Abstract
Inflammation underlies most age-related diseases, including cancer, but the etiology is poorly understood. One proposed factor is the presence of senescent cells, which increase with age. The senescence response arrests the proliferation of potentially oncogenic cells, and most senescent cells secrete high levels of proinflammatory cytokines and other proteins. The complex senescence-associated secretory phenotype is likely regulated at multiple levels, most of which are unknown. We show that cell surface-bound IL-1α is essential for signaling the senescence-associated secretion of IL-6 and IL-8, 2 proinflammatory cytokines that also reinforce the senescence growth arrest. Senescent human fibroblasts expressed high levels of IL-1α mRNA, intracellular protein, and cell surface-associated protein, but secreted very little protein. An IL-1 receptor (IL1R) antagonist, neutralizing IL-1α antibodies, and IL-1α depletion by RNA interference all markedly reduced senescence-associated IL-6/IL-8 secretion. Depletion of the key IL-1R signaling component IRAK1 also suppressed this secretion, and IL-1α neutralizing antibodies prevented IRAK1 degradation, indicating engagement of the IL-1R signaling pathway. Furthermore, IL-1α depletion reduced the DNA binding activity of NF-κB and C/EBPβ, which stimulate IL-6/IL-8 transcription. IL-1α was a general regulator of senescence-associated IL-6/IL-8 secretion because IL-1α blockade reduced IL-6/IL-8 secretion whether cells senesced owing to DNA damage, replicative exhaustion, oncogenic RAS, or chromatin relaxation. Furthermore, conditioned medium from IL-1α-depleted senescent cells markedly reduced the IL-6/IL-8-dependent invasiveness of metastatic cancer cells, indicating that IL-1α regulates the biological effects of these cytokines. Thus, cell surface IL-1α is an essential cell-autonomous regulator of the senescence-associated IL-6/IL-8 cytokine network.
Keywords: aging, cancer, inflammation, invasion, IRAK1
Cellular senescence is a potent anticancer mechanism that arrests the proliferation of cells at risk for neoplastic transformation. Several lines of evidence suggest this cellular response may also contribute to aging (1, 2). This apparent paradox—that cell senescence can be both beneficial (tumor suppressive) and deleterious (pro-aging)—is consistent with the evolutionary theory that aging phenotypes are a consequence of the declining force of natural selection with age (3, 4). Under conditions in which most organisms evolve, high rates of mortality from extrinsic causes preclude selection for traits that are beneficial at old age. Moreover, traits that are beneficial to young organisms (e.g., tumor suppression) can even be detrimental later in life, a scenario termed “antagonistic pleiotropy” (5, 6).
How might the senescence response be antagonistically pleiotropic? While the senescence growth arrest can suppress cancer, the accumulation of nondividing senescent cells might compromise tissue regeneration or repair. Furthermore, senescent cells develop a complex senescence-associated secretory phenotype (SASP) (7–10). The SASP includes high level secretion of inflammatory cytokines, principally IL-6 and IL-8. Chronic inflammation is a near-universal feature of aging mammals and can drive the pathogenesis of many age-related diseases, ranging from atherosclerosis to late-life cancer (11–13).
Cellular senescence can be caused by myriad stimuli. These causes include dysfunctional telomeres, nontelomeric DNA damage, strong mitogenic signals (especially those caused by oncogene activation), and perturbations to chromatin organization (2). All of these situations are implicated in the etiology of cancer and increase with age.
Senescent cells have been identified in vivo in preneoplastic lesions and aging tissues (1, 2, 14). Furthermore, damaged or senescent cells in preneoplastic or neoplastic tissues also express the inflammatory cytokines IL-6 and IL-8 (15–17), which are major components of the SASP (10, 15, 16). Thus, there is significant evidence that both cellular senescence and the associated inflammatory cytokine secretion occur in vivo.
The SASP entails over-expression and secretion of numerous proteins that can alter tissue microenvironments. The SASP inflammatory cytokines are of particular interest because they promote so many age-related pathologies (11–13). IL-6 and IL-8 are the most robustly expressed of the SASP cytokines (10, 15, 16). In addition to being inflammatory mediators, IL-6 and IL-8 were recently shown to belong to a small number of secreted factors that also reinforce the senescence growth arrest through autocrine and paracrine mechanisms (15, 16, 18, 19).
Very little is known about mechanisms that initiate and maintain the SASP. IL-1α and IL-1β are minor SASP components (10). Both proteins are secreted at low levels, compared to IL-6 and IL-8. IL-1 (both α and β forms) is a multifunctional cytokine that regulates inflammatory and immune responses primarily by initiating a signal transduction cascade that ultimately induces IL-6 and IL-8 expression (20). Recombinant IL-1α and IL-1β bind the same receptor (IL-1R) and exert similar biological effects. However, IL-1β is active solely as a mature secreted form, whereas IL-1α is rarely secreted at high levels and acts either intracellularly or as a cell surface-bound protein (20). Moreover, IL-1α, in contrast to IL-1β, can function as an uncleaved precursor protein (pIL-1α) as well as a processed (cleaved) protein.
Upon binding its receptor, IL-1R, IL-1 induces the formation of a complex containing IL-1R and its coreceptor (IL-1RAcP). This complex triggers a series of cytoplasmic events that ultimately activate the transcription factor NF-κB. NF-κB then transactivates numerous genes, including those encoding IL-6 and IL-8.
Here we show that the expression and secretion of IL-6 and IL-8 by senescent human fibroblasts depend on cell surface-bound IL-1α. Our findings identify a hierarchy by which the SASP is controlled and provide a unique context (the senescence response) in which IL-1α acts.
Results
IL-1α and IL-1β Expression Increase in Senescent Cells.
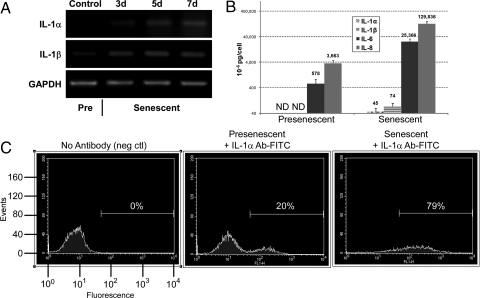
IL-1 expression is known to increase when human fibroblasts undergo senescence, but little is known about its functional role in the senescence response (21). We verified the senescence-associated rise in expression by quantifying mRNA levels for the 2 major agonistic IL-1 family proteins, IL-1α and IL-1β. We exposed presenescent human fibroblasts (strain HCA2) to a senescence-inducing dose of bleomycin, which generates extensive DNA damage. RT-PCR showed that, relative to untreated presenescent controls, transcripts encoding both proteins increased steadily over 7 days (Fig. 1A). Quantitative real time PCR (qPCR) confirmed a 6- to 7-fold senescence-associated increase in these transcript levels [supporting information (SI) Fig. S1A]. The gradual increase over several days matched the kinetics with which the SASP develops (10). In these and subsequent experiments, we confirmed senescence by growth arrest (not shown), morphology, and staining for senescence-associated β-galactosidase (SA-β-gal) (Fig. S1B).
Fig. 1.
IL-1α and IL-1β mRNA and protein levels increase in senescent cells. (A) HCA2 fibroblasts were either untreated or treated with bleomycin (50 μg/ml) for 3 h. Total RNA was collected at the indicated intervals thereafter, and transcript levels for IL-1α, IL-1β, and GAPDH were determined by semiquantitative RT-PCR. Pre, presenescent cells. (B) Cells were either untreated or treated with bleomycin and allowed to recover for 6 d. Conditioned media were collected over the next 24 h and analyzed for IL-1α, IL-1β, IL-6 and IL-8 by ELISA. (C) Cells were either untreated or treated with bleomycin and allowed to recover for 7 d. The cells were collected, washed, incubated in PBS with or without FITC-labeled monoclonal antibodies against IL-1α, and processed by FACS analysis to determine the amount of cell surface-bound IL-1α. The low-intensity peak is background fluorescence for unlabeled cells. The histograms were gated as indicated by the horizontal lines and the percent of total fluorescence signal is shown.
To determine the significance of the rise in mRNA levels, we analyzed intracellular IL-1α and IL-1β protein levels by immunofluorescence. Both proteins were barely detectable in presenescent cells, but the levels increased significantly when cells became senescent (see Fig. S1B). Intracellular IL-1α and IL-1β were distributed throughout the cell.
IL-1α Is Primarily Cell Surface-Associated in Senescent Cells.
IL-1α is rarely secreted or detected in body fluids, although low levels were detected by antibody arrays in conditioned media (CM) from senescent, but not presenescent, cells (10). We validated the antibody array results for IL-1α and IL-1β using more quantitative ELISAs (Fig. 1B). CM from presenescent cells contained undetectable levels of either form of IL-1, although basal levels of IL-6 and IL-8 were detectable. Senescent cells secreted nearly undetectable levels of IL-1α, averaging 45 × 10−6 pg/cell, which was within experimental error. They secreted slightly higher levels of IL-1β (74 × 10−6 pg/cell). As expected (10), secreted IL-6 and IL-8 levels were extremely high (25,366 × 10−6 and 129,836 × 10−6 pg/cell, respectively). Thus, despite high levels of IL-1α and IL-1β mRNA and intracellular protein, neither protein is secreted at high levels by senescent cells.
Because IL-1α, but not IL-1β, is often cell surface-associated, we examined cells for surface-associated IL-1α by FACS analysis using a fluorescent-labeled anti-IL-1α monoclonal antibody (Fig. 1C). The proportion of detectably labeled cells was consistently higher in the senescent population (79%) compared to the presenescent population (20%). This finding suggests that IL-1α acts primarily in a juxtacrine manner.
IL-1α, but Not IL-1β, Regulates IL-6 and IL-8 Secretion by Senescent Cells.
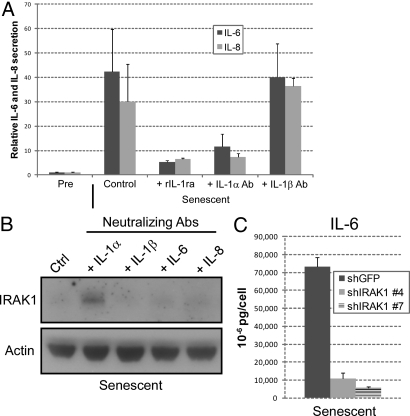
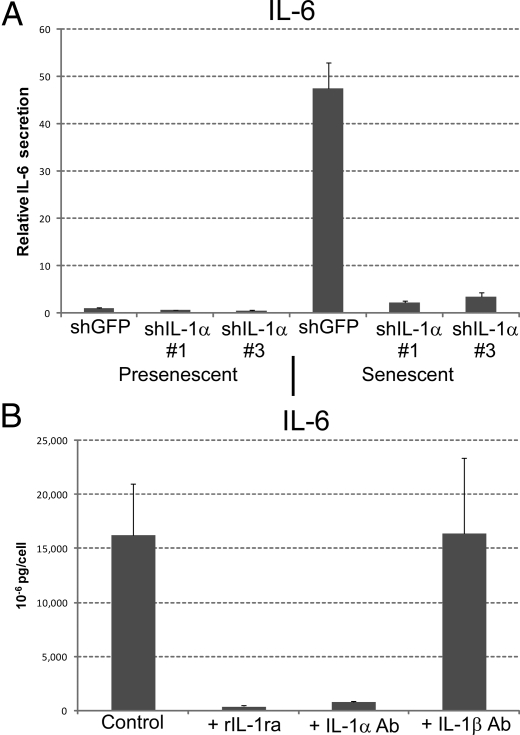
Among the genes known to be induced by IL-1 are the SASP factors IL-6 and IL-8 (20). To examine the role of IL-1 in IL-6/IL-8 secretion by senescent cells, we used recombinant IL-1 receptor antagonist (rIL-1ra), a selective competitive inhibitor of IL-1α and IL-1β binding (22). We added rIL-1ra to cells induced to senesce by bleomycin, and 6 days later collected CM over the next 24 h. rIL-1ra markedly reduced IL-6 and IL-8 secretion by senescent cells (Fig. 2A). To determine the contributions of IL-1α and IL-1β to this reduction, we repeated the experiment using IL-1α or IL-1β monoclonal antibodies, which block interactions between IL-1 ligands and IL-1R (23, 24). IL-1α, but not IL-1β, antibody substantially reduced IL-6 and IL-8 secretion (see Fig. 2A), comparable to the reduction caused by rIL-1ra. Thus, senescence-associated IL-6/IL-8 secretion is controlled by IL-1α, not IL-1β, and is mediated by IL-1R.
Fig. 2.
Expression and secretion of IL-6 and IL-8 depend on IL-1α signaling. (A) HCA2 cells were either untreated or treated with bleomycin and allowed to recover for 6 d. Neutralizing antibodies or rIL-1ra were added for 24 h. CM were collected and analyzed for IL-6 (black bars) and IL-8 (gray bars) by ELISA. Cytokine levels were normalized to those secreted by untreated control cells. Neutralizing antibodies and recombinant proteins were added at the following final concentrations: IL-1α, 0.6 μg/ml; IL-1β, 0.8 μg/ml; rIL-1ra, 240 ng/ml. (B) Whole cell lysates were prepared from cells treated with the indicated neutralizing antibodies 7 d after bleomycin treatment and analyzed by Western blotting for IRAK1 and actin (control) protein levels. (C) Cells infected with shGFP or shIRAK1-expressing lentiviruses were treated with bleomycin and allowed to recover for 6 d. CM were collected over the next 24 h and analyzed for IL-6 by ELISA.
IL-1α Stimulates Senescence-Associated IL-6/IL-8 Secretion Through IL-1R Signaling.
Interleukin-1 receptor-associated kinase 1 (IRAK1) is a key component of the IL-1/IL-1R signaling pathway (25). When IL-1 binds IL-1R, IRAK1 is stimulated by myeloid differentiation primary-response protein 88 and IRAK4 to bind the IL-1R/IL-1RAcP complex. IRAK4 then phosphorylates IRAK1, which recruits or activates additional factors, ultimately allowing nuclear translocation of NF-κB. NF-κB transactivates a variety of genes, including IL-6 and IL-8 (26). After participating in IL1R signaling, IRAK1 is degraded (25). We therefore assessed the status of the IL-1/IL-1R signaling pathway by analyzing steady state IRAK1 protein levels. We incubated senescent cells with neutralizing antibodies against IL-1α, IL-1β, IL-6 or IL-8, and measured IRAK1 levels by Western blotting (Fig. 2B). IRAK1 was barely detectable in control senescent cells and cells exposed to IL-1β, IL-6, or IL-8 antibodies, indicating active IL-1R signaling. By contrast, IRAK1 levels were markedly higher in cells exposed to IL-1α antibody, indicating blocked IL1R signaling.
We also depleted cells of IRAK1 protein by RNA interference (RNAi) using lentiviruses encoding short hairpin (sh) RNAs against IRAK1 or GFP (control) (Fig. S2). IRAK1 depletion (>90%) prevented the increased IL-6 secretion that occurs 7 days following treatment with a senescence-inducing dose of bleomycin (Fig. 2C). Together, these findings suggest that IL-1/IL-1R signaling is engaged solely by IL-1α in senescent cells, and that IL-1R signaling is responsible for IL-6 and IL-8 secretion by senescent cells.
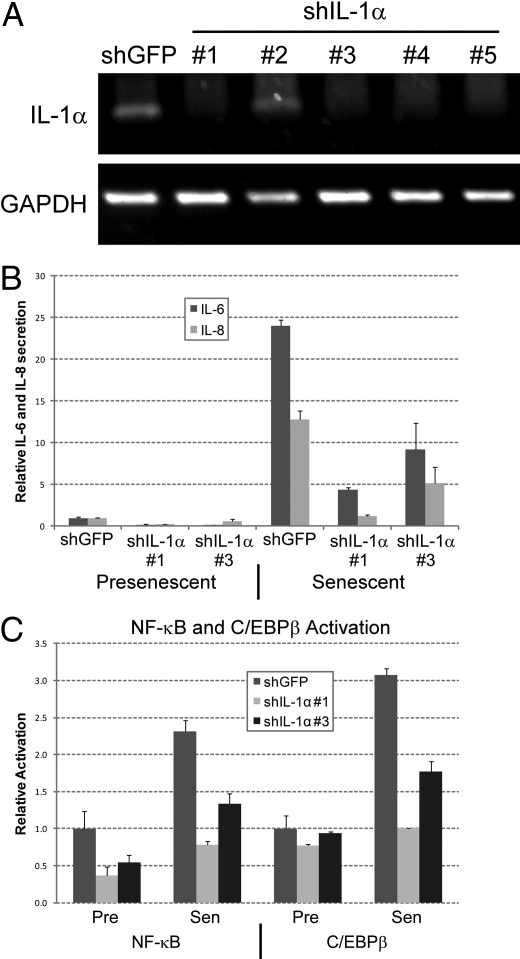
We confirmed that IL-1α is crucial for IL-6/IL-8 secretion by senescent cells by depleting cells of IL-1α by RNAi. We verified loss of IL-1α expression by RT-PCR (Fig. 3A) and immunofluorescence (Fig. S3). Two shRNAs (#1 and #3) strongly depleted IL-1α, with shRNA #1 being more potent than #3. We analyzed IL-1α-deficient cells for IL-6 and IL-8 secretion when presenescent and after bleomycin-induced senescence (Fig. 3B). Consistent with the neutralizing antibody and IRAK1 depletion experiments (see Fig. 2), reduced IL-1α expression suppressed senescence-associated IL-6/IL-8 secretion.
Fig. 3.
Inhibition of IL-1α suppresses the IL-6/IL-8 cytokine response. (A) HCA2 cells were infected with shGFP- or shIL-1α-expressing lentiviruses, selected, and expanded. Total mRNA was collected and transcripts for IL-1α and GAPDH were quantified by RT-PCR. (B) Cells infected with shGFP or shIL-1α-expressing lentiviruses were either untreated or treated with bleomycin and allowed to recover for 6 d. CM were collected over the next 24 h and analyzed for IL-6 (black bars) or IL-8 (gray bars) by ELISA. Levels were normalized to those found in untreated shGFP control cells. (C) Nuclear extracts were prepared from presenescent (Pre) and bleomycin-induced senescent (Sen) cells expressing shGFP or shIL-1α and analyzed for NF-κB (p65) and C/EBPβ activities using TransAM DNA binding ELISAs.
Finally, NF-κB and C/EBPβ, transcription factors that are important for IL-6/IL-8 expression in senescent cells (15, 16), were targets of IL-1α. NF-κB is a known target of IL-R signaling (26); it is not known whether this is true for C/EBPβ. We prepared nuclear extracts from control and IL-1α-depleted presenescent and bleomycin-induced senescent cells, and measured NF-κB (p65) and C/EBPβ DNA binding activities (Fig. 3C). As expected, senescent control cells had more NF-κB (p65) and C/EBPβ DNA binding activity than presenescent control cells. IL-1α depletion significantly reduced NF-κB and C/EBPβ DNA binding activities in senescent cells and reduced NF-κB activity in presenescent cells. These findings indicate that both NF-κB and C/EBPβ are targets of the IL-R signaling that regulates IL-6/IL-8 expression in human fibroblasts.
IL-1α Regulates IL-6 and IL-8 Secretion Caused by Multiple Senescence Inducers.
To determine whether regulation of IL-6/IL-8 secretion by IL-1α is general or specific to DNA damage-induced senescence, we treated cells with sodium butyrate (NaB), a histone deacetylase inhibitor that induces a senescence-like arrest (27, 28). NaB does not cause DNA damage, but rapidly induces a SASP, presumably by relaxing chromatin, which histone deacetylase inhibitors do, although they affect only a small fraction of genes (29). IL-1α-depleted cells treated with NaB secreted strikingly less IL-6 (Fig. 4A) and IL-8 (not shown), compared to NaB-treated control cells. Moreover, IL-1α neutralizing antibodies or rIL-1ra inhibited IL-6/IL-8 secretion by replicatively senescent cells (Fig. 4B), which senesce primarily because of short dysfunctional telomeres (10). Similar results were obtained using cells induced to senesce by oncogenic RASV12 expression (30) (Fig. S4). Thus, IL-1α is an essential positive regulator of senescence-associated IL-6/IL-8 secretion, regardless of the senescence inducer.
Fig. 4.
IL-1α regulates IL-6/IL-8 secretion by cells induced to senesce by different stimuli. (A) HCA2 cells infected with shGFP or shIL-1α-expressing (#1 and #3) lentiviruses were either untreated or treated with 2 mM NaB for 2 d. CM were collected over the next 24 h and analyzed for IL-6 by ELISA. Levels were normalized to those found in untreated shGFP control cells. (B) CM with or without neutralizing antibodies or rIL-1ra were collected for 24 h from replicatively senescent HCA2 cells, and IL-6 was assessed by ELISA. The neutralizing antibodies and rIL-1ra were added at the following final concentrations: IL-1α, 0.6 μg/ml; IL-1β, 0.8 μg/ml; rIL-1ra, 240 ng/ml.
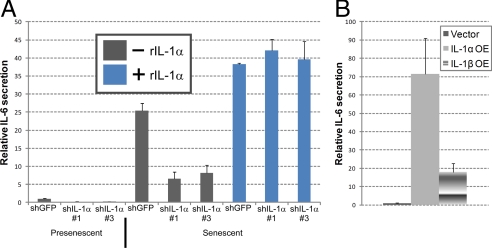
Recombinant IL-1α Restores IL-6/IL-8 Secretion to IL-1α-Deficient Senescent Cells.
To more critically test the idea that IL-1α is the primary trigger for IL-1R signaling and IL-6/IL-8 expression in senescent cells, we added recombinant IL-1α (rIL-1α) to presenescent cells or IL-1α-deficient senescent cells, which express low levels of IL-1α. rIL-1α stimulated presenescent fibroblasts to secrete IL-6 and IL-8 in a dose-responsive manner (Fig. S5). Moreover, rIL-1α completely restored IL-6 (Fig. 5A) and IL-8 (not shown) secretion to IL-1α-deficient senescent cells. rIL-1α stimulated ∼60% more IL-6 secretion compared to untreated senescent cells, possibly because the rIL-1α concentration (10 ng/ml) exceeded endogenous IL-1α levels in senescent cells. It is difficult to estimate the total amount of active IL-1α in cells because both intracellular and surface-associated forms contribute to this value. We conclude that extracellular stimulation of IL-1R by rIL-1α is sufficient to induce IL-6/IL-8 in presenescent cells and rescue the diminished IL-6/IL-8 secretion by IL-1α-deficient senescent cells.
Fig. 5.
Recombinant IL-1α complements IL-1α deficient senescent cells. (A) HCA2 cells infected with shGFP or shIL-1α-expressing (#1 and #3) lentiviruses were treated with bleomycin and allowed to recover for 6 d. Recombinant IL-1α (10 ng/ml) was added over the next 24 h and CM were collected for IL-6 assessment by ELISA. (B) CM collected for 24 h from IL-1α- or IL-1β-expressing HCA2 cells were analyzed for IL-6 secretion by ELISA.
We also stably expressed IL-1α and IL-1β in presenescent cells. Lentiviral vectors efficiently over-expressed IL-1α and IL-1β proteins, as determined by immunofluorescence (Fig. S6A). ELISAs showed that IL-1α-expressing cells secreted 72-fold more IL-6 compared to unmodified or insertless vector-expressing cells. By contrast, IL-1β-expressing cells secreted only 18-fold more IL-6 (Fig. 5B). Morever, IL-1α, but not IL-1β, expression reduced cell proliferation (not shown) and increased p21 expression (Fig. S6B), consistent with the finding that the IL-6/IL-8 network can reinforce a growth arrest (15, 16) and supporting the conclusion that IL-1α regulates IL-6/IL-8 secretion by senescent cells.
IL-1α-Deficiency Reduces the Ability of Senescent Cells to Promote Cancer Cell Invasion.
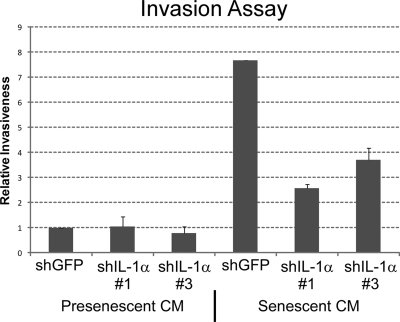
IL-6/IL-8 can stimulate cancer cells to invade a basement membrane, a crucial step in metastasis (31–33). Morever, both cytokines are important for the cancer cell invasiveness stimulated by the SASP (10). We therefore assayed CM from control and IL-1α-deficient senescent cells for their ability to stimulate human breast cancer cells (MDA-MB-231) to invade a basement membrane (Fig. 6). CM from IL-1α-depleted cells were significantly less able to stimulate MDA-MB-231 cell invasion compared to CM from control cells. Moreover, the extent of decreased invasion correlated with the degree of IL-1α depletion (see Fig. 3). This result confirms the importance of IL-1α for stimulating IL-6/IL-8 secretion, and shows that IL-1α is also responsible for at least this (cancer cell invasiveness) biological activity of IL-6 and IL-8.
Fig. 6.
CM from IL-1α deficient cells fails to support cancer cell invasiveness. The Boyden chamber invasion assay was used to determine the effects of CM from senescent HCA2 cells infected with shGFP or shIL-1α-expressing (#1 and #3) lentiviruses on the invasiveness of MDA-MB-231 human breast cancer cells. Data are presented as relative invasiveness of the cells through the Matrigel, where the levels are normalized to invasiveness stimulated by CM from shGFP control cells.
Discussion
Inflammation is the first immune response to infection or irritation. It entails a number of events, including recruitment of immune cells, tissue remodeling, and increased blood flow. As a short-term response, inflammation is crucial for killing invading organisms or eliminating foreign particles. However, chronic inflammation is implicated in almost every major disease associated with aging, including cancer (11–13). Cellular senescence, a well-established tumor suppressive mechanism (2, 34), has also been proposed to drive aging and age-related disease (1, 2), most recently through the SASP (10). Senescent cells secrete proinflammatory cytokines, proteases, and other proteins that can alter tissue microenvironments. IL-6 and IL-8 are among these secreted proteins and can act in an autocrine manner to reinforce the senescence growth arrest (15, 16). Other SASP factors affect neighboring cells, promoting degenerative or hyperproliferative changes (7–9, 35) and stimulating cancer cell invasiveness (10). IL-6 and IL-8 play major roles in the invasion promoting activity. Thus, the mechanisms that control IL-6/IL-8 expression and secretion by senescent cells are of great interest, and potential importance for developing strategies to restrain their inflammatory effects.
We identified the multifunctional cytokine IL-1α as a key positive regulator of IL-6/IL-8 expression and secretion by senescent human fibroblasts. This IL-1α-dependence was observed for multiple senescence inducers. Moreover, IL-1α was essential for the senescence-associated secretion of IL-6/IL-8 through continuous stimulation of the IL-1R by cell surface-associated IL-1α. Furthermore, CM from IL-1α-deficient senescent cells markedly reduced the invasiveness of a highly metastatic human breast cancer cell line, which we previously showed depended on IL-6 and IL-8 (10). This result identifies IL-1α as crucial for the ability of senescent cells to create a tissue microenvironment that promotes cancer progression (11, 12).
Early research on IL-1α demonstrated that it initiates an inflammatory response by binding its plasma membrane-associated IL-1R (36). However, IL-1α is rarely secreted in vivo and not commonly detected in body fluids, except in severe pathological states (in which cell death might account for its release) (20). As an intracellular molecule, IL-1α can regulate cell growth, senescence, differentiation, and gene expression (37–42). In addition, an active form of IL-1α was shown to be anchored to the plasma membrane, and surface-bound IL-1α was shown to act in a juxtacrine manner through the IL-1R (43, 44). Our findings suggest this is also the case in senescent human fibroblasts. We detected IL-1α intracellularly and on the plasma membrane. Because IL-6/IL-8 secretion was neutralized by extracellular anti-IL-1α antibodies and CM from senescent cells contained nearly undetectable IL-1α levels, we conclude that IL-1α up-regulates IL-6/IL-8 secretion by senescent cells principally as the plasma membrane-bound form. Senescent cells secreted low levels of IL-1β, but IL-1β was incapable of engaging IL-1R signaling because IL-6/IL-8 secretion continued unabated in the presence of IL-1β neutralizing antibodies. Thus, IL-1α, not IL-1β, is the primary upstream driver of IL-6/IL-8 secretion by senescent human cells.
Engagement of the cell surface IL-1R was essential for the IL-6/IL-8 response of senescent cells. As a competitive inhibitor of ligands that bind IL-1R, rIL-1ra prevented IL-1α (and IL-1β) from binding IL-1Rs. This interference disrupted the autocrine, receptor-mediated, positive feedback loop that entails IL-1α stimulating its own expression through activation of NF-κB (45). This autocrine production of IL-1α was halted by IL-1ra. Previous findings showed that intracellular IL-1α can provide a complete signal for IL-6/IL-8 expression—in the absence of cell surface receptor signaling (42). We cannot exclude the possibility that intracellular IL-1α also plays a role in regulating IL-6/IL-8 secretion by senescent cells. However, the rIL-1ra and neutralizing antibody experiments clearly demonstrated that sustained IL-1R stimulation by surface-bound IL-1α is required to maintain senescence-associated IL-6/IL-8 secretion.
IL-1α is known to stimulate IL-6/IL-8 expression through NF-κB (26, 45). In addition, C/EBPβ was recently shown to be important for IL-6/IL-8 expression by senescent cells (15, 16). IL-1α depletion reduced the DNA binding activities of both these transcription factors in senescent cells. Thus, IL-1α regulates the senescence-associated IL-6/IL-8 cytokine network through both NF-κB and C/EBPβ.
Inflammation is a vital physiological mechanism for survival in an environment replete with pathogenic microorganisms. When chronic, however, inflammation contributes to a variety of pathologies, including many age-related diseases (11–13). Although cellular senescence is an effective tumor suppressor mechanism, it also entails the secretion of proinflammatory cytokines, possibly to alert neighboring cells to tissue damage (17). Recent findings show that mice harboring chronic DNA damage caused by short telomeres also secrete inflammatory mediators (46). Our finding that IL-1α signaling is an upstream regulator of senescence-associated IL-6/IL-8 secretion suggests possibilities for mitigating the proinflammatory effects of senescent cells by specifically inhibiting IL1R signaling.
Materials and Methods
Cells.
HCA2 primary foreskin fibroblasts were obtained from J. Smith (University of Texas, San Antonio) and cultured as described (8, 10, 17, 47). To generate lentiviruses, 293FT packaging cells (Invitrogen) were used. Presenescent cells completed <35 population doublings and had a 24 h BrdU labeling index of >75%. Cultures were considered replicatively senescent when they had labeling indices of <5%. For damaged-induced senescence, cells were treated with bleomycin at 50 μg/ml for 3 h. On the day 7 following treatment, cells were stained for SA-β-gal (47).
Viruses and Infections.
Lentivirus-expressing oncogenic RASV12 was described (17). Lentiviruses encoding shRNAs against GFP and IL-1α were purchased from Open Biosystems. The shRNA sequences are provided in SI Methods. Virus titers were adjusted to infect 95% to 99% of cells. Infected cells were selected in puromycin for 4 d.
Immunofluorescence.
Cells were cultured in 4-well chamber-slides (Nunc), fixed in paraformaldehyde, and permeablized in PBS (PBS)/0.5% Triton X-100, as described (10, 17). Slides were blocked with 5% FBS in PBS. Primary antibodies were diluted (1:200) in blocking buffer and incubated with fixed cells for 1 h at room temperature. Slides were washed, incubated with secondary antibodies (1:500 dilution) for 1 h at room temperature, washed, and mounted with ProLong Gold antifade reagent (Invitrogen).
Antibodies.
IL-1α (MAB200), IL-1β (MAB601), IL-6 (AF-206-NA), IL-8 (MAB208), and IL-1β-Fluorescein (FAB200F) antibodies were obtained from R&D Systems, IRAK1 (sc-5288) and p53 (sc-126) antibodies were from Santa Cruz Biotechnology, Inc., and p21 (556430) antibody was from BD Biosciences.
Recombinant Proteins.
Recombinant IL-1α (200-LA), IL-1b (201-LB), IL-1ra (280-RA) and EGF (236-EG) were obtained from R&D Systems.
NF-κB and C/EBPβ Binding Activity Assays.
Nuclear extracts were prepared using the Nuclear Extract Kit (40010) from Active Motif. NF-κB and C/EBPβ DNA binding activities were determined using the TransAM NF-κB p65 kit (40096) or C/EBP α/β kit (44196), respectively, both from Active Motif.
Semiquantitative RT-PCR and Real Time qPCR.
Details regarding the RT-PCR and real time qPCR are provided in SI Methods.
CM and ELISA.
CM were prepared by washing cells twice in PBS and incubating in serum-free DMEM containing antibiotics for 24 h. Cell number was determined in every experiment. ELISAs were performed using kits and procedures from R&D Systems (IL-6 #D6050, IL-8 #D8000C, IL-1α #DLA50 and IL-1β #DLB50). The data were normalized to cell number and reported as 10−6 pg secreted protein per cell per day.
Western Blots.
Detection of Membrane-Bound IL-1α by Flow Cytometry.
For detection of membrane-bound IL-1α by flow cytometry, 2.5 × 105 cells were washed twice with PBS, removed from the culture plate with 10 mM EDTA/PBS, pelleted, washed twice with 0.5% BSA/PBS, blocked with human IgG, and incubated with FITC-labeled monoclonal IL-1α antibodies (10 μl/1.0 × 105 cells) for 45 min at 4 °C in the dark. The cells were washed twice with 0.5% BSA/PBS and resuspended in PBS for analysis using a BD LSR flow cytometer.
Invasion Assay.
Details regarding the invasion assay are provided in SI Methods.
Acknowledgments.
We thank Drs. Pierre Desprez and Francis Rodier for valuable comments and critical reading of the manuscript. This work was supported by Grants P01-AG025901, P30-AG025708, R37-AG09909, U54-CA12654, and T32-AG000266 from the National Institutes of Health, and the Larry L. Hillblom Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905299106/DCSupplemental.
References
- 1.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 3.Medawar PB. An Unresolved Problem of Biology. HK Lewis: London; 1952. [Google Scholar]
- 4.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- 6.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 7.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 8.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 10.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apte RN, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Millis AJ, Baglioni C. Expression of interleukin 1-inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA. 1992;89:4683–4687. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf JS, et al. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001;7:1812–1820. [PubMed] [Google Scholar]
- 23.Giri D, Ittmann M. Interleukin-1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2000;157:249–255. doi: 10.1016/s0002-9440(10)64535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lottaz D, Beleznay Z, Bickel M. Inhibition of ATP-binding cassette transporter downregulates interleukin-1beta-mediated autocrine activation of human dermal fibroblasts. J Invest Dermatol. 2001;117:871–876. doi: 10.1046/j.0022-202x.2001.01451.x. [DOI] [PubMed] [Google Scholar]
- 25.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 26.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munro J, Barr NI, Ireland H, Morrison V, Parkinson EK. Histone deacetylase inhibitors induce a senescence-like state in human cells by a p16-dependent mechanism that is independent of a mitotic clock. Exp Cell Res. 2004;295:525–538. doi: 10.1016/j.yexcr.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Ogryzko VV, Hirai TH, Russanova VR, Barbie DA, Howard BH. Human fibroblast commitment to a senescence-like state in response to histone deacetylase inhibitors is cell cycle dependent. Mol Cell Biol. 1996;16:5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gialitakis M, et al. Coordinated changes of histone modifications and HDAC mobilization regulate the induction of MHC class II genes by Trichostatin A. Nucleic Acids Res. 2006;34:765–772. doi: 10.1093/nar/gkj462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 31.Kellokumpu-Lehtinen P, et al. Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. Int J Cancer. 1996;66:515–519. doi: 10.1002/(SICI)1097-0215(19960516)66:4<515::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Harris AL, et al. Gene therapy through signal transduction pathways and angiogenic growth factors as therapeutic targets in breast cancer. Cancer. 1994;74:1021–1025. doi: 10.1002/1097-0142(19940801)74:3+<1021::aid-cncr2820741508>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Edwards DR, Murphy G. Cancer. Proteases—invasion and more. Nature. 1998;394:527–528. doi: 10.1038/28961. [DOI] [PubMed] [Google Scholar]
- 34.Ohtani N, Mann DJ, Hara E. Cellular senescence: Its role in tumor suppression and aging. Cancer Sci. 2009;100:792–797. doi: 10.1111/j.1349-7006.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 37.Cozzolino F, et al. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci USA. 1990;87:6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garfinkel S, Brown S, Wessendorf JH, Maciag T. Post-transcriptional regulation of interleukin 1 alpha in various strains of young and senescent human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 1994;91:1559–1563. doi: 10.1073/pnas.91.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawaguchi Y, Hara M, Wright TM. Endogenous IL-1alpha from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest. 1999;103:1253–1260. doi: 10.1172/JCI4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier JA, Statuto M, Ragnotti G. Endogenous interleukin 1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol Cell Biol. 1994;14:1845–1851. doi: 10.1128/mcb.14.3.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozaki S, Sledge GW, Jr, Nakshatri H. Cancer cell-derived interleukin 1alpha contributes to autocrine and paracrine induction of pro-metastatic genes in breast cancer. Biochem Biophys Res Commun. 2000;275:60–62. doi: 10.1006/bbrc.2000.3241. [DOI] [PubMed] [Google Scholar]
- 42.Werman A, et al. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplanski G, et al. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- 44.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci USA. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura H, et al. Molecular analysis of constitutive IL-1alpha gene expression in human melanoma cells: autocrine stimulation through NF-kappaB activation by endogenous IL-1alpha. Cytokine. 1998;10:872–879. doi: 10.1006/cyto.1998.0369. [DOI] [PubMed] [Google Scholar]
- 46.Jiang H, et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci USA. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]