Abstract
Recently, various approaches for controlling the embryonic stem (ES) cell microenvironment have been developed for regulating cellular fate decisions. It has been reported that the lineage specific differentiation could be affected by the size of ES cell colonies and embryoid bodies (EBs). However, much of the underlying biology has not been well elucidated. In this study, we used microengineered hydrogel microwells to direct ES cell differentiation and determined the role of WNT signaling pathway in directing the differentiation. This was accomplished by forming ES cell aggregates within microwells to form different size EBs. We determined that cardiogenesis was enhanced in larger EBs (450 μm in diameter), and in contrast, endothelial cell differentiation was increased in smaller EBs (150 μm in diameter). Furthermore, we demonstrated that the EB-size mediated differentiation was driven by differential expression of WNTs, particularly noncanonical WNT pathway, according to EB size. The higher expression of WNT5a in smaller EBs enhanced endothelial cell differentiation. In contrast, the increased expression of WNT11 enhanced cardiogenesis. This was further validated by WNT5a-siRNA transfection assay and the addition of recombinant WNT5a. Our data suggest that EB size could be an important parameter in ES cell fate specification via differential gene expression of members of the noncanonical WNT pathway. Given the size-dependent response of EBs to differentiate to endothelial and cardiac lineages, hydrogel microwell arrays could be useful for directing stem cell fates and studying ES cell differentiation in a controlled manner.
Keywords: hydrogel microwells, stem cell differentiation, WNT signal pathway
The developmental versatility of embryonic stem (ES) cells offers a powerful approach for directing cell fate and is a promising source of progenitors for cell replacement therapy and tissue regeneration (1). ES cells can differentiate into a wide spectrum of cell types, such as cardiomyocytes and endothelial cells, by forming embryoid bodies (EBs) (2). Those lineages arise from distinct mesoderm subpopulations that develop sequentially from premesoderm cells (3). Such lineage specification is highly coordinated with differential changes in gene expression (4–8).
Despite the therapeutic potential of ES cells, one of the significant challenges to their widespread clinical use, is the inability to homogeneously direct ES cell differentiation into specific lineages. One reason for the heterogeneity in EB differentiation is caused from variations in EB size (9, 10). To address this challenge for controlling the differentiation of ES cells, various microscale technologies (i.e., surface patterning, hydrogel microwells, and microfluidic systems) have been developed for directing the stem cell fate (11–15). Micropatterning techniques have been used to evaluate the effect of EB size on ES cell differentiation. For instance, microfabricated adhesive stencils were used to pattern ES cells for controlling initial ES cell aggregate sizes, which influenced the early differentiation to different germ layers (12). In another approach, microcontact printed substrates were used to generate islands of ES cells to regulate the self-renewal of ES cells by local modulation of self-renewal signaling molecules (13). However, although the size of ES cell aggregates has been shown to influence lineage specific differentiation (16, 17), the underlying biology and EB-size mediated factors for ES cell fate has not been well elucidated.
In this study, we elucidated the biological events that regulate EB-size mediated cell fate into cardiac and endothelial lineages by using nonadhesive poly(ethylene glycol) (PEG) hydrogel microwells of various diameters (150, 300, and 450 μm) ES cells formed homogenous EBs with different sizes. In the study of ES cell differentiation into cardiac and endothelial lineage, we found a highly size dependent response. Furthermore, we demonstrated that the differential expression of WNT5a and WNT11, two members of the noncanonical WNT pathway, was directly involved in the size mediated response of the cell aggregates. We further validated these responses by performing the studies to show that the size mediated response could be altered by the modulations of these signaling molecules. These results suggest that microwell-based templates could be important in directing the differentiation of ES cells and for elucidating stem cell differentiation mechanisms by enabling the formation of controlled microenvironments.
Results
Hydrogel Microwell Arrays to Culture ES Cells.
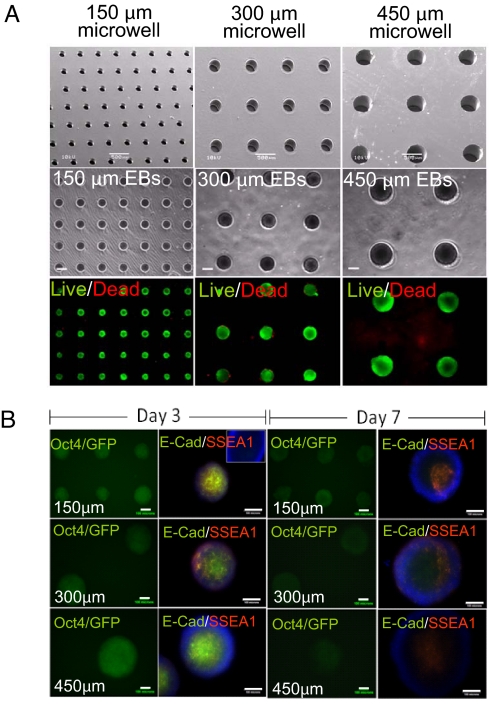
We used a templating approach based on hydrogel microwell arrays to control the size and shape of mouse ES (mES) cell aggregates. To fabricate hydrogel microwells of different diameters (Fig. S1A), we used a micromolding technique that we have previously described (16–18). SEM images (Fig. 1A) showed that microwells were engineered with controllable diameters (150, 300, and 450 μm.) In addition, the results of the cell viability assay demonstrated that although cells were cultured within microwells with different diameters, they remained highly viable after 7 days (Fig. 1A).
Fig. 1.
Arrays of hydrogel microwells for culturing ES cells. (A) Analysis of EBs cultured within microwells for 7 days. Scanning electron microscopy (SEM) images show the formation of uniform arrays of PEG microwells with different diameters (150 μm, 300 μm, and 450 μm) (Top). Phase contrast (Middle) and fluorescent images (Bottom) of EBs cultured within microwells after 7 days. (Scale bar, 100 μm.) Live and dead cells were stained with calcein AM (green) and ethidium homodimer (red). (Scale bar, 200 μm.) (B) The molecular expression of ES cell pluripotency markers after 3 and 7 days. Oct4 expression of ES cells expressing the Oct4/GFP reporter gene (left column for each day). Immunocytochemical staining of SSEA1 (red)/E-cadherin (green) in EBs within microwells (right column for each day). (Scale bar, 100 μm.)
Green fluorescent protein (GFP) expression in Oct4/GFP transfected R1 cell line derived EBs gradually decreased (Fig. 1B). The decrease of Oct4 expression was confirmed by immunocytochemical staining against pluripotency markers, SSEA1 and E-cadherin. At day 3 of culture, relatively strong expression of SSEA1 and E-cadherin on individual cell surface in EBs was detected in all microwells. But, after culturing for 7 days, EBs showed weak SSEA1 and E-cadherin expressions, indicating that they were on the way to differentiate.
Cardiogenesis.
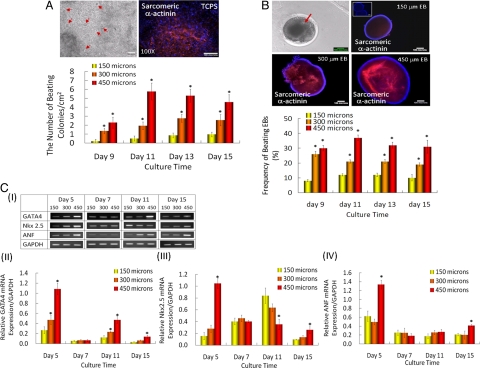
We analyzed the effects of EB size on cardiogenic differentiation by counting the frequency of beating EBs, as well as characterizing the cardiac gene expression. Beating EBs were easily detectable in microwells, which indicated the spontaneous cardiogenic differentiation of mES cells even in basic EB medium. In a parallel study, EBs were retrieved from microwells after 5 days and replated in six-well plates. Within these cultures, cardiomyocytes were readily identifiable from the EB outgrowth due to their spontaneous contractions during the differentiation culture. Cardiomyocytes within beating colonies were small and round (Fig. 2A) and their numbers increased with the initial sizes of the EBs. As shown in Fig. 2B, a higher frequency of beating EBs was also observed in the culture of larger EBs (300 and 450 μm in diameter) that were maintained in the microwells for up to 15 days.
Fig. 2.
EB size-mediated cardiogenic differentiation of ES cells. (A) Morphology and characterization of beating foci (red arrows) in EB outgrowths. Immunocytochemical characterization of cardiomyogenic differentiation and quantification of beating colonies from EB outgrowths that were replated from microwells. Sarcomeric α-actinin expression (red) of EB outgrowths at day 15 of culture. (Scale bar, 100 μm.) (B) Morphology of beating EBs, immunocytochemical characterization of cardiomyogenic differentiation identified by sarcomeric α-actinin and evaluation of beating EBs cultured in microwells. Inset for 150 μm EB figure indicates control stained only with secondary antibody. (Scale bar, 100 μm.) (C) Time course of cardiomyogenic gene expression from EBs within microwells. (I) Gel pictures, (II) GATA4 mRNA expression, (III) Nkx2.5 mRNA expression, and (IV) ANF mRNA expression. Data shown as mean normalized mRNA expression intensity ± SEM (n = 3, * indicates P < 0.05 compared to 150 μm EB).
Figure 2B shows the strong staining of sarcomeric α-actinin in EBs cultured in microwells (450 μm in diameter). In comparison, α-actinin expression was not detectable from smaller EBs (150 μm in diameter). Similar to cardiogenic differentiation of intact EBs within microwells, the outgrowths of EBs that were replated from microwells (450 μm in diameter) also showed strong sarcomeric α-actinin and tropomyosin staining with elongated cardiomyocytes. Adjacent cardiomyocytes showed different degrees of sparse and irregularly organized myofibrillar structure (Fig. 2A).
EB-size dependent cardiomyogenic differentiation was also characterized by evaluating the gene expression of cardiogenic markers, Nkx2.5, GATA4, and atrial natriuretic factor (ANF). Two of the key transcription factors controlling cardiomyogenic differentiation, Nkx2.5 and GATA4, were highly expressed in EBs cultured within larger microwells (450 μm in diameter) (Fig. 2C). Interestingly, GATA4 and Nkx2.5 expression was higher in EBs from 450 μm microwells at early culture time (day 5). This result was consistent with the higher number of beating colonies and strongly positive expression of sarcomeric α-actinin in 450 μm EBs. In cardiogenesis, it is known that GATA4 and Nkx 2.5 are expressed at the early stages during heart development and their expressions occur in a time-dependent manner. GATA4 and Nkx2.5 induce the expression of other genes related with cardiogenic functions, such as those for cell contraction or beating (19–23). This is consistent with the functional analysis, which showed increased beating in 450-μm EBs during the entire culture period and higher expression of cardiogenic markers at early culture time (day 5).
Endothelial Cell Differentiation.
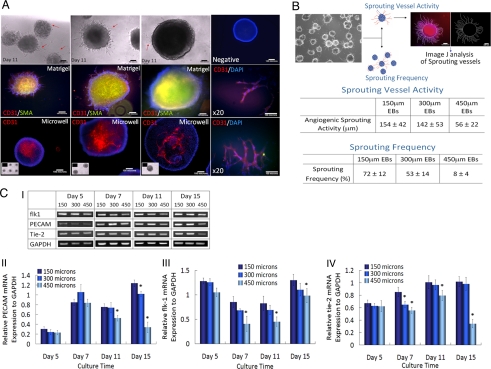
We analyzed the EB-size mediated tendency of ES cells to differentiate into endothelial cells. After EB formation within microwell arrays for 5 days, the EBs were transferred to Matrigel coated substrates in the presence of endothelial cell growth medium. It has been reported that mesoderm and progenitors for endothelial cell lineage are generated in EBs between days 3–5. Vasculogenesis is also achieved by replating the EBs on Matrigel or Type I collagen gel, following 3–5 days of EB formation with the appropriate endothelial supplements (24–27). Hence, in this study, the EBs were transferred to Matrigel for inducing endothelial lineage differentiation after 5 days of culture. Distinct vessel sprouting from EBs could be observed after 6 days (total 11 days) of culture. The EBs from 150-μm and 300-μm microwells showed much higher vessel sprouting activity as compared to EBs from 450-μm microwells (Fig. 3A). These vessel sprouting structures were characterized by immunocytochemical staining with CD31(PECAM) and smooth muscle actin (SMA). Fig. 3A shows the strongly positive reaction against CD31 and SMA in vessel sprouting region and the internal region of EBs from microwells that were 150 μm and 300 μm in diameter. In a parallel study, we analyzed the internal vessel structure within microwells for all EBs that were cultured in microwells. This study revealed that 150 μm and 300 μm EBs showed significantly higher internal vascular structures in comparison with 450 μm EBs. To further quantify the vessel sprouting activities, we measured the average length of sprouting and the percentage of sprouting EBs. A higher frequency of sprouting EBs and a longer sprouting length was observed from EBs with 150 and 300 μm in diameter as compared to EBs with 450 μm in diameter (Fig. 3B).
Fig. 3.
EB size-mediated endothelial cell differentiation of ES cells. (A) Phase contrast and immunocytochemical characterization of endothelial cell differentiation indentified by CD31 (red) and SMA (green) at day 11. EBs retrieved from microwells (Top) and negative control staining only with secondary antibody (Right fluorescent image). ES cells cultured within microwells for 5 days were plated onto Matrigel substrates and were cultured for additional 6 days (Middle) and a fluorescent image of vessel sprouting from EBs on Matrigel (Right fluorescent image). EBs cultured within microwells for 11 days (Bottom) and a fluorescent image of vessel sprouting from EBs on Matrigel (Right fluorescent image). Each inset indicates phase contrast image of corresponding EBs cultured within microwells. (Scale bar, 100 μm.) (B) Characterization of sprouting vessel activity and frequency of EBs cultured within microwells. The sprouting activity and frequency of ES cells cultured within smaller microwells were higher than those within larger microwells. (Scale bar, 100 μm.) (C) Analysis of gene expression of ES cells to identify endothelial cell differentiation. (I) Gel pictures, (II) PECAM mRNA expression, (III) Flk-1 mRNA expression, and (IV) Tie-2 mRNA expression. Data shown as mean normalized mRNA expression intensity ± SEM (n = 3, * indicates P < 0.05 compared to 150 μm EB).
We also characterized the EB-size dependent-endothelial cell differentiation by evaluating the endothelial cell-specific gene expression of flk-1, PECAM, and tie-2. As shown in Fig. 3C, flk1, which is a receptor for vascular endothelial growth factor and normally expressed in endothelial cell or vascular progenitors, is highly expressed in EBs cultured within microwells that were 150 μm and 300 μm in diameter. In addition, EBs cultured within microwells that were 150 μm in diameter showed much higher induction of PECAM transcript in the late stage of culture as compared to larger microwells with 300 and 450 μm in diameter (Fig. 3C). Interestingly, tie-2 expression, a marker indicating endothelial cells, in 150 μm and 300 μm EBs was much higher at late culture time (day 15). Cumulatively, these results suggest that cardiogenesis was developed in larger EBs with the highly reduced endothelial cell differentiation. In contrast, small EBs resulted in much higher endothelial cell differentiation with reduced cardiogenesis.
Differential Expression of WNT5a and WNT11.
To characterize the factors that influence EB-size dependent differentiation into cardiac and endothelial cells, we evaluated the gene and protein expression of various extracellular matrix (ECM) molecules and soluble factors. Specifically, the basement membrane components that can be generated by endoderm within EBs (28) and WNT signaling family, which is known to be important in cardiogenesis and endothelial cell differentiation (4, 26, 29, 30) were evaluated. The genes for various key components of basement membrane were evaluated and showed no significant difference in expression (Fig. S2A) at day 5 of EB formation. This was also supported by the similarity in laminin distribution within EBs of distinct sizes (Fig. S2B). In addition, the canonical WNT2 and noncanonical WNT5a and WNT11 pathways were evaluated. We did not observe significant difference in WNT2 expression and β-catenin expression at day 3 and 5 of EB formation within microwells. In contrast, we detected differential expression of WNT5a and WNT11 in EBs with different sizes (Fig. S2A). The smaller EBs (150 μm in diameter) expressed high levels of WNT5a without WNT11 expression. Meanwhile, the larger EBs (450 μm in diameter) expressed WNT11 with the highly reduced WNT5a expression at day 5 of EB formation. Thus, smaller EBs showed higher expression of WNT5a and higher endothelial cell differentiation while suppressing WNT11 expression. In contrast, larger EBs showed highly increased expression of WNT11 and higher cardiogenesis with low expression of WNT5a (Fig. S2C).
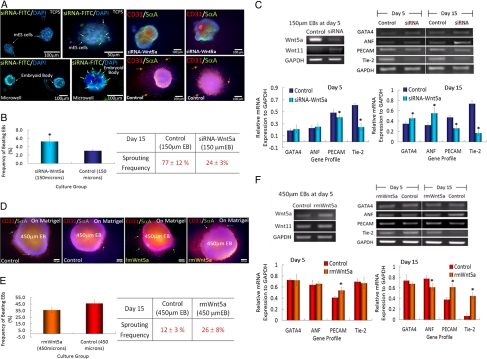
To further confirm the role of WNT5a and WNT11 on regulating EB-size mediated cardiogenesis and endothelial cell differentiation, inhibition and activation test were performed. For the inhibition assay, we evaluated vessel sprouting activity and beating activity of smaller EBs (150 μm in diameter) by silencing the WNT5a gene by WNT5a-siRNA transfection. As shown in Fig. 4A, WNT5a gene silenced 150-μm EBs did not show vessel sprouting structure on Matrigel in endothelial cell culture condition, but showed relatively high expression of sarcomeric α-actinin. This suggests an increase in cardiogenesis and a decrease in vessel sprouting activity, altering the behavior of normal 150-μm EBs (Fig. 4B). The WNT5a gene silencing was confirmed by the highly reduced expression of WNT5a mRNA in WNT5a-siRNA transfected 150-μm EBs (Fig. 4C). Cardiogenic gene (GATA4 and ANF) expression was highly induced in WNT5a gene silenced 150-μm EBs with distinctly low expression of endothelial cell markers, PECAM and tie-2. Accompanied with the inhibition assay, the activation assay was performed by the addition of recombinant mouse WNT5a in the culture of 450-μm EBs on Matrigel. The addition of recombinant mouse WNT5a increased vessel sprouting structure on Matrigel in endothelial cell culture condition, but there was no significant difference in beating activity as compared to normal 450-μm EBs (Fig. 4 D and E). Despite the addition of recombinant mouse WNT5a, there was a slight increase in WNT5a mRNA expression, but no difference in WNT11 mRNA expression. Also, cardiogenic gene (GATA4 and ANF) expression did not show any significant difference except for a slight reduction in ANF expression in 450-μm EBs with the addition of recombinant mouse WNT5a at day 15 of culture. However, the expression of endothelial cell markers, PECAM and tie-2, was increased by the addition of recombinant mouse WNT5a (Fig. 4F).
Fig. 4.
The inhibition and activation of WNT5a for directing EB-size mediated cardiogenic and endothelial cell differentiation of ES cells cultured within microwells. (A) Inhibition analysis: Immunocytochemical characterization for WNT5a-siRNA transfection (green) indicates that siRNA is delivered throughout the cell aggregates (Left). Endothelial cell (red, CD31) and cardiogenic (green, sarcomeric α-actinin) differentiation for siRNA transfected and control EBs. (B) Characterization of EB beating and sprouting frequency. (C) Analysis of gene expression of ES cells cultured within microwells (150 μm diameter) to identify endothelial cell and cardiogenic differentiation. (D) Activation analysis: Immunocytochemical characterization of ES cells cultured with the addition of recombinant mouse WNT5a within microwells (450 μm diameter). (Scale bar, 100 μm.) (E and F) Analysis of cardiogenic and endothelial cell differentiation confirmed by EB beating, vessel sprouting frequency, and gene expression. (n = 3, * indicates P < 0.05 compared to controls).
Discussion
Due to its similarity to the embryonic gastrulation process, EB formation has been commonly used to induce spontaneous differentiation of ES cells (2). The size of EBs is considered as an important parameter that influences ES cell differentiation (31). However, the complex series of interactions within a differentiating cell aggregate is difficult to analyze and is further increased in complexity in that most methods to form EB cultures are either too cumbersome or unable to uniformly control the size of EBs. To address these challenges, various microengineering approaches have been recently developed (12–14). It has been observed that size directs the early germ layer formation within EBs (12, 16). Another approach showed relatively homogenous EB formation using rotating bioreactor culture system (32) that enhanced differentiation into cardiogenic lineage (33). However, previous studies did not analyze the effects of EB size on various tissue types derived from the mesoderm tissue, nor did they elucidate the biological role of morphogenetic signaling molecules (i.e., WNT pathway) on EB-size mediated response. In our work, we extend on these studies to show that cardiac and endothelial cell differentiation, two derivatives of mesoderm lineage, occurs based on developmentally distinct WNT signals that are initiated based on the size of the EBs.
Our results show that uniformly formed EBs within microwells differentiated upon removal of self-renewing factors by losing expression of Oct4, E-cadherin, and SSEA-1. Similar to embryogenesis, in vitro EB formation results in primordial precursor cells that further differentiate to highly specialized phenotypes of cardiac (34) and vascular tissue (27). Interestingly, larger EBs supported cardiac differentiation, while smaller ones generated endothelial cells. This was despite the fact that at early stages the degree of mesoderm formation in the EBs appeared to be independent of the EB size. Thus, this suggests that signals derived from other tissues may have played a critical role in directing the mesoderm tissue to differentiate differently. Based on a previously published report (12), it is known that larger EBs generate a larger degree of early endoderm tissue. Thus, it may be that the inductive signals of early endoderm drive the differentiation of the mesoderm cells in the larger EBs. This is further supported in that endoderm has been reported to be important in both Xenopus and chick embryos (35, 36). Alternatively, the endothelial cell differentiation in smaller EBs may be the result of absence of cues from the endoderm tissue or the presence of inductive cues from the ectoderm and mesoderm tissue.
To further test these hypotheses, we analyzed the role of various signaling molecules on the EBs of various sizes. Given the importance of ECM in regulating the surrounding microenvironment by modulating the biomechanical and biochemical signaling, we tested the level of expression of different ECM components in the EBs. Surprisingly, despite differences in the early expression of endoderm tissue, we did not observe significant difference in the expression of various ECM molecules in the EBs of different sizes. We, therefore, analyzed the role of WNT family members on regulating early patterning and morphogenesis in the developing embryos. WNT5a and WNT11, which have been known to participate in cardiogenic and endothelial lineage differentiation (4), were selected and evaluated. We also studied their differential expression according to EB size mediated ES cell fate specification. By performing these studies, we demonstrated that noncanonical WNT family members, WNT5a and WNT11, were differentially expressed in EBs of different sizes. Previous studies have shown that WNT signaling pathway played an important role in controlling morphogenesis in cardiac (4) and vascular (5) tissue development. WNT5a is a key signaling molecule that mediates endothelial cell proliferation and differentiation (5, 29), while WNT11 plays an important role for cardiac development (4, 30). Our results directly support the notion that the differential expression of these molecules within the EBs, potentially from nonmesoderm tissues, directly contributes to the differentiation of mesoderm precursors to either cardiac or endothelial pathways. This is further supported by other studies that report the differential WNT5a and WNT11 gene expression during embryonic development (37). In our study, we did not observe difference in the WNT2 expression between EBs of different sizes, which has been known to participate in mesoderm formation (38).
Another important feature of our studies may be related to the size of the EBs and their influence on regulating the temporal expression of signaling molecules. It has been previously shown that WNT5a expression was induced from approximately day 4 of EB formation (5) and WNT11 expression was induced from approximately day 6 (4). This change in expression is also correlated by an increase in EB size (Fig. S3). Therefore, it appears that as the size of the EBs is increased with culture time, there is a tendency of WNT5a to down-regulate and to express WNT11, which correlates with our results, showing that WNT11 is expressed in larger EBs. Although this is yet to be proven, it may be that by regulating the size of the EBs and it may be possible to program aspects of the EB's temporal signaling.
The addition and silencing of WNT5a experiments yielded a number of other interesting features. First, although the addition of recombinant WNT5a increased endothelial cell activity in large EBs, we did not observe significant effect on cardiogenic differentiation. This result was similar to a previous study, in which WNT signaling through a β-catenin pathway did not change cardiogenic potential over a range of WNT5a concentrations (39). Thus, the size of EBs can preferentially enhance cardiogenesis simply by suppressing the expression of WNT5a. Second, the silencing of WNT5a in smaller EBs resulted in a decrease in endothelial cell differentiation and an increase in cardiac differentiation. This suggests that upon silencing of WNT5a, the mesoderm precursors in the EBs may change their differentiation pathway from endothelial to cardiac.
Given these features, our results show a deterministic mechanism in which noncanonical WNT pathway controls embryonic cardiac and vascular development as a function of EB size. These data suggest that homogeneous EBs formed within PEG microwells can control WNT signaling pathway to direct cell fate specification. Although it appears that WNT pathway plays an important role in directing cardiac and endothelial cell specification, the relationships between WNT5a and WNT11 or their interactions with other WNT family members remain to be further studied.
Conclusion
We demonstrate that microengineered hydrogel wells can be used to direct ES cell differentiation in a size dependent manner. In particular, cardiogenesis was enhanced in larger EBs, while endothelial cell differentiation was increased in smaller EBs. Furthermore, we found that noncanonical WNT pathway played an important role in controlling cardiogenic and endothelial cell differentiation. Specifically, the higher expression of WNT5a in smaller EBs enhanced endothelial cell differentiation while suppressing WNT11 expression. Given the size-mediated differentiation response, the homogeneous formation of EBs within microwells could be a potentially useful tool for directing ES cell differentiation for regenerative medicine and drug discovery applications.
Methods
Fabrication of Hydrogel Microwell Platforms.
Silicon masters were developed by using published microfabrication procedures (16). Detail methods of microwell fabrication are available in SI Methods.
ES Cell Culture and Embryoid Body Formation.
Methods of ES cell expansion, EB formation, cardiogenic cell differentiation, and following morphological observation and cell viability, as well as, evaluation of beating and vessel sprouting activity are available in SI Methods.
Immunocytochemical Staining.
Detailed method of inhibition and activation assay is available in SI Methods.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR).
Detailed method of RT-PCR is available in SI Methods. Primers and product sizes are detailed in Table S1.
Inhibition and Activation Assay.
Detailed method of inhibition and activation assay is available is SI Methods.
Supplementary Material
Acknowledgments.
This research was partly supported by the National Institutes of Health (DE019024, HL092836, EB007249) and the U.S. Army Corps of Engineers. D. Ortmann was supported by the German Academic Exchange Service (DAAD).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905550106/DCSupplemental.
References
- 1.Wobus AM, Boheler KR. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 2.Itskovitz-Eldor J, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 3.Kouskoff V, et al. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terami H, et al. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 5.Yang DH, et al. Wnt5a Is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving both Wnt/β-Catenin and Protein Kinase C alpha. Circ Res. 2009;104:372–379. doi: 10.1161/CIRCRESAHA.108.185405. [DOI] [PubMed] [Google Scholar]
- 6.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 7.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 8.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nat. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 9.Leahy A, Xiong JW, Kuhnert F, Stuhlmann H. Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J Exp Zool. 1999;284:67–81. doi: 10.1002/(sici)1097-010x(19990615)284:1<67::aid-jez10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Koike M, Sakaki S, Amano Y, Kurosawa H. Characterization of embryoid bodies of mouse embryonic stem cells formed under various culture conditions and estimation of differentiation status of such bodies. J Biosci Bioeng. 2007;104:294–299. doi: 10.1263/jbb.104.294. [DOI] [PubMed] [Google Scholar]
- 11.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J, et al. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip. 2007;7:1018–1028. doi: 10.1039/b704739h. [DOI] [PubMed] [Google Scholar]
- 13.Peerani R, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torisawa Y, et al. Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab Chip. 2007;7:770–776. doi: 10.1039/b618439a. [DOI] [PubMed] [Google Scholar]
- 15.Meyvantsson I, Beebe DJ. Cell culture models in microfluidic systems. Annu Rev Anal Chem. 2008;1:423–449. doi: 10.1146/annurev.anchem.1.031207.113042. [DOI] [PubMed] [Google Scholar]
- 16.Karp JM, et al. Controlling size, shape, and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 17.Moeller HC, et al. A microwell array system for stem cell culture. Biomaterials. 2008;29:752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khademhosseini A, et al. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Durocher D, et al. The cardiac transcription factors Nkx2–5 and GATA-4 are mutual cofactors. EMBO J. 1997;16:5687–5696. doi: 10.1093/emboj/16.18.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang WY, Cukerman E, Liew CC. Identification of a GATA motif in the cardiac alpha-myosin heavy-chain-encoding gene and isolation of a human GATA-4 cDNA. Gene. 1995;155:219–223. doi: 10.1016/0378-1119(94)00893-w. [DOI] [PubMed] [Google Scholar]
- 21.Molkentin JD, Kalvakolanu DV, Markham BE. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Mol Cell Biol. 1994;14:4947–4957. doi: 10.1128/mcb.14.7.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtsu Y, et al. Stimulation of P19CL6 with multiple reagents induces pulsating particles in vivo. Curr Med Res Opin. 2005;21:795–803. doi: 10.1185/030079905X41499. [DOI] [PubMed] [Google Scholar]
- 24.Fehling HJ, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 25.Nakagami H, et al. Model of vasculogenesis from embryonic stem cells for vascular research and regenerative medicine. Hypertension. 2006;48:112–119. doi: 10.1161/01.HYP.0000225426.12101.15. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, et al. Gene expression profile signatures indicate a role for Wnt signaling in endothelial commitment from embryonic stem cells. Circ Res. 2006;98:1331–1339. doi: 10.1161/01.RES.0000220650.26555.1d. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita J, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nat. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara H, et al. Regulation of mesodermal differentiation of mouse embryonic stem cells by basement membranes. J Biol Chem. 2007;282:29701–29711. doi: 10.1074/jbc.M611452200. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin AM, Kitajewski J, D'amore PA. Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors. 2007;25:25–32. doi: 10.1080/08977190701272933. [DOI] [PubMed] [Google Scholar]
- 30.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nat. 2002;418:6360641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 31.Bauwens CL, et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 32.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 33.Niebruegge S, et al. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 34.Boheler KR, et al. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 35.Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–342. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Devlopment. 1995;121:515–523. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- 37.Hardy KM, et al. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, et al. Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. J Biol Chem. 2007;282:782–791. doi: 10.1074/jbc.M606610200. [DOI] [PubMed] [Google Scholar]
- 39.Chen VC, et al. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol. 2008;26:1169–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.