinedisulfonic acid. Values in parentheses represent rates of Fe(II) production from dark controls. VF photolysis was conducted in natural sunlight attenuated by 75% to an intensity of 500 μE·m−2·s−1 [1 einstein (E) is 1 mol of photons]; thus, our measured rate was multiplied by a factor of 4 to give the full sunlight rate for comparison with aquachelin C. Rates of photolysis were determined by monitoring changes in the LMCT bands at 330, 490, and 407 nm of the ferric complexes of VF, petrobactin, and marinobactin, respectively, as described in Methods.
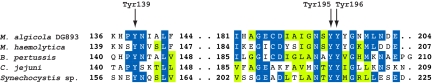
Growth promotion assays indicate that VF is a functional siderophore for Marinobacter sp. DG879 (Fig. 1B), and 55Fe-labeled VF chelates were taken up by the bacteria in a time- and concentration-dependent manner with a Km of 17.6 μM. Fe(III)-VF uptake showed active transport behavior as indicated by its inhibition by an uncoupler of oxidative phosphorylation or low temperature (Fig. 2B). In experiments with Fe(III)-VF chelates photolyzed in situ with attenuated sunlight, 55Fe was assimilated at a 70% higher rate than observed with unphotolyzed Fe(III)-VF (Fig. 2B). Because the photolysis completely destroyed the VF, and VF* shows no significant affinity for either Fe(II) or Fe(III), the cells must have acquired iron from photochemically released inorganic Fe(II) or Fe(III). In addition to siderophore-mediated transport systems, many Gram-negative bacteria possess siderophore-independent Fe(III) uptake systems such as the FbpABC transporter, which utilizes dissolved ferric hydrolysis species, Fe(III)′, and other partially chelated forms of ferric ion (26). Examination of the genome of the closely related VF-producer Marinobacter algicola DG893 reveals the presence of putative homologs to FbpA and FbpB containing the functionally important and universally conserved dityrosine unit in FbpA (Fig. 3) involved in the binding of ferric iron (27). We were not able to find homologs of specific Fe(II) transport systems indicating that the bacteria may not be able to assimilate ferrous iron. Indeed, bacterial 55Fe uptake from photolyzed Fe-VF was diminished >4-fold in the presence of the reductant ascorbate, confirming that the bacteria are most likely assimilating photochemically produced ferric iron species (Fig. 2B).
Fig. 3.
Partial predicted translation of M. algicola DG893 FbpA homolog aligned with known FbpA proteins. M. algicola DG893 accession no. EDM49388. Protein PDB ID nos.: Mannheimia haemolytica (1SI0), Bordetella pertussis (1Y9U), Campylobacter jejuni (1Y4T), and Synechocystis sp. FutA1 (2PT2). Alignment was performed using Muscle as implemented in CLC Sequence Viewer (Version 6). Identical residues are depicted with blue shading and similar residues with green shading. Y195 and Y196 are universally conserved in FbpA proteins and are responsible for binding Fe3+. Y139 is only found in a subset of FbpA proteins and is present in the coordination sphere of Fe3+ as well.
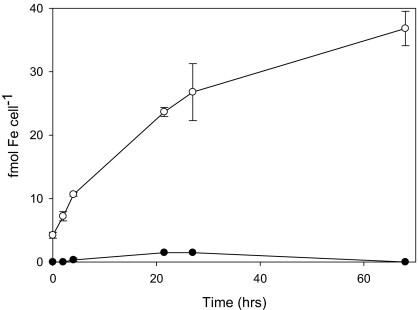
Although the uptake of iron from VF was expected for the producing bacteria, the question of its availability to a phytoplanktonic partner remained unresolved. For these studies, we used axenic cultures of the bloom-forming dinoflagellate Scrippsiella trochoidea to eliminate complications that would arise in a mixed culture experiment. In the dark, S. trochoidea assimilated iron from SW media containing 50 nM Fe(III) and 500 nM VF at a rate of 0.74 amol of Fe per cell per h. However, this rate increased >20-fold with in situ photolysis of Fe(III)-VF in the presence of attenuated sunlight (Fig. 2C). The photolysis initially produces dissolved Fe(II), which at the pH of SW, should have rapidly reoxidized to dissolved Fe(III)′ and colloidal ferric hydroxide particles. Thus, the enhanced uptake appeared to be linked to the much higher biological availability of these inorganic iron photolysis products, in agreement with previous photolysis experiments with other ferric chelates (20, 23). Similar results were obtained when the Fe-VF complex was photolyzed separately and then added to cultures of S. trochoidea in the dark. The uptake of the photochemically produced Fe(III)′ by S. trochoidea could involve a cell surface reductase/Fe(II) permease system similar to that described in diatoms and green algae (13, 22). Preliminary evidence showing such ferrireductase activity for S. trochoidea cells is consistent with this model (Fig. 4). Consequently, Fe(III)′ acts as a convergence point, from which both the bacteria and the algae use the newly “bioavailable” inorganic iron produced from photochemical redox cycling.
Fig. 4.
Ferrireductase assay of axenic cultures of S. trochoidea. Formation of Fe(II) as measured as its BPDS complex in the presence (open circles) or absence (filled circles) of S. trochoidea cells.
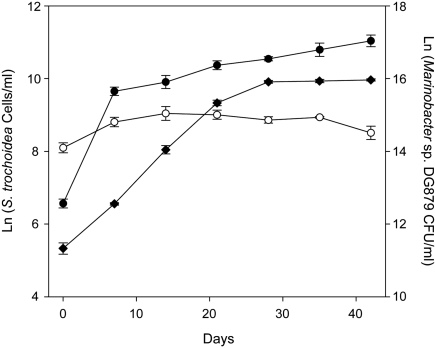
If it is evident from our data that phytoplankton stand to gain from this bacterial-algal interaction, what of the bacteria? Heterotrophic marine bacteria such as Marinobacter ultimately depend on organic matter produced by algae and other phototrophs for their growth and reproduction. However, our observations suggest that a more direct, mutualistic, relationship has evolved between the bacterial and algal partners. We have observed that Marinobacter spp. growth in binary dinoflagellate cultures with S. trochoidea depends on algal growth, whereas the same bacterium growing in algal medium only (no algae present) shows by comparison only static growth (Fig. 5). Marine algae are known to release a complex array of organic compounds during growth, such as sugars, amino acids, and lipids (28), and Marinobacter species are capable of using such compounds as sole carbon and energy sources (14). Genome data of the VF-producing M. algicola DG893 corroborates this apparent adaptation to the utilization of algal exudates, because a range of genes have been identified for sugar and amino acid transport and utilization, as well as metabolism of glycolate, lipids, esters, and aliphatics. This genome data also suggests that an even more intimate relationship can exist between the bacterium and the algal host, because a number of “eukaryotic-like” proteins and secretion systems more typically associated with bacterial pathogens or symbionts have also been identified in M. algicola DG893 and in the genomes of other bacteria associated with algae (29).
Fig. 5.
Growth pattern of a binary culture of the bloom-forming S. trochoidea and an associated VF-producing Marinobacter strain (DG879) in f/2 medium. Growth of S. trochoidea (filled diamonds) in the presence of Marinobacter sp. DG879 (closed circles) is shown alongside Marinobacter sp. DG879 growing alone in f/2 medium (open circles). Error bars represent SD of cell counts from triplicate cultures.
Discussion
The production of VF may be particularly well suited to the cellular characteristics and behavior of bloom-forming dinoflagellates, which may help explain why dinoflagellate–bacterial associations are so widespread. Dinoflagellates are large motile cells (≈10–100 μm in diameter), which often migrate vertically between sunlit, nutrient-depleted surface waters during the day, and deeper, more nutrient-enriched waters at night (30). They are positively phototactic, which causes them to accumulate in dense aggregations at the sea surface during the day. It has recently been shown that hydrodynamic effects also tend to force their accumulation into narrow concentrated subsurface layers (31). Such a dense accumulation of algal cells and associated bacteria should lead to more elevated concentrations of VF in the surrounding SW than would occur if the cells were evenly distributed throughout the water column. Together the high photolysis rate constants of Fe-VF chelates, the lack of competing complexation by the photoproduct, and the accumulation of cells near the sea surface should greatly enhance Fe(III)′ production rates and lead to a highly efficient system for the formation of bioavailable iron. Last, the large size of dinoflagellates causes a further increase in VF concentrations near the cell surface relative to that in the surrounding bulk SW owing to their thick surface diffusive boundary layers (32, 33). The resulting high surface concentrations of VF would help overcome any negative effect of its lower stability constant on its ability to solubilize iron oxides or to compete with the strong iron binding ligands found in SW, whose conditional stability constants (3 × 1011 to 1 × 1013 M−1) are higher than those for VF, but whose concentrations are extremely low (1–3 nM) (19).
It is likely that the low stability of ferric VF chelates is not an accident and has been favored by natural selection to make the chelate more readily reducible, both in terms of photoreduction and bioreduction at cell surfaces, which in turn would increase the bioavailability of the iron to the host algal cells (22, 23). There is an inverse relationship between the stability of Fe(III) siderophore chelates and their ease of reduction, because high chelate stability energetically favors the maintenance of the bound iron in the +3 oxidation state and discourages its reduction to Fe(II) (34). Thus, weakly bound chelates may have been selected evolutionarily in bacterial symbionts to favor iron reduction and iron availability to phytoplankton. There is a tradeoff of course, because if the Fe(III) binding is too weak, the chelators will not compete with the strong Fe-binding ligands in SW or with the formation of Fe hydroxides. However, as noted above, dinoflagellates and other algae may have found a way around this difficulty by increasing the ligand concentration in the vicinity of the cells through the formation of dense blooms and the limited diffusive flux of VF [and of photoproduced Fe(II)′ and Fe(III)′ species] in the surface boundary layer of the cells.
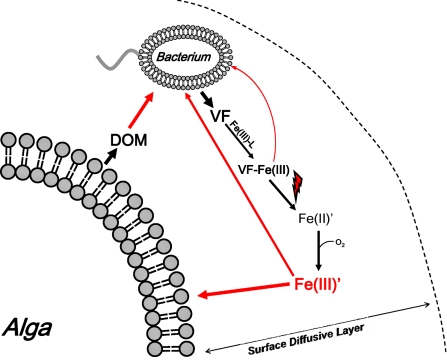
Thus, it is evident from our findings that both bacteria and their algal hosts stand to gain in evolutionary selection through close association with one another. The labile iron produced from photolysis of ferric chelates with VF provides iron to the phytoplankton, which need iron in large amounts to support the photosynthetic fixation of carbon. This fixed carbon subsequently fuels the growth and reproduction of both the phytoplankton and their bacterial associates, and is ultimately used to synthesize the siderophore VF (Fig. 6). Such mutualistic sharing of fixed carbon and iron, some aspects of which have been noted earlier (35), has far reaching implications for the biogeochemical cycling of iron and carbon, and the overall influence of phytoplankton and bacteria on each others' evolution. We need a better understanding of these mutualistic interactions if we are to understand and predict the creation of algal blooms and the response of populations of marine algae and bacteria to changes in their environment. Such an understanding is particularly important given the rapid changes now occurring with coastal eutrophication and current and future climate change.
Fig. 6.
Bacterial–algal mutualism based on the photoreductive dissociation of Fe(III)-VF chelates. On binding iron in the light, Fe(III)-VF photolyzes to ultimately produce Fe(III)′, which is then assimilated by both organisms. In the algae, the assimilated iron is needed in large amounts to support photosynthetic fixation of carbon. Some of this fixed carbon is excreted and used to support bacterial growth and VF production. Due to the thick boundary layer surrounding large algal cells such as dinoflagellates, diffusion of VF and excreted organic carbon away from the algal cell is highly diminished.
Methods
Bacterial Isolation, Culturing, and Growth.
Marinobacter spp. were isolated from algal cultures by serial 10-fold dilution and spread-plating on either a low-strength modified Zobell's marine agar (ZM/10) (14) or artificial (A)SW agar (ONR7a) (36) supplied with n-hexadecane (Sigma–Aldrich) in the vapor phase as the sole carbon source. Incubation was at room temperature in the dark for 1–4 weeks. Routine culture of Marinobacter isolates was on marine agar (2216 or ZM/1) at 30 °C, and cultures were stored frozen at −80 °C in 20% glycerol.
Marinobacter Phylogeny.
Genomic DNA was extracted, and the 16S rRNA gene amplified by PCR according to Green et al. (14). Phylogenetic affiliation was established after automatic alignment by the NAST aligner (http://greengenes.lbl.gov) (37) and importation of the aligned sequences into ARB software using the ARB parsimony tool (38). The alignment was refined and ambiguous positions were masked from the analysis. Phylogenetic inference of the masked alignment was based on maximum likelihood (PHYML) using the GTR model of nucleotide substitutions, with bootstrap support (100 resamplings) for nodes, as implemented in ARB. Gamma-proteobacteria of the genus Salicola were used as an outgroup.
VF Production and Uptake.
VF was isolated as previously described (18) and LC-MS spectra were obtained on a Finnigan LCQ ion-trap mass spectrometer equipped with an ESI source (Finnigan MAT). NMR characterization was carried out on a Varian 500-MHz instrument using standard pulse sequences. Siderophore growth bioassay was performed according to Amin et al. (18).
Determination of the Inability of the Photoproduct to Bind Fe.
The inability of the photoproduct (VF*) to coordinate iron was determined by five different methods: (i) the absence of any ligand-to-metal charge-transfer (LMCT) bands in the UV–visible region always observed for Fe(III)-siderophore complexes; (ii) VF* showed negative results in the universal CAS assay often used to test for siderophores in solution; (iii) at SW pH, VF* failed to prevent the precipitation of an equimolar amount of Fe(III) as the hydroxide (added as FeCl3 solution); (iv) in growth-promotion assays, VF* failed to support the growth of the producing bacteria compared with an equimolar amount of VF, indicating that VF* is not used as an iron scavenger; and (v) solutions of Fe + VF* produced by photolysis subjected to HPLC yielded only pure VF* and not an Fe-VF* complex.
PCR Amplification of VF Biosynthetic Genes pvsAB.
Degenerate PCR primers were designed to amplify a region between the N terminus of PvsA (PvsAf1: GARTGYGAYGTNTTYAAYCC) and C terminus of the PvsB (PvsBr1: CCRTARAAYTTRTTDATRTC), two of the enzymes involved in VF biosynthesis. PCR amplification used standard PCR buffer conditions with 1 μM each primer and 2 mM Mg2+. Cycling conditions used an initial denaturation step of 94 °C for 5 min, followed by 10-cycle step-down annealing profile starting at 58 °C, extension at 72 °C for 3 min, and denaturation at 94 °C for 10 s, then, a further 30 cycles of annealing at 48 °C (30 s), 72 °C for 3 min, and 94 °C for 10 s, and a final 72 °C for 10-min extension. The expected PCR product was ≈3 kbp. PCR products were cloned into pGEM-TEasy (Promega) according to the manufacturer's instructions and sequenced using the forward and reverse M13 sequencing primers and ver 1.1 BigDye terminator chemistry (Applied Biosystems), and electropherograms were obtained by using an ABI 3730xl (Applied Biosystems). Nucleotide sequences were deposited with GenBank under accession numbers EU241703–EU241707.
Axenic Algal Culture Generation and Growth.
S. trochoidea cultures were maintained in f/2 medium prepared with SW collected either from the Santa Barbara Pier or the Tiree passage near Oban, Scotland, and supplemented with selenium (39). They were grown either in borosilicate glass flasks or vented 25-cm2 tissue culture flasks (Greiner Bio-One). Axenic S. trochoidea cultures were generated using a two-stage procedure. Stage 1: S. trochoidea CCAP 1134/1 was induced to form sexual resting cysts by growth in f/2 medium deficient in nitrate and phosphate. Cysts were harvested by micropipette, washed in sterile f/2 medium, and surface-sterilized with 0.1% H2O2. The cysts were then washed with sterile f/2 medium to remove residual H2O2. The cysts were added to sterile f/2 in Petri dishes (Nunc), to which ≈1 × 104 colony-forming units per mL of Marinobacter sp. DG879 was added, and were allowed to germinate and grow out under standard light and temperatures regimes suitable for Scrippsiella. Stage 2: Approximately 1 mL of viable vegetative culture was added to 100 mL of f/2 medium in glass flasks and amended with streptomycin sulfate (30 μg·mL−1; Sigma–Aldrich), and the culture was grown through to stationary phase. Bacterial presence was monitored weekly by spread-plate technique using ZM/10 agar. Cultures from which bacteria had been eliminated were subcultured by adding 1 mL of the axenic culture to 100 mL of sterile f/2 medium containing streptomycin (30 μg·mL−1), and the culture was again grown through to stationary phase. Bacterial contamination was then monitored using agar culture, and the absence of PCR product amplified using the universal bacterial primer pair 27f and 1492r was confirmed (40).
All cultures were grown in a Thermo-818 Illuminated incubator at 18 °C with 14:10 light/dark cycle at a photon flux density of 80 microeinstein (μE)·m−2·s−1. Algal cell counts were routinely monitored using a Trilogy fluorometer (Turner Designs 7200) or using a Sedgwick-Rafter counting slide.
Algal–Bacterial Binary Culture Growth.
S. trochoidea and Marinobacter sp. DG879 binary cultures, or Marinobacter sp. DG879 alone, were grown in triplicate 100-mL flasks containing f/2 medium prepared with natural SW and amended with selenium (39). The Marinobacter-only flasks were inoculated with a suspension of DG879 prepared by resuspending several colonies of DG879 in sterile f/2 medium. S. trochoidea and DG879 binary culture flasks were inoculated with 1 mL of stationary-phase binary culture. Growth was at 15 °C with a 12:12 light/dark cycle at a photon flux density of ≈80 μE·m−2·s−1. All flasks were sampled weekly. Lugol's fixed S. trochoidea cell were enumerated by using a Sedgewick–Rafter chamber. Bacterial colony-forming units were determined by spread-plating 10-fold serial dilutions of Marinobacter on ZM/1 agar (14).
55Fe Uptake by S. trochoidea and Marinobacter sp. DG879.
55Fe-VF complex was prepared by adding a standard solution of FeCl3·6H2O (1 mg/mL; Aldrich) and 55FeCl3 (1,522 MBq/mL; Perkin–Elmer) to VF (final Fe:ligand = 1:10 for algal uptake, and 1:3 for bacterial uptake; final FeCl3:55Fe = 1:0.1) in the dark. The complex was allowed to equilibrate for at least 24 h in the dark before use.
For bacterial iron uptake, cells were grown in ASW (18) with the final pH adjusted to 8.0. The ASW was passed through Chelex-100 resin (Bio-Rad) to remove trace metals and supplemented with 100 nM FeCl3. Cultures were harvested at midexponential growth phase by centrifuging at 6,000 rpm for 20 min in a Sorvall RC5C+ centrifuge followed by washing three times with iron-free ASW. The harvested cells were then diluted with ASW to an optical density at 600 nm of 0.4 and incubated at 4 °C until further use (24). Before the start of the uptake, cells were shaken at 130 rpm and 20 °C for 30 min, after which the experiment was started by adding a 1 μM final 55FeVF concentration. Ascorbate (10 mM) and carbonyl cyanide 3-chlorophenyhydrazone (CCCP; 30 μM) were added 1 h before adding 55FeVF. Uptake experiments at low temperature or in the presence of CCCP were kept in the dark and maintained at 20 °C. Uptake in the presence of the reductant ascorbate was performed by photolyzing the required amount of 55FeVF by using a mercury vapor lamp (175 W) for 30 min at acidic pH and in the presence of 10 mM ascorbate before addition to the culture. Cultures not kept in the dark were exposed to attenuated sunlight (450 μE·m−2·s−1) and maintained at 20 °C using a refrigerated circulating water bath (Neslab). Aliquots were withdrawn at each time point and filtered using a Millipore 1225 sampling vacuum manifold onto 0.6-μm pore size polycarbonate filters (Millipore). Filtered cells were washed with 5 mL of ASW followed by 5 mL of Ti(III)-citrate-EDTA reagent (41) and a final 5-mL rinse with ASW to reductively remove iron oxides and iron bound to cell surfaces. 55Fe cellular uptake was measured using a Beckman–Coulter LS6500 liquid scintillation counter.
Axenic S. trochoidea cultures used in iron uptake experiments were grown in Aquil (886 μM NO3−/36.3 μM PO43−/100 μM EDTA/100 nM Fe supplemented as FeEDTA) (42). All cultures were grown in a Thermo-818 Illuminated incubator at 18 °C with 14:10 light/dark cycle at a photon flux density of 80 μE·m−2·s−1. Exponentially growing cells were harvested by gravity filtration into Aquil medium containing no EDTA, vitamins, iron, or other trace metal additions. The culture sample was concentrated by passage of ≈90% of the culture medium through a 1.2-μm pore polycarbonate filter [(Millipore) housed in an acid-washed Nalge polysulfone filter holder] followed by redilution of the culture with EDTA and trace-metal-free Aquil medium. This procedure was repeated at least four more times to free the culture of residual EDTA. The final diluted culture contained ≈104 algal cells per mL. 55FeVF was then added as previously described for the bacteria, and cultures were either kept in the dark or exposed to attenuated sunlight (450 μE·m−2·s−1). Both cultures were maintained at 20 °C. Aliquots were withdrawn at each time point and filtered onto 3-μm pore size polycarbonate filters (Millipore). Sample washings and counts were done as previously described.
Determination of the Conditional Stability Constant of VF in SW.
To determine the conditional stability constant of VF in SW, 10-mL subsamples of open-ocean surface SW that had been UV-irradiated for 8 h and “chelexed,” were aliquoted into acid-cleaned Teflon cups; 1 nM VF was then added to each cup, followed by boric acid buffer (pH 8) and iron additions ranging from 0 to 6 nM. Subsamples were allowed to equilibrate for 2 h before adding 10 μM salicylaldoxime (SA). After equilibrating for 15 min with SA, samples were analyzed (three independent titrations) by competitive ligand-exchange adsorptive cathodic stripping voltammetry (CLE-ACSV) according to Buck et al. (43). All titrations were done in the dark to prevent photodegradation of VF. The total dissolved iron concentration in the chelexed, UV-irradiated SW (0.1 nM) was determined via ACSV with SA (43).
Photolysis of Fe(III)-VF.
Fe(III)-VF (5, 10, and 15 μM, three replicates each) solutions containing a 10-fold molar excess of total VF were irradiated in the presence of 20, 40, and 45 μM ferrous trapping agent, bathophenanthrolinedisulfonic acid (BPDS; Fluka), respectively. The solutions contained 5 mM Hepes buffer (pH 8.1) and were made up in Aquil (without nutrients, trace metals, EDTA, or vitamins). One set of solutions was exposed to attenuated sunlight (500 μE·m−2·s−1) at 20 °C, whereas a set of control samples was kept in the dark. Initial rates of Fe(II) production were measured by monitoring Fe(BPDS)32+ LMCT band at 535 nm (22,140 M−1·cm−1). For the low light flux experiment, 0.1 mM ferric complexes of VF, petrobactin, and marinobactin solutions were exposed to a fluorescent lamp (80 μE·m−2·s−1, Sylvania Ecologic Cool White, 34 W) at 20 °C in the presence of 50 mM Hepes buffer (pH 8.2) and 0.7 M NaCl. UV–visible spectra were recorded using a Cary 50 spectrophotometer.
Ferrireductase Assay.
Axenic S. trochoidea was grown in acid-washed 250 mL Erlenmeyer flasks in f/2 supplemented with 10−5 M Fe. Late log-phase cells were harvested by gentle filtration onto 10-μm pore polycarbonate membranes, washed twice with equal volumes of sterile natural SW, and finally suspended in an equal volume of sterile natural SW. Triplicate S. trochoidea cell suspensions were incubated at 21 °C in the dark in the presence of 130 μM Hepes buffer (pH 8.1), 10 μM Fe (chelated with 10-fold excess EDTA), and 100 μM of the Fe(II) chelator BPDS. Sterile natural SW was used as a background control. Ferrireductase activity was measured by pelleting the algal cells by centrifugation and measuring the absorbance of Fe(II)BPDS chelates in the supernatant at a wavelength of 535 nm.
Supplementary Material
Acknowledgments.
We thank C. Bolch and T. Gutierrez for access to additional bacteria isolated from algae and Prof. Kathy Barbeau and Randelle M. Bundy (Scripps Institute for Oceanography, University of California, San Diego) for the measurement of the conditional stability constant of VF in SW. This work was supported by National Oceanographic and Atmospheric Administration Grants NA04OAR4170038 and NA08OAR4170669, and California Sea Grant College Program Projects R/CZ-198 and R/CONT-205. D.H.G. was supported in part by a New Zealand Foundation for Research, Science, and Technology postdoctoral fellowship. DNA sequencing was supported by a Natural Environment Research Council (London) Molecular Genetics Facility Grant MGF 122 (to D.H.G.). Genome sequencing of M. algicola DG893 was supported by the Gordon and Betty Moore Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU241703–EU241707).
This article contains supporting information online at www.pnas.org/cgi/content/full/0905512106/DCSupplemental.
References
- 1.Coale KH, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. doi: 10.1038/383495a0. [DOI] [PubMed] [Google Scholar]
- 2.Dymond J, Lyle M. Flux comparisons between sediments and sediment traps in the eastern tropical Pacific: Implications for atmospheric CO2 variations during the Pleistocene. Limnol Oceanogr. 1985;30:699–712. [Google Scholar]
- 3.Keller MD, Bellows WK, Guillard RRL. Dimethyl sulfide production in marine phytoplankton. In: Saltzman ES, Cooper WJ, editors. Biogenic Sulfur in the Environment. Washington, DC: Am Chem Soc; 1989. pp. 167–182. [Google Scholar]
- 4.Cloern JE. Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser. 2001;210:223–253. [Google Scholar]
- 5.Cho BC, Azam F. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature. 1988;332:441–443. [Google Scholar]
- 6.Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438:90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science. 2005;307:1598. doi: 10.1126/science.1105486. [DOI] [PubMed] [Google Scholar]
- 8.Mayali X, Franks PJS, Azam F. Cultivation and ecosystem role of a marine Roseobacter clade-affiliated cluster bacterium. Appl Environ Microbiol. 2008;74:2595–2603. doi: 10.1128/AEM.02191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayali X, Doucette GJ. Microbial community interactions and population dynamics of an algicidal bacterium active against Karenia brevis (Dinophyceae) Harmful Algae. 2002;1:277–293. [Google Scholar]
- 10.Bruland KW, Donat JR, Hutchins DA. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol Oceanogr. 1991;36:1555–1577. [Google Scholar]
- 11.Tortell PD, Maldonado MT, Granger J, Price NM. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol Ecol. 1999;29:1–11. [Google Scholar]
- 12.Vraspir JM, Butler A. Chemistry of marine ligands and siderophores. Annu Rev Mar Sci. 2009;1:43–63. doi: 10.1146/annurev.marine.010908.163712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kustka AB, Allen AE, Morel FMM. Sequence analysis and transcriptional regulation of Iron acquisition genes in two marine diatoms. J Phycol. 2007;43:715–729. [Google Scholar]
- 14.Green DH, Llewellyn LE, Negri AP, Blackburn SI, Bolch CJS. Phylogenetic and functional diversity of the cultivable bacterial community associated with the paralytic shellfish poisoning dinoflagellate Gymnodinium catenatum. FEMS Microbiol Ecol. 2004;47:345–357. doi: 10.1016/S0168-6496(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 15.Alavi M, Miller T, Erlandson K, Schneider R, Belas R. Bacterial community associated with Pfiesteria-like dinoflagellate cultures. Environ Microbiol. 2001;3:380–396. doi: 10.1046/j.1462-2920.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- 16.Hold GL, et al. Characterisation of bacterial communities associated with toxic and non-toxic dinoflagellates: Alexandrium spp. and Scrippsiella trochoidea. FEMS Microbiol Ecol. 2001;37:161–173. [Google Scholar]
- 17.Yamamoto S, Okujo N, Yoshida T, Matsuura S, Shinoda S. Structure and iron transport activity of vibrioferrin, a new siderophore of Vibrio parahaemolyticus. J Biochem. 1994;115:868–874. doi: 10.1093/oxfordjournals.jbchem.a124432. in Japanese. [DOI] [PubMed] [Google Scholar]
- 18.Amin SA, Küpper FC, Green DH, Harris WR, Carrano CJ. Boron binding by a siderophore isolated from marine bacteria associated with the toxic dinoflagellate Gymnodinium catenatum. J Am Chem Soc. 2007;129:478–479. doi: 10.1021/ja067369u. [DOI] [PubMed] [Google Scholar]
- 19.Rue EL, Bruland KW. Complexation of Fe(III) by natural organic ligands in the central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem. 1995;50:117–138. [Google Scholar]
- 20.Barbeau K, Rue EL, Bruland KW, Butler A. Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature. 2001;413:409–413. doi: 10.1038/35096545. [DOI] [PubMed] [Google Scholar]
- 21.Barbeau K. Photochemistry of organic iron(III) complexing ligands in oceanic systems. Photochem Photobiol. 2006;82:1505–1516. doi: 10.1562/2006-06-16-IR-935. [DOI] [PubMed] [Google Scholar]
- 22.Shaked Y, Kustka AB, Morel FMM. A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol Oceanogr. 2005;50:872–882. [Google Scholar]
- 23.Anderson MA, Morel FMM. The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol Oceanogr. 1982;27:789–813. [Google Scholar]
- 24.Küpper FC, Carrano CJ, Kuhn J-U, Butler A. Photoreactivity of iron(III)-aerobactin: Photoproduct structure and iron(III) coordination. Inorg Chem. 2006;45:6028–6033. doi: 10.1021/ic0604967. [DOI] [PubMed] [Google Scholar]
- 25.Abergel RJ, Zawadzka AM, Raymond KN. Petrobactin-mediated iron transport in pathogenic bacteria: Coordination chemistry of an unusual 3,4-catecholate/citrate siderophore. J Am Chem Soc. 2008;130:2124–2125. doi: 10.1021/ja077202g. [DOI] [PubMed] [Google Scholar]
- 26.Shouldice SR, et al. Structural basis for iron binding and release by a novel class of periplasmic iron-binding proteins found in Gram-negative pathogens. J Bacteriol. 2004;186:3903–3910. doi: 10.1128/JB.186.12.3903-3910.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexeev D, et al. A novel protein-mineral interface. Nat Struct Mol Biol. 2003;10:297–302. doi: 10.1038/nsb903. [DOI] [PubMed] [Google Scholar]
- 28.Myklestad SM. Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Sci Tot Environ. 1995;165:155–164. [Google Scholar]
- 29.Worden AZ, Cuvelier ML, Bartlett DH. In-depth analyses of marine microbial community genomics. Trends Microbiol. 2006;14:331–336. doi: 10.1016/j.tim.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Kamykowski D, Milligan EJ, Reed RE. Relationships between geotaxis/phototaxis and diel vertical migration in autotrophic dinoflagellates. J Plankton Res. 1998;20:1781–1796. [Google Scholar]
- 31.Durham WM, Kessler JO, Stocker R. Disruption of vertical motility by shear triggers formation of thin phytoplankton layers. Science. 2009;323:1067–1070. doi: 10.1126/science.1167334. [DOI] [PubMed] [Google Scholar]
- 32.Richardson LL, Stolzenbach KD. Phytoplankton cell size and the development of microenvironments. FEMS Microbiol Ecol. 1995;16:185–191. [Google Scholar]
- 33.Jonsson PR, Pavia H, Toth G. Formation of harmful algal blooms cannot be explained by allelopathic interactions. Proc Natl Acad Sci USA. 2009;106:11177–11182. doi: 10.1073/pnas.0900964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis BL, et al. Voltammetric estimation of iron(III) thermodynamic stability constants for catecholate siderophores isolated from marine bacteria and cyanobacteria. Mar Chem. 1995;50:179–188. [Google Scholar]
- 35.Kirchman DL, et al. Carbon versus iron limitation of bacterial growth in the California upwelling regime. Limnol Oceanogr. 2000;45:1681–1688. [Google Scholar]
- 36.Dyksterhouse SE, Gray JP, Herwig RP, Lara JC, Staley JT. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int J Syst Bacteriol. 1995;45:116–123. doi: 10.1099/00207713-45-1-116. [DOI] [PubMed] [Google Scholar]
- 37.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig W, et al. ARB: A software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH, editors. Culture of Marine Animals. New York: Plenum; 1975. pp. 26–60. [Google Scholar]
- 40.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson RJM, Morel FMM. Distinguishing between extra- and intracellular iron in marine phytoplankton. Limnol Oceanogr. 1989;34:1113–1120. [Google Scholar]
- 42.Price NM, et al. Preparation and chemistry of the artificial algal culture medium Aquil. Biol Oceanogr. 1988/1989;6:443–461. [Google Scholar]
- 43.Buck KN, Lohan MC, Berger C, Bruland K. Dissolved iron speciation in two distinct river plumes and an estuary: Implications for riverine iron supply. Limnol Oceanogr. 2007;52:843–855. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.